Highlights
-
•
High STAT3 is correlated with poor prognosis in GBM.
-
•
Bazedoxifene targets IL-6-mediated sustained STAT3 activation.
-
•
STAT3 drives glioma stem cell maintenance, bazedoxifene targets this pathway.
-
•
Bazedoxifene crosses the BBB and prolongs survival in GBM bearing mice.
-
•
Bazedoxifene-treated tumors have less activated STAT3.
Keywords: Glioblastoma, IL6, STAT3, Bazedoxifene, Glioma stem cells, Cancer stem cells
Abbreviations: GBM, glioblastoma; BBB, blood-brain barrier; STAT3, signal transducer and activator of transcription 3; IL6, interleukin-6; BZA, Bazedoxifene; EGFR, Epidermal growth factor receptor; JAK1, Janus Kinase 1; SOCS3, suppressor of cytokine signaling -3; IL6Rα, Interleukin-6 receptor chain alpha; GSC, glioma cancer stem-like cell; CNS, central nervous system; PDX, Patient-derived xenograph; eLDA, extreme limiting dilution assay; IP, intraperitoneal
Abstract
An important factor correlated with poor survival in glioblastoma (GBM) is the aberrant and persistent activation of STAT3, a critical transcription factor that regulates multiple genes with key roles in cell survival, proliferation, resistance to chemotherapy, and stem cell maintenance. The Interleukin-6 (IL6)-STAT3 signaling axis has been studied extensively in inflammation and cancer. However, it is not completely understood how high levels of activated STAT3 are sustained in tumors. Previously, we identified a novel mechanism of biphasic activation of STAT3 in response to gp130-linked cytokines, including IL6, in which activation of STAT3 is prolonged by circumventing the negative regulatory mechanisms induced by its initial activationTo target prolonged STAT3 activation, we used the small molecule inhibitor bazedoxifene (BZA), which blocks formation of the IL6 receptor-gp130 complex. Glioma stem-like cells (GSCs) are more tumorigenic and more resistant to therapy. STAT3 is a key driver of the expression of stem cell transcription factors, making it a therapeutically important target in GBM. We show that treating GSCs with BZA decreases their self-renewal capacity and the expression of GSC markers in vitro. Additionally, BZA crosses the blood-brain barrier and confers a survival advantage in an orthotopic syngeneic mouse model of GBM. Although IL6-STAT3 signaling is important for GSC survival, a therapeutic agent that inhibits this pathway without toxicity has yet to be identified. Our findings reveal a mechanism of sustained STAT3 signaling in GBM and reveal its role in GSC maintenance, and we identify BZA as a novel candidate for treating GBM.
Introduction
Glioblastoma (GBM) is one of the most aggressive forms of brain tumor, with a 5-year survival rate of less than 8% [1]. GBM is challenging to treat for many reasons, including its aggressiveness, its diffuse nature, and the inability of therapeutic agents to cross the blood-brain barrier (BBB). These challenges have left patients diagnosed with GBM few treatment options beyond the standard of care, which involves surgical resection followed by treatment with temozolomide and radiation [2]. These therapies may slow the progression of the disease, but provide only a slight survival advantage, with an average increase in survival of 3 months following treatment [3].
A high level of activated STAT3 is frequently observed in GBM and has been correlated with decreased patient survival [4]. STAT3, a transcription factor activated by several different cytokines, plays a major role within the microenvironment of nervous system tumors [5,6]. Prolonged activation of STAT3 significantly contributes to tumor progression by driving high expression of many oncogenes [7,8].
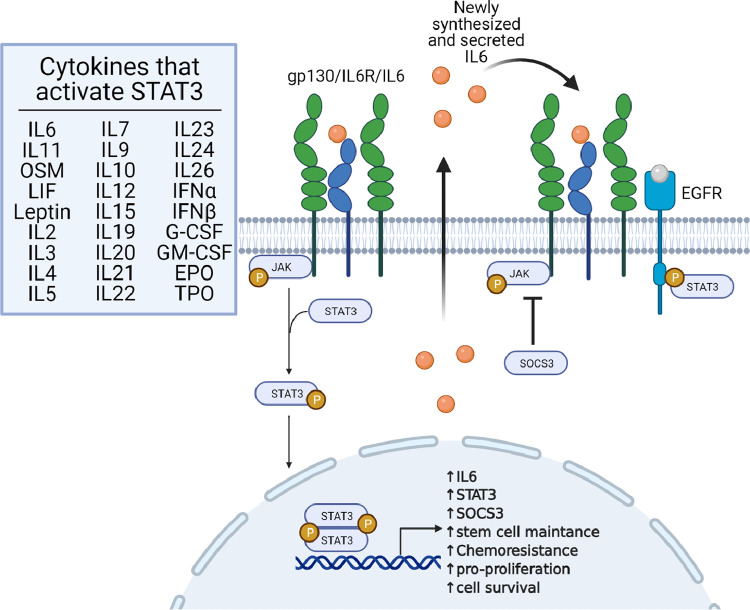
The canonical mechanism of interleukin-6 (IL6)-induced tyrosine phosphorylation of STAT3 (pY705 STAT3) has been studied extensively in the context of inflammation and cancer. However, it is not completely understood how high levels of pSTAT3 are sustained in tumors, since powerful negative regulatory mechanisms inhibit its phosphorylation soon after cells are exposed to STAT3-activating cytokines. Our previous work led to the discovery of a biphasic activation of STAT3 in response to gp130-linked cytokines, including IL6 and oncostatin M (OSM) [9]. We have formulated a working model (Fig. 1) to explain how, after the initial negative regulation of STAT3 activation, a late, sustained phase of STAT3 activation occurs that allows for continuous transcriptional activation of the pSTAT3 target genes. We propose that the sustained activation of STAT3 occurs through a mechanism distinct from that of the initial activation, involving the phosphorylation of STAT3 through EGFR rather than gp1309.
Fig. 1.
Illustration of the biphasic activation of STAT3. The image is adapted from our original article describing the mechanism of sustained activation of STAT3 in cancer cells [9]. The image was created with BioRender.com.
The canonical signaling pathway for IL6-mediated STAT3 activation involves the binding of IL6 to the IL6Rα/gp130/JAK1/JAK2 receptor complex [10,11]. The JAKs autophosphorylate, leading to their full activation, and then phosphorylate gp130 to provide docking sites for the subsequent recruitment of STAT3, followed by its phosphorylation on Y705 [12]. Phosphorylated STAT3 dimerizes and translocates into the nucleus where it activates the transcription [13] of many genes, including STAT3, IL6, and SOCS3 [14,15]. The negative regulator SOCS3 binds to JAKs and inhibits their ability to phosphorylate STAT3, thus blocking the signaling cascade [14,16]. Our previous data show that association of the IL6 receptor complex with EGFR prolongs the activation of STAT3 by avoiding SOCS3-dependent inhibition, thus increasing and prolonging the expression of many STAT3-dependent genes involved in the progression of cancers, including GBM, where both IL6 secretion and EGFR levels are often elevated [9,17,18]. Therefore, it is critical to develop therapies that target IL6 or IL6Rα to block sustained STAT3 activity.
Recurrence is a major obstacle for GBM patients, 70% of whom experience recurrent disease [19]. A small proportion of the tumor includes glioma stem-like cells (GSCs) that are resistant to therapy and are highly tumorigenic [20], [21], [22], [23]. GSCs play a significant role in progressive and recurrent disease [24]. STAT3 knockdown inhibits the ability of GSCs to self-renew [25], because STAT3 drives the expression of three well-known neural stem cell transcription factors: Sox2, Oct4, and Nanog [26,27], making STAT3 an obvious therapeutic target in GSCs.
Many inhibitors have been developed that target the IL6/STAT3 pathway. Antibodies currently used in the clinic that directly target IL6 or IL6R do not cross the blood-brain barrier, making them poor candidates for treating central nervous system (CNS) tumors. Small molecule inhibitors that target JAKs are used to treat some cancers, but JAKs are essential components of many cytokine signaling pathways, raising important concerns regarding specificity [28,29]. Several small molecule inhibitors target STAT3 itself; however, some target the SH2 domain [28,29], which is conserved among the STAT proteins, decreasing their specificity [30]. Furthermore, even STAT3-specific inhibitors target the entire family of cytokines that use the gp130 common receptor subunit, preventing a targeted approach against IL6 alone.
Although several different STAT3 inhibitors have entered clinical trials, they have shown strong toxicity and were discontinued [31]. Finding a novel target responsible for sustaining prolonged STAT3 activation could lead to a therapy that preferentially inhibits GSCs, which the current standard of care fails to do. The present work explores the therapeutic potential of the small molecule inhibitor Bazedoxifene (BZA) because of its ability to inhibit IL6-mediated STAT3 activation in GBM cells. BZA is currently used in the clinic as a third-generation selective estrogen receptor modulator to treat postmenopausal osteoporosis [32]. More recently, it was discovered that BZA binds to gp130, a receptor subunit that cooperates with several partners, including the IL6 receptor [33]. Importantly, BZA preferentially disrupts the gp130-IL6 receptor complex and has only minor effects on the binding of gp130 to its other partners (e.g., OSM receptor) [33]. We hypothesized that BZA should have a signifiant inhibitory effect on IL6-mediated STAT3 activation, which is critical in many cancers, and greater specificity than a general STAT3 inhibitor.
In this study, we explored the effect of BZA on STAT3 activation in GBM cells in vitro and used an orthotopic syngeneic mouse model to assess the potential therapeutic application of BZA.
Materials and methods
Bioinformatics analysis of patient data
Data sets downloaded from GlioVis (gliovis.bioingo.cnio.es) were sorted by expression levels and divided at the medians. The survival data were analyzed and graphed using GraphPad Prism version 9.1.0 for Windows (GraphPad Software, La Jolla, CA).
Cell culture
GL261, a mouse GBM cell line acuiqured from the NCI, was cultured in high glucose DMEM supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 U/mL). Human GBM PDX lines L1 and L2 were a gift from Dr. Brent Reynolds and were cultured in Neurobasal media (complete recipe in supplemental table 1). The PDX lines were grown adherently using Petri dishes treated with geltrex (ThermoFisher Scientific, Waltham, MA). HEK293T cells were obtained from ATCC and grown in high glucose DMEM, supplemented with 5% FBS, penicillin (100 U/mL), and streptomycin (100 U/mL). All the cell lines were maintained at 37°C and 5% CO2.
Inhibitor treatment and IL6 stimulation
To examine the pattern of STAT3 activation, cells were treated with 50 ng/mL recombinant human IL6 (Peprotech, Cranbury, NJ) for 30 min. The medium containing IL6 was removed and the cells were washed with PBS. Fresh medium was added, and the cells were incubated at 37°C until the indicated times. To assess the effects of BZA (Selleck chemical, Houston, TX) on the activation of STAT3, cells were pretreated with the drug for 2 h prior to IL6 treatment. Cells were then cultured in the presence of BZA throughout the remainder of the experiment. To examine the effects of BZA on downstream IL6/STAT3 signaling, cells were plated at low density in 10 cm plates and treated with BZA for up to 72 h.
Transient transfections
HEK293T cells were used to ectopically express IL6Rα for immunoprecipitation experiments. Transient transfections were performed using the PEI method. The construct used was HG-10974-CM human IL6R cDNA ORF from Sinobiologicals (US headquarters, Wayne, PA). All transiently transfected cells were used within 36 h, for optimum construct expression.
Cell lysis and western blotting
Cell pellets were suspended in 0.5% NP-40 lysis buffer containing phosphatase and protease inhibitors. The mixture was vortexed for 15 sec and incubated on ice for 5 min. The vortexing and incubation were repeated for a total of 3 cycles. The suspension was centrifuged at 4°C for 30 min at 16,000 x g. The protein concentration in the supernatant solution was measured using the Bio-rad protein assay (Bio-rad, Hercules, CA). SDS-PAGE was performed using 30-50 μg of protein and hand-cast 8% or 10% polyacrylamide gels. Proteins were transferred to a PVDF membrane using a wet transfer system. Membranes were blocked in 5% NFDM followed by incubation with primary antibodies, which are listed in Supplementary Table 2. Membranes were washed 3 x in Tris-buffered saline with Tween (TBST) for 10 min, followed by incubation with secondary antibody for 1 h. Membranes were washed and treated with SuperSignal West Pico PLUS (ThermoFisher Scientific, Waltham, MA). Bands were recorded either by exposing and developing film or by using a ChemiDoc.
RNA extraction and qPCR
RNA was extracted using Trizol and Bromochloropropane. Isolated RNA was treated with a DNA-free DNA removal kit (Invitrogen, Waltham, MA) and measured using a Nanodrop ND-1000 spectrophotometer (ThermoScientific, Waltham, MA). Reverse transcription PCR was performed using 2 μg of RNA and the SuperScriptTM III kit (ThermoFisher Scientific, Waltham, MA). Real-time PCR was performed using Bullseye Evagreen qPCR 2X master mix (MidSci, St. Louis, MO). The primers used are listed in Supplementary Table 3.
In vitro extreme limiting dilution assay
Cells grown in suspension were dissociated, counted, and plated at approximately 20, 10, 5, or 1 cell per well in ultra-low adherent 96-well plates (Corning, Corning, NY). Plates were incubated at 37°C and 5% CO2 for 14 days. The wells were examined by eye and the number of wells that contained at least one tumorsphere was counted. Analysis was performed by usig the eLDA: Limiting dilution analysis software [34].
In vivo studies
All experiments were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and performed following established protocols. Four-week old C57BL/6 mice were purchased from Jackson Laboratory and housed in the Cleveland Clinic Biological Research Unit. Equal numbers of male and female mice in the 4-8 week age rangewere injected in the left brain hemispheres with approximately 25,000 cells suspended in 5 uL of DMEM-null medium. Mice were monitored daily for hunched posture, proper grooming, lethargy, and neurological symptoms that would indicate degree of tumor burden.
One week post injection, the mice were randomly assigned to control or treatment groups. The treatment group was intraperitoneally injected with 40 mg/kg BZA in DMSO, diluted with corn oil (Sigma-Aldrich, St. Louis, MO) to a total of 50 μl volume per injection. The control group was intraperitoneally injected with a matched volume of DMSO diluted in corn oil. Mice were treated 3 times a week on alternating days. The mice were monitored daily for the endpoint criteria described above. After sacrifice, the tumor tissue was kept for histology or biochemical assays.
To examine the ability of BZA to cross the blood-brain barrier, 8 week-old mice were treated daily for 5 days with intraperitoneal injections of vehicle only, or 10, 40, or 100 mg/kg of BZA. One day after treatment, the mice were sacrificed and perfused with cold PBS. The brain tissue was homogenated in 0.5% NP-40 lysis buffer with phosphatase and protease inhibitors. The homogenate was analyzed by the proteomics core at the Lerner Research Institute for LC-MS detection of BZA.
Mouse tissue protein extraction
Tumor tissue collected from the in vivo experiments was flash frozen and kept at -80°C for future Western analysis. The tissue was lysed with 0.5% NP-40 buffer containing phosphatase and protease inhibitors and homogenized using a Misonix sonicator 3000 (Misonix Incorporated, Farmingdale, NY). Protein samples were prepared as described above.
Statistical analysis
GraphPad Prism version 9.1.0 for Windows (GraphPad Software, La Jolla, CA) was used for statistical analysis.
Results
Patient-derived data confirm that high STAT3 expression correlates with poor prognosis
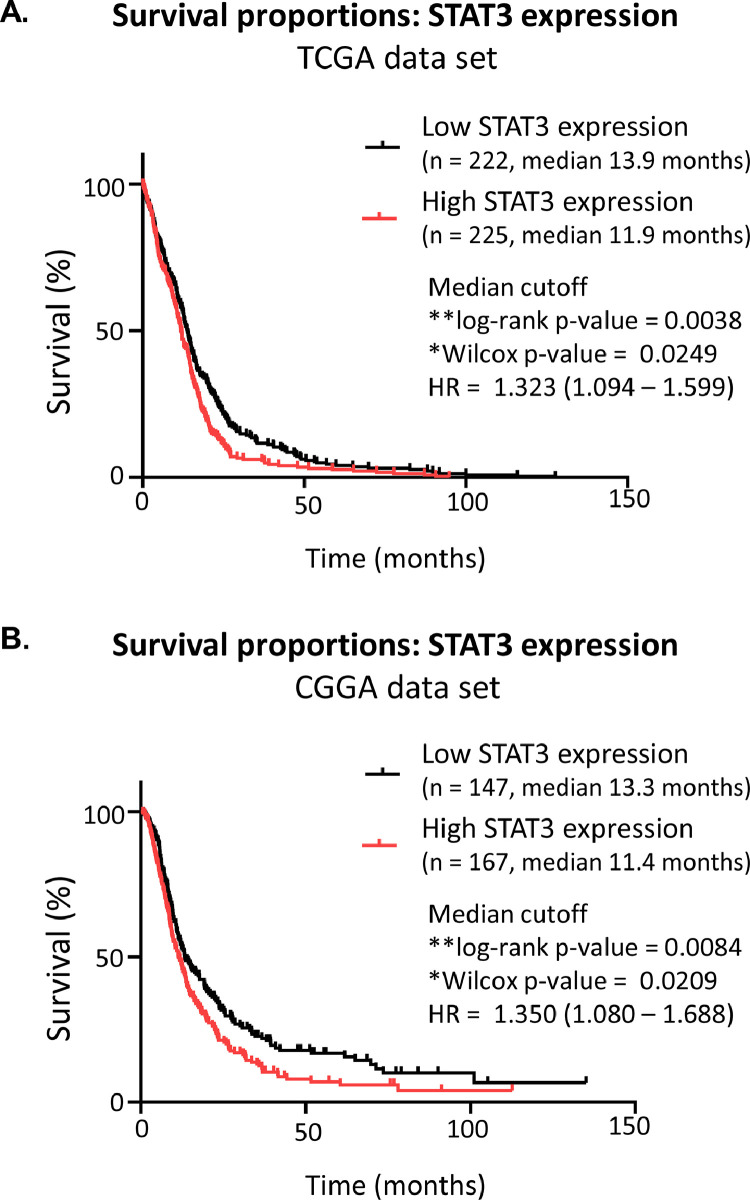
Previously, we found that cancer cells sustain Y705 STAT3 phosphorylation in a biphasic, EGFR-dependent manner [9]. STAT3 has long been known to be an oncogene, and there is a well-established literature demonstrating its protumor effects in GBM [7]. Data obtained from the TCGA_GBM (HG-U133A) data set are shown after separation into subsets of tumors that express high or low levels of STAT3, using the median value plotted against the time of survival (https://www.cancer.gov/tcga) (Fig. 2A). The patient group with high STAT3 expression shows a significant decrease in survival. Comparing the survival times of patients with high or low STAT3 expression, the hazard ratio is 1.323 (Mantel-Haenszel, 95% CI = 1.094-1.599), indicating that increased STAT3 expression is correlated with a worse prognosis. To confirm these results we also examined the Chinese Glioma Genome Atlas mRNA seq data sets (mRNAseq_693 and mRNAseq_325) for STAT3 mRNA expression in GBM tumors (Fig. 2B). The data were separated by median mRNA expression values and plotted as high or low expression vs survival time (http://www.cgga.org.cn/index.jsp). Similarly, there is a significant difference in patient survival between tumors with high or low STAT3 expression (log-rank p-value = 0.0084, Wilcox p-value = 0.209). The hazard ratio is very similar to that of the TCGA data set at 1.350 (Mantel-Haenszel, 95% CI – 1.080-1.688). When the expression of STAT3 is compared among tumor grades in both datasets, there is, on average, higher STAT3 expression in grade IV (GBM) tumors than in lower grade gliomas (Sup. Fig 1.). Previous appreciation of the pro-tumor role of STAT3, combined with the patient data presented here, illustrate the importance of STAT3 as a therapeutic target.
Fig. 2.
High STAT3 expression correlates with poor survival. Patients’ data exported from (A) TCGA_GBMLGG or (B) CGGA. Only data points from diagnosed GBM tumors were used, and low-grade glioma data points were removed. Data points were divided by high STAT3 expression or low STAT3 expression within tumor samples, with a median cutoff.
GBM cells maintain sustained STAT3 activation in vitro
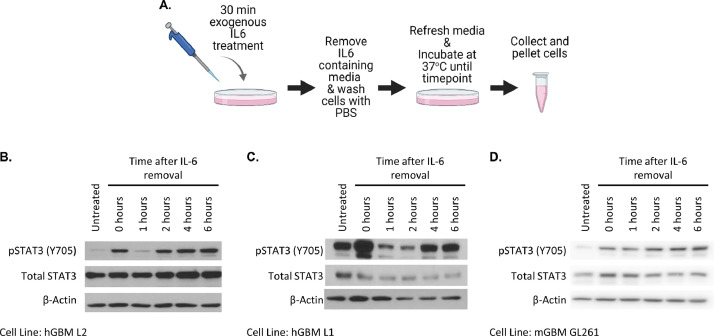
Currently, little is known about sustained activation of STAT3 in GBM. By adapting the experimental design of our previous work (Wang, et. al., 2013), we determined that STAT3 activation is sustained in a similar manner in GBM cell lines (Fig. 3A). Cells were treated with 50 ng/µL of recombinant human IL6 for 30 min and the medium containing IL6 was removed, followed by washing to remove any remaining IL6. Fresh medium was added to the cells and, 6 h after IL6 treatment, the cells were collected and prepared for analysis by the Western method. We observed the typical initial phosphorylation of STAT3 (Fig 3B, lane 2) and its subsequent rapid down regulation by the cell's endogenous negative feedback mechanism (Fig 3B, lane 3). However, 2-4 h after IL6 removal, there was a re-emergence of pY705 STAT3 expression, seen in three GBM cell lines (Figs 3B-3D), which was sustained for at least 6 h after removal of exogenous IL6. These results indicate that the GBM cell lines can sustain STAT3 phosphorylation without continuous external stimuli.
Fig 3.
GBM cells maintain sustained STAT3 activation in vitro. (A) Experimental design for panels B-D. The image was created with BioRender.com. Figs. B-D show the sustained pY705 STAT3 and total STAT3 levels after IL6 treatment in (B) L2 PDX GSCs, (C) L1 PDX GSCs, (D) GL261 mouse glioma cells.
BZA inhibits sustained tyrosine phosphorylation of STAT3
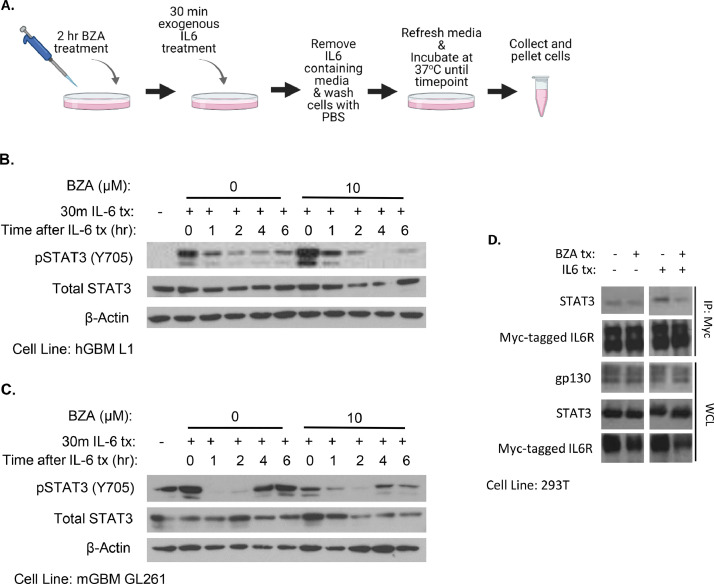
BZA binds to gp130, obstructing its interaction with IL6Rα [33]. BZA has the most potent inhibitory effect on IL6-mediated STAT3 activation when compared to the other cytokines that signal through the gp130 subunit [33]. The IL6 gene is a STAT3 target [14], and newly synthesized and secreted IL6 is required for cancer cells to maintain STAT3 activation [9]. To assess the effectiveness of BZA's ability to inhibit IL6-mediated STAT3 activation, we modified the experimental design by pretreating the cells with the drug for 2 h and then maintaining the same concentration throughout the experiment (Fig. 4A). BZA-treated cells showed similar Y705 STAT3 phosphorylation immediately following IL6 treatment, but pY705 STAT3 was significantly decreased at later times compared to controls not treated with BZA (Fig. 4B). These data indicate that BZA inhibits sustained Y705 STAT3 phosphorylation,but not the initial phosphorylation stimulated by exogenous IL6.
Fig. 4.
BZA inhibits sustained pY705 STAT3 and disrupts the IL6R complex. (A) Experimental design for panels B and C. Image created using BioRender.com. Cells were treated with BZA for 2 h before being treated with 50 ng/µL of recombinant human IL6 for 30 min, and the drug concentration was maintained throughout the experiment. Levels of pY705 STAT3 and total STAT3 after treatment with BZA and IL6 in (B) L1 PDX GSCs and (C) GL261 mouse glioma cells. (D) HEK293T cells were transiently transfected to express myc-tagged IL6Rα. The cells were treated with BZA and IL6 for 2 h before collection and lysis. The lysates were used to immunoprecipitate myc-tagged IL6Rα. The IP samples and whole cell lysates were analyzed by the Western method for components that bind to IL6R.
Since BZA disrupts the IL6Rα-gp130 complex, we expected to observe a decrease in the amount of STAT3 bound to this complex in BZA-treated cells. The GBM cell lines used here have constitutive STAT3 phosphorylation. To avoid this complication in assessing the interaction between IL6R and STAT3 after treatment with BZA, we used HEK293T cells, which have very low levels of both constitutively activated STAT3 and IL6Rα. We increased the expression of IL6Rα by transfecting myc-tagged IL6Rα into the HEK293T cells. Following treatment with IL6, with or without BZA, for 2 h, the collected cells were lysed and IL6Rα was immunoprecipitated using the myc tag. When the cells were treated with IL6 alone, more STAT3 was bound to IL6Rα than in untreated cells, as expected (Fig. 4C). However, when the cells were treated with BZA in addition to IL6, the amount of STAT3 bound to IL6Rα decreased substantially, with no change in the expression of total STAT3 (Fig. 4C). These results indicate that treatment with BZA does prevent the binding of STAT3 to the IL6Rα complex.
The use of BZA in vitro decreases GSC marker expression and self-renewal
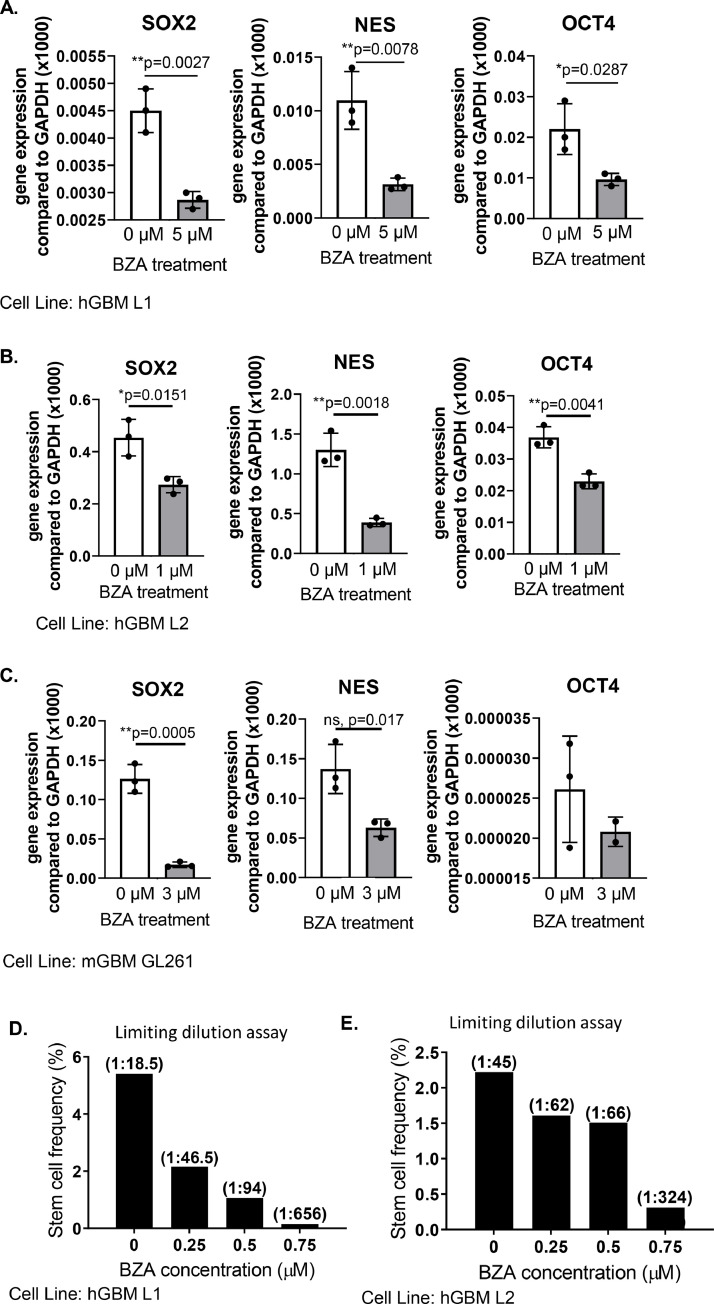
STAT3 enhances the expression of stem cell transcription factors and cancer cell stemness in vitro [7,[25], [26], [27],35]. To investigate BZA's ability to inhibit STAT3 activation of GSC characteristics, we monitored the expression of stem cell markers. When cells were treated with BZA for up to 72 h, the expression of the GSC markers SOX2, Nestin, and OCT4, were decreased. These results were observed in both human and mouse cell lines (Figs 5A-5C). One characteristic that defines a cancer stem-like cell is its ability to self-renew and to form tumor spheres in vitro [36]. The in vitro extreme limiting dilution assay (eLDA) provides an estimate of the frequency of stem cells in a cell population, based on the number of tumor spheres that form [34]. We treated the hGBM GSCs with BZA continuously for the entire 14 day duration of the eLDA because of their high level of constitutive STAT3 activation. It is possible that some of the decrease in number of spheres is due to an anti-proliferative effect. To help mitigate this complicating factor we used doses at or below a previously determined IC50 (Sup. Fig. 2). The IC50 value was determined using the MTT assay to indirectly measure cell survival. When cells were treated with BZA, we observed a dose-dependent decrease in stem cell frequency (Figs 5D & 5E). Together, reduced stem cell marker gene expression and reduced self-renewal capacity reveal that BZA effectively targets GSCs in vitro.
Fig. 5.
STAT3 inhibition by BZA decreases GSC markers and characteristics. Cells were treated with BZA for the indicated times concentrations before RNA isolation. The levels of mRNA expression of the stem cell markers SOX2, Nestin, and OCT4 were assessed by qPCR in (A) L1 PDX GSCs, 5µM for 72 h (B) L2 PDX GSCs 1µM for 48 h and (C) GL261 mouse glioma cells, 3µM for 72 h. (D-E) In vitro extreme limiting dilution assays (eLDA) were analyzed with eLDA software (http://bioinf.wehi.edu.au/software/elda/). (C) A representative eLDA graph of L1 PDX GSCs, repeated three times, and (D) a representative graph of L2 PDX GSCs, repeated twice.
BZA crosses the blood-brain barrier
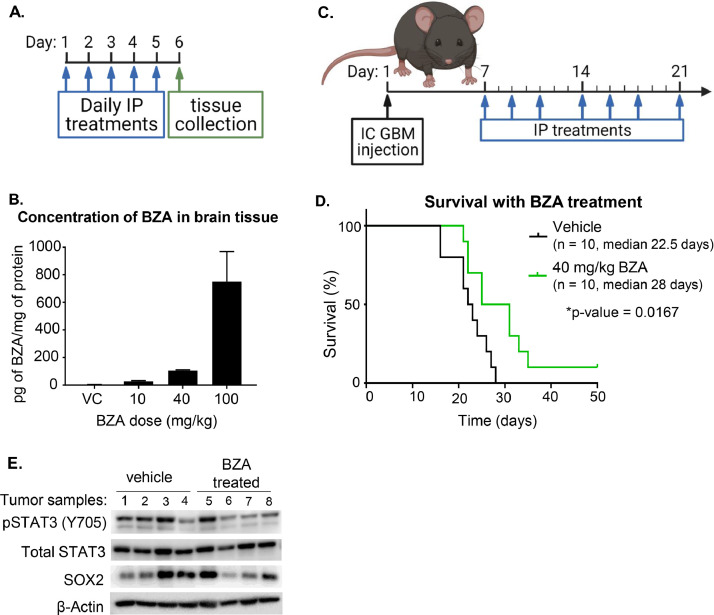
Although BZA is a small molecule, its ability to cross the blood-brain barrier (BBB) has not yet been studied. Non-tumor-bearing mice were treated with BZA at 0, 10, 40, and 100 mg/kg by daily intraperitoneal (IP) injection for 5 days. On the sixth day, the mice were sacrificed and perfused with cold PBS (Fig. 6A). The brain tissue was collected and homogenized in NP-40 lysate buffer and analyzed by liquid chromatography-mass spectrometry by the Lerner Research Institute Proteomics core. We observed a dose-dependent increase in the concentration of BZA in the brains (Fig. 6B). One of the therapeutic hurdles for GBM treatment is the ability of a drug to cross the BBB. Revealing that BZA can indeed do this increases its potential for use as an effective GBM therapy.
Fig. 6.
BZA crosses the blood-brain barrier and provides a survival advantage in an orthotopic syngeneic GBM model. (A) Experimental design for panel B. Mice were treated with BZA daily for 5 days before the tissues were collected and analyzed by the Lerner Research Institute Proteomics Core. Image created using BioRender.com. (B) Brain tissue lysate was analyzed by LC/MS for BZA concentration. The graph shows the concentration of BZA within the brain lysates, normalized by protein concentration. (C) Experimental design for panel D. The mice were observed for 50 days post-IC GBM injection for signs of neurological or physical distress to signify tumor burden. Image created using BioRender.com. (D) Kaplan-Meier curve depicting survival of mice treated with vehicle control or BZA. (E) Western analysis of pY705 STAT3, STAT3, and SOX2 levels in tumor samples from vehicle control or treated mice.
BZA confers a survival advantage in an orthotopic syngeneic mouse model
Since BZA crosses the BBB, we next examined its effect in an orthotopic syngeneic tumor mouse model. STAT3 is therapeutically important in GBM but, to date, specific STAT3 inhibitors have only been tested clinically in other diseases. However, none of the therapeutics have succeeded in clinical trials due to systemic toxicity. To measure toxicity, the weights of the mice in the BBB penetrance experiment were monitored. Mice treated with 40 or 100 mg/kg of drug experienced significant weight loss: 7-12% and 17-20% of starting weight, respectively (Sup. Fig. 3). Together with the knowledge that BZA has a half-life of 30 h in humans [37], these observations guided us to use concentrations of 40 mg/kg or less in subsequent experiments
For the orthotopic syngeneic tumor model, we used 4-8 week-old C57B/L6 mice intracranially injected with 25,000 GL261 cells. After 7 days to allow for tumor engraftment, the mice were treated with vehicle or 40 mg/kg BZA IP, three days a week for a total of 50 days, or less if the mice needed to be sacrificed sooner (Fig. 6C). Mice treated with BZA had a median survival time of 28 days, 5.5 days longer than the median survival time of the vehicle-treated mice. One mouse in the BZA-treated group did not exhibit any of the endpoint criteria that indicate high tumor burden and was sacrificed on day 50. Although the mouse that reached day 50 showed no behavioral or physical indications of tumor burden, it was later observed that a tumor was indeed present. These results further indicate the potential utility of BZA as a therapy for GBM. We also tested 20 mg/kg BZA, following the same protocol. In this experiment there was no difference in median survival compared to the vehicle control (Sup. Fig. 4). Together our results show that BZA has potential as a novel therapy for GBM patients.
BZA-treated mouse tumors show a decrease in STAT3 activation
All the experiments supporting BZA's mechanism of action in sustaining IL6-mediated STAT3 phosphorylation were done in vitro. To assess the ability of BZA to change the levels of pY705 STAT3 in vivo, tumor samples from the survival experiments were lysed and analyzed by the Western method for levels of pY705 STAT3 (Fig. 6E). The samples analyzed include four vehicle controls and four BZA-treated tumors. Each group comprises samples from two females and two males. In three of the four vehicle-treated tumors, there are high levels of pY705 STAT3, while in three of the four BZA-treated samples, there are relatively low levels of pY705 STAT3. SOX2 levels were also assessed by Western. Since the levels varied greatly among samples from the same treatment protocol, we conclude that there was not a significant difference in SOX2 expression. However, the decreased levels of STAT3 phosphorylation do show that BZA treatment inhibits the activation of STAT3, helping to account for the survival advantage seen in Fig. 6D.
Conclusions
It is expected that almost 13,000 new GBM cases will be diagnosed in the USA in 2021 [1], yet there has not been a significant advance in the standard of care protocol since the introduction of temozolomide in 2005 [3], [19]. GBM tumors are a heterogeneous mixture of cells, including GSCs, which contribute to high therapeutic resistance and increased tumorigenicity [20], [21], [22], [23], [24]. With 70% of GBM patients experiencing recurrent disease [19], developing a therapeutic strategy that targets both the bulk tumor and the GSC population of cells is of the utmost importance.
STAT3 signaling contributes to tumor cell survival, proliferation, and the maintenance of cancer stem cells, and has been studied extensively as a therapeutic target in cancer. However, drugs that target STAT3 have yet to progress past clinical trials due to lack of specificity or toxicity [28]. Antibodies that target signaling components upstream of STAT3, including IL6 or the IL6R, are not promising therapeutics because the BBB prevents them from reaching the tumor unless the BBB is breached.
We show that BZA, a small molecule STAT3 inhibitor, can penetrate the BBB, that BZA is effective in preventing sustained STAT3 activation in GBM cell lines, and that it increases the time of survival in an orthotopic syngeneic mouse model. Furthermore, when we treat human GSC cells with BZA, the expression of stem cell markers decreases, as does the self-renewal capacity.
BZA has the potential to decrease sustained IL6-mediated STAT3 activation without a significant effect on other gp130-dependent signaling pathways, allowing for more specific targeting of sustained STAT3 activation. A problem with previous STAT3-specific therapeutics has been toxicity due to poor specificity [28]. Many of the STAT3-specific small molecule inhibitors target areas of the protein that are conserved across the entire STAT family, such as the SH2 domain [28,30]. Since BZA specifically targets the gp130-IL6R interaction, inhibition of other STAT family members is not a concern [33]. In previous work, we have shown that newly synthesized and secreted IL6 is necessary for the ability of cancer cells to sustain pY705 STAT3 levels [9]. We now propose that BZA targets the ability of cells to sustain STAT3 activation by targeting the IL6R complex. Our current work shows that sustained STAT3 activation is more sensitive to BZA than the STAT3 activation that is driven by initial treatment with a cytokine such as IL6. BZA is currently used to treat post-menopausal osteoporosis as a selective estrogen receptor modulator [32]. Considering that BZA has already been used clinically [32], the rigor of the safety trials already conducted gives hope that it can be well tolerated as a novel therapy to treat various cancers, including but not limited to GBM.
In this study, we evaluated BZA as a single therapeutic agent in a mouse model. If BZA is used to treat GBM in the future, it is likely to be used in combination with standard-of-care therapies, including temozolomide and radiation. To follow our findings on BZA's effects on stem cell characteristics, we will test its efficacy in preclinical models of other tumors, especially those with high cancer stem cell frequencies: for example, small cell lung carcinoma. In conclusion, STAT3 activation is a therapeutically significant target in GBM, and BZA holds promise as a novel therapy not only because it decreases the levels of persistently activated STAT3 in GBM tumors and cell lines, but also because it inhibits the ability of cells to maintain GSC characteristics. Similarly, the results of treating an orthotopic syngeneic mouse model of GBM with BZA are encouraging, extending the average survival time in the treated mice. Our work illustrates the therapeutic potential of BZA, a well-tolerated, FDA-approved drug, in the treatment of a devastating disease that is in desparate need of novel therapeutic options.
Declarations of Competing Interest
The authors have no conflicts of interest.
Acknowledgments
Acknowledgments
We would like to thank current and past members of the Stark and Lathia laboratories, who provided constructive feedback and support throughout this project, and the members of SW's thesis committee, Drs. Mark Jackson, Alexandru Almasan, David Peereboom, and Micheal Vogelbaum, for their continued guidance and support.
Author contributions
Samantha M. Wightman: Conceptualization, Investigation, Writing – Original draft preparation, Writing – Review & Editing.
Yuxin Wang: Conceptualization, Methodology, Investigation, Writing – Review & Editing
Tyler J. Alban: Methodology, Writing – Review & Editing
Xing Chen: Resources, Methodology
Justin D. Lathia: Resources, Conceptualization, Writing – Review & Editing
George R. Stark: Supervision, Conceptualization, Writing – Review & Editing
Funding
This work was supported by National Cancer Insitute grant P01CA062220
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101192.
Contributor Information
Samantha M. Wightman, Email: wightms@ccf.org.
Tyler J. Alban, Email: albant@ccf.org.
Xing Chen, Email: chenx@ccf.org.
Justin D. Lathia, Email: lathiaj@ccf.org.
Yuxin Wang, Email: wangy7@ccf.org.
George R. Stark, Email: starkg@ccf.org.
Appendix. Supplementary materials
References
- 1.Ostrom QT, P. N., Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro-oncol. 2020;22:96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandes C, C. A., Osorio L, Costa Lago R, Linhares P, Carvalho B, Caeiro C. Codon Publications; 2017. Glioblastoma. ed De Vleeschouwer S. [PubMed] [Google Scholar]
- 3.Stupp R. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Birner P., Toumangelova-Uzeir K., Natchev S., Guentchev M. STAT3 tyrosine phosphorylation influences survival in glioblastoma. J. Neurooncol. 2010;100:339–343. doi: 10.1007/s11060-010-0195-8. [DOI] [PubMed] [Google Scholar]
- 5.Levy D.E., Lee C.-K. What does Stat3 do? J. Clin. Invest. 2002;109:1143–1148. doi: 10.1172/jci200215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villalva C. STAT3 is essential for the maintenance of neurosphere-initiating tumor cells in patients with glioblastomas: a potential for targeted therapy? Int. J. Cancer. 2011;128:826–838. doi: 10.1002/ijc.25416. [DOI] [PubMed] [Google Scholar]
- 8.Chang Q. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia. 2013;15:848. doi: 10.1593/neo.13706. -IN845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., van Boxel-Dezaire A.H., Cheon H., Yang J., Stark G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. U S A. 2013;110:16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibi M, M. M., Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 11.Taga T, H. M., Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 12.Guschin D, R. N., Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark G, Ihle JN, Kerr IM. A major role for the protein tyrosine kinase JAK1 in the JAK-STAT signal transduction pathway in response to interleukin-6. EMBO. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker S, G. B., Muller CW. Three dimensional structure of the STAT3-beta homodimer bound to DNA. Nature. 1998:394. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 14.Naka T, N. M., Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 15.Ichiba M., Nakajima K., Yamanaka Y., Kiuchi N., Hirano T. Autoregulation of the Stat3 Gene through Cooperation with a cAMP-responsive Element-binding Protein. J. Biol. Chem. 1998;273:6132–6138. doi: 10.1074/jbc.273.11.6132. [DOI] [PubMed] [Google Scholar]
- 16.Babon Jeffrey J. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–250. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan C.W. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolhion C. Interleukin-6 overexpression as a marker of malignancy in human gliomas. J. Neurosurg. 2001;94 doi: 10.3171/jns.2001.94.1.0097. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 20.Galli R. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer. Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 21.Chen J. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao S. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 23.Lathia J.D. Direct In Vivo Evidence for Tumor Propagation by Glioblastoma Cancer Stem Cells. PLoS One. 2011;6:e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson M., Hassiotou F., Nowak A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015;36:177–185. doi: 10.1093/carcin/bgu243. [DOI] [PubMed] [Google Scholar]
- 25.Wang H. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S.Y. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foshay K.M., Gallicano G.I. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev. 2008;17:269–278. doi: 10.1089/scd.2007.0098. [DOI] [PubMed] [Google Scholar]
- 28.Yu C.-L., Jove R., Turkson J. 2016. Historical Development of STAT3 Inhibitors and Early Results in Clinical Trials; pp. 69–94. [DOI] [Google Scholar]
- 29.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:15. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim C.P., Cao X. Structure, function, and regulation of STAT proteins. Mol. Biosyst. 2006;2:14. doi: 10.1039/b606246f. [DOI] [PubMed] [Google Scholar]
- 31.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 32.Miller P.D. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J. Bone Miner. Res. 2007;23:525–535. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 33.Wu X., Cao Y., Xiao H., Li C., Lin J. Bazedoxifene as a novel GP130 inhibitor for pancreatic cancer therapy. Mol. Cancer Ther. 2016;15:2609–2619. doi: 10.1158/1535-7163.MCT-15-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y., Smyth G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Kim E. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23:839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lathia J.D., Mack S.C., Mulkerns-Hubert E.E., Valentim C.L.L., Rich J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazedoxifene, < https://go.drugbank.com/drugs/DB06401 >.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.