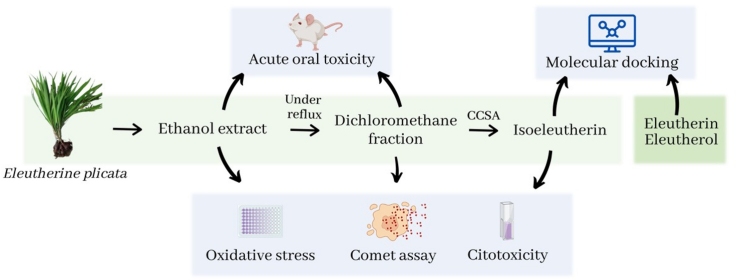
Graphical abstract
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCRJ, Cell bank of Rio de Janeiro; BFS, bovine fetal serum; DARP, dopamine releasing protein; DMEM, Dulbecco's Modified Eagle's Medium; DMSO, dimethyl sulfoxide; DPPH, 2,2-diphenyl-1-picrylhydrazyl; EDTA, ethylenediaminetetraacetic; EEEp, ethanol extract of Eleutherine plicata; FADD, Fas associated death domain; FDMEp, dichloromethane fraction of Eleutherine plicata; FrAE, ethyl acetate fraction of Elutherine plicata; GA, Genetic Algorithm; GOLD, Genetic Optimization for Ligand Docking; HPLC, high performance liquid chromatography; IC50, 50 % cytotoxic concentration; MTT, ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]); MD, molecular dynamics; NMR, nuclear magnetic resonance; NMU, N-methyl-N-nitrosurea; OECD, Organization for Economic Co-Operation and Development; PDB, Protein Data Bank; RPMI, Roswell Park Memorial Institute medium; ROS, reactive oxygen species; rpm, rotations per minute; RSMD, root mean square deviation; TLC, tin layer chromatography; TNFR, tumour necrosis fator receptor
Keywords: Eleutherine plicata, Toxicity, Isoeleutherin, Eleutherin, Eleutherol, Caspase-8
Highlights
-
•
Ethanol extract of Eleutherine plicata showed low in vitro and in vivo cytotoxic potential.
-
•
The dichloromethane fraction was cytotoxic to HepG2 and caused DNA. However, no toxicity was observed in vivo.
-
•
Isoeleutherin caused DNA damage by the comet method and activated caspase-8 in the in silico study.
Abstract
Eleutherine plicata has been shown to be a promising medicinal plant, and its activity has been associated with naphthoquinones. The present study aimed at evaluating the cytotoxicity, genotoxicity, and oral toxicity of the ethanol extract (EEEp), dichloromethane fraction (FDMEp) of E. plicata, and isoeleutherin. For the cytotoxicity evaluation, the viability test (MTT) was used. Genotoxicity was accessed through the Comet assay (alkaline version), acute and subacute oral toxicities were also evaluated. The antioxidant capacity of the samples in the wells where the cells were treated with E. plicata was evaluated. Furthermore, the participation of caspase-8 in the possible mechanism of action of isoeleutherin, eleutherin, and eleutherol was also investigated through a docking study. FDMEp and isoeleutherin were cytotoxic, with higher rates of DNA fragmentation observed for FDMEp and isoeleutherin, and all samples displayed higher antioxidant potential than the control. In the acute oral toxicity test, EEEp, FDMEp, and isoeleutherin did not cause significant clinical changes. In the subacute toxicity assay, EEEp and FDMEp also did not cause clinical, hematological, or biochemical changes. The three compounds bound similarly to caspase-8. Despite the results of cytotoxicity, in vitro studies demonstrated that the use of EEEp appears to be safe and cell death may involve its binding to caspase-8.
1. Introduction
E. plicata is used in the Brazilian Amazon to treat parasitic diseases [1], diarrhea [2], bellyache, cramps, other intestinal problems [3,4], intestinal bleeding, vomiting with blood [5], stomachache [6], wound healing [7,8], anemia, and blood purification [9].
The following compounds were isolated from this species: reducing sugars, phenols, tannins, steroids, terpenoids, azulenes, coumarin derivatives, and mainly quinones (naphthoquinones and anthraquinones; [10]). Among the identified quinones are eleutherinone, isoeleutherol [11], hongconine [12], elecanacin [11], chrysofanol [13], naphthol eleutherol, and naphthoquinones eleutherin and isoeleutherin. Studies suggest that the naphthoquinones eleutherol, eleutherin, and isoeleutherin are the major constituents of the species and can serve as markers for it [10,14].
Notwithstanding, naphthoquinones have been reported to possess antiinflammatory, anti-viral, anti-bacterial, anti-fungal properties [15], and could induce apoptosis through a mitochondrial-dependent pathway [16,17], involving a high concentration of reactive oxygen species (ROS), which result in macromolecular damage, growth arrest, apoptosis, and ultimately, cell death [[17], [18], [19], [20]].
Mitochondrial-mediated apoptosis is produced by the loss of the potential of the mitochondrial membrane and proceeds through the release of cytochrome C and ROS. In the cytosol, cytochrome C forms a complex with apoptotic protease activating factor 1, ATP, and procaspase-9. This complex is known as apoptosome and results in the activation of caspase-9, executing the cleavage and activation of executioner caspases - such as caspase-3, caspase-6, and caspase-7 - initiating a cascade of caspase activation that eventually ends in cell death [21,22].
Caspase-8 and caspase-10 are the initiator caspases for death receptors of the TNF receptor (TNFR) family, binding to the receptor Fas-associated death domain protein (FADD), regulating the expression of IL-1β [23]. However, the biochemical mechanism that stimulates caspase 8-mediated pro-IL-1β processing is still undefined [24].
Bearing these mechanisms in mind, a study evaluated the in vitro antimalarial activity of the ethanol extract of Eleutherine plicata (EEEp), ethyl acetate fraction (FrAE; from which naphthoquinones and eleutherol were isolated), and the isolated molecules eleutherol, eleutherin, and isoeleutherin, against a chloroquine-resistant strain of Plasmodium falciparum (W2). Eleutherin and isoeleutherin showed the best activity (IC50 = 10.45 and 8.70 μg/mL, respectively). Furthermore, docking studies were carried out in the cytochrome bc1 complex binding cavity, with eleutherin and isoeleutherin also showing their interaction with conserved residues in the cytochrome bc1 complex binding cavity, similarly to the mechanism of action for atovaquone [25].
Moreover, another study evaluated the antimicrobial activity of the EEEp, FDMEp, FrAE, and isoeleutherin isolated from Eleutherine bulbosa (E. plicata synonym) against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. In the agar diffusion assay, EEEp, FDMEp, and FrAE were active against S. aureus. In the microdilution test, the most promising sample was FDMEp (minimum inhibitory concentration = 125 μg/mL). For all samples, the Minimum Bactericidal Concentration was > 1000 μg/mL [26].
The antitumoral activity of EEEp, FDMEp, eleutherol, eleutherin, and isoeleutherin was also evaluated in vitro (using oral cancer-SCC-9 cell line and human keratinocytes – HaCaT, as a control normal cell line). The greatest inhibition of cell proliferation and selectivity index were observed with EEEP, and cell disaggregation was influenced by the exposure time and concentration of the extract [27].
The genotoxicity and mutagenicity of isoeleutherin and eleutherin were also evaluated in the bioassay with Allium cepa, both in silico, using the PreADMET software, and by binding to topoisomerase II, through molecular docking and molecular dynamics (MD) simulations. In silico studies have demonstrated identical toxicity and mutagenicity profiles for algae, Daphinia, and fish. However, eleutherin proved to be more genotoxic, increasing the mitosis index, aberration frequency, and micronucleus, nuclear buds, and mitotic irregularities were observed during the metaphase. The results of docking and MD simulations showed that the compounds were able to interact with the residues present in the enzyme binding site. Throughout the MD trajectories, the compounds showed molecular stability and the free energy results proved that the compounds formed a stable complex with topoisomerase II [28].
It is noticed that this species has great medicinal potential, however, to date, no in vitro and in vivo toxicity studies have been found to support its medicinal application. Therefore, the present study evaluated the cytotoxicity of EEEp, FDMEp, and isoeleutherin, and their genotoxic potential through the comet assay. In addition, evaluations of acute and subacute oral toxicities of these compounds were performed in mice. The antioxidant potential of samples treated in vitro and the molecular docking of the caspase-8 binding capacity of isoeleutherin, eleutherin, and eleutherol were also evaluated.
2. Materials and methods
2.1. Plant material and extract preparation
Eleutherine plicata Herb. is a plant of the Eleutherine genus, belonging to the Iridaceae family and widely distributed in Brazil. This plant is a bulbous herbaceous plant with an approximate size of 20–30 cm in height, with simple entire leaves, longitudinally pleated and inflorescence in pink flower panicles. They also have styloid crystals, inferior ovaries, and flowers with three stamens. The bulbs are composed of onion-like scales, but with a burgundy color and exude white latex when cut [29,30].
Bulbs of E. plicata were collected in the municipality of Traquateua - PA, Brazil, BR 318, Lat. 1,1436 °, Long. 46,95511 °. The botanical identification was carried out by Dr. Márlia Coelho, and the exsiccate was deposited in the João Murça Pires Herbarium (MG) of the Museu Paraense “Emílio Goeldi” under the MG registry no. 202631. The collected plant material was washed in running water and dried under forced air in an oven for two weeks, until it acquired constant weight. After this period, the dry material was crushed in a knife mill, yielding the powder from which 500 g was used for the preparation of the ethanol extract, which was obtained by maceration in 96°GL ethanol (1718733; Sigma-Aldrich; Saint Louis, USA) for seven days, with daily agitation. This process was repeated, monitoring the extraction of naphthoquinones by Thin Layer Chromatography (TLC), eluted with dichloromethane (34856; Sigma-Aldrich; Saint Louis, USA).
From the bulbs of E. plicata, the EEEp, FDMEp, and a pure substance were obtained and characterized in another study of our research group [25,28]. The extract was obtained by maceration, followed by fractionation under reflux, and the dichloromethane fraction was fractionated in an open column of silica gel, using solvents of increasing polarity (hexane, dichloromethane, ethyl acetate, methanol), thus obtaining four fractions: hexane (0.21 g), dichloromethane (0.65 g), ethyl acetate (1.10 g), methanolic (3.5 g). The substance was identified by High Performance Liquid Chromatographic (HPLC) and Nuclear Magnetic Resonance (NMR; [28]). After preparing the plant extract, it was dissolved in 0.05 % dimethyl sulfoxide (DMSO; 506008; Sigma-Aldrich; Saint Louis, USA) for in vitro and in vivo experiments on the day of treatment.
The components of E. plicata extracts were previously studied and characterized [10], and naphthoquinones isoeleutherine, eleutherine, and eleutherol are the majoritarian constituents. Moreover, the procedures taken for extraction and isolation in the present study were the same as described by those authors and verified by thin layer chromatography.
2.2. In vitro assays
2.2.1. Cultivation and subculture of cells for in vitro experiments
VERO cells were cultivated in Dulbecco's Modified Eagle's Medium (DMEM; PRD.0.ZQ5.10000038041; Sigma-Aldrich; Saint Louis; USA) - supplemented with 10 % bovine fetal serum (BFS; 630111; Laborclin; Pinhais; Brazil), 100 μg/mL streptomycin (3894995; Sigma-Aldrich; Saint Louis; USA), and 60 μg/mL penicillin (3834217; Sigma-Aldrich; Saint Louis; USA). HepG2 cells were grown in Roswell Park Memorial Institute Medium - 1640 medium (RPMI; MFCD00217820; Sigma-Aldrich; Saint Louis; USA) supplemented with 10 % BFS, 100 μg/mL streptomycin (3894995; Sigma-Aldrich; Saint Louis; USA) and 60 μg/mL penicillin. These strains were cultivated in culture bottles with a surface equal to 25cm2, stored in a CO2 gas incubator (8539.29.10; Laboven; São Paulo; Brazil), at 37 °C in a humid atmosphere with 5 % CO2, according to the recommendation of the protocol of the cell bank of Rio de Janeiro (BCRJ; Rio de Janeiro; Brazil), from where they were obtained.
For the subculture, the medium used by the cells was discarded and the cell monolayer (between 80 % and 90 % confluence) was washed twice with 1.0 mL of Hanks' balanced salt solution (0.4 g of KCl, 0.06 g of KH2PO4, 0.04 g of Na2HPO4, 0.35 g of NaHCO3, 1.0 g of glucose, 8.0 g of NaCl, in H2O sufficient quantity for 1000 mL; 60REAQMO016378; Quimica Moderna Formula; Jaraguá do Sul; Brazil). After washing, 0.125 g of trypsin (9002-07-7; Sigma-Aldrich; Saint Louis; USA), 0.02 g of EDTA (24907; Neon; São Paulo; Brazil) diluted in 100 mL of Hanks' solution (H6648; Sigma-Aldrich; Saint Louis; USA) was added to the cells, in the amount of 1.0 mL for each culture bottle for approximately 2 min to allow cell dissociation from them. Subsequently, trypsin was inactivated by adding 6.0 mL of culture medium containing 10 % BFS and the cell suspension was homogenized. This procedure applies to plating (transfer of cells to the culture plates).
2.2.2. Cytotoxicity assay
The method was developed according to Mosmann [31]. In a 96-well plate, VERO cells (BCRJ) were distributed (8 × 103cells/mL DMEM medium supplemented with 10 % BFS) and the plates were incubated at 37 °C in a humid atmosphere under 5 % CO2. After 24 h of incubation, cells were treated with five increasing concentrations (12.5 μg/mL; 25 μg/mL; 50 μg/mL; 100 μg/mL, and 200 μg/mL) of EEEp and FDMEp from E. plicata, as well as isoeleutherin, solubilized at the time of testing in culture medium, and compared to the positive control, N-methyl-N-nitrosurea (NMU; 684-93-5; Sigma-Aldrich; Saint Louis; USA). After 24 h of exposure, the supernatant was discarded and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT; 4081397; Sigma-Aldrich; Saint Louis; USA) was added at a concentration of 500 μg/mL. The absorbances were read on a multiwell scanning spectrophotometer (Varian Mercury 300), using a reference wavelength of 570 nm. The IC50 values (50 % cytotoxic concentration) were calculated using dose-response curves from three independent experiments for each treatment.
2.2.3. Antioxidant capacity evaluation
To access the antioxidant capacity of the samples, 3 × 105 HepG2 cells/mL (BCRJ) were treated with the same samples and concentrations used in the cytotoxicity assay, by the DPPH test (1846081; Sigma-Aldrich; Saint Louis; USA; [32]). After 24 h of treatment, the well content were discarded, the cells were trypsinized, and 50 μL of each well was transferred to test tubes with 950 μL of 0.1 mM alcoholic DPPH solution. The tubes were placed in a water bath at 37 °C for 30 min. The absorbances were read on a spectrophotometer at 517 nm. The absorbance values found for each sample were subtracted from the initial absorbance value of DPPH [33,34].
The construction of the standard curve was performed in triplicate, allowing the calculation of the regression equation, which was used to calculate the values of antioxidant capacity of the samples from three independent experiments for each treatment.
2.2.4. Comet assay (alcaline version)
Using 12-well plates, 1.5 × 105 HepG2 cells/Ml (BCRJ) were seeded in RPMI-1640 medium supplemented with 10 % BFS, the cells were cultured at 37 °C in a humidified atmosphere under 5 % CO2. After 21 h in culture, the cells were treated for three hours with three concentrations of each of the samples: EEEp (9.8 μg/mL, 4.9 μg/mL, and 2.45 μg/mL), FDMEp (9.5 μg/mL, 4.75 μg/mL, and 2.375 μg/mL), and isoeleutherin (15.55 μg/mL, 7.77 μg/mL, and 3.88 μg/mL). In addition to this treatment, a negative control was made with cells and culture medium, and a positive control in which the cells were treated with 0.02 μg/mL of doxorubicin, due to their previously known genotoxicity. Cell exposure lasted three hours. Three independent experiments were performed for each treatment.
After exposure, the supernatant was discarded, and the cells were trypsinized. A sample of 450 μL from each group was collected and underwent centrifugation of 1000 rpm for 5 min in a micro centrifuge. After centrifugation, the supernatant was discarded, leaving 100 μL for resuspension. Of this content, 30 μL were added to 300 μL of low melting point agarose (0.8 %). Homogenization was performed, and 100 μL of this content was placed on a microscope slide (previously covered with agarose solution with normal melting point).
Each slide was covered with a coverslip (24 × 60 mm) and maintained at 4 °C for 5 min until solidification of agarose. After solidification, coverslips were carefully removed, and slides were dipped into a lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris - 17-1321-01; Sigma-Aldrich; Saint Louis; USA- 1 % Triton X-100 - 2315025; Sigma-Aldrich; Saint Louis; USA - and 10 % DMSO; pH 10) and kept at 4 °C and protected from light for 24 h.
After removal of the lysis solution, the slides were placed in an electrophoresis tank. Electrophoresis was performed at 34Vx300 mA for a period of 20 min. After this procedure, the slides were removed from the tube and dipped quickly into cold distilled H2O (4 °C) and then transferred to a new dip in cold distilled H2O for 5 min. The slides were fixed with 100 % ethanol for 3 min and then stained with 50 μL of ethidium bromide solution (20 μg/mL). In sequence, the slides were covered with coverslips for analysis [35]. Fluorescence microscope was used to visualize the slides at 400x, analyzing 100 cells per group. The analysis was performed by the pattern of scores according to Mota et al. [36], where the degree of injury suffered by the cells was evaluated according to the size and intensity of the comet's tail, which represents the level of DNA fragmentation.
2.3. Acute and subacute oral toxicity tests
Sixty Balb C-An mice (Mus musculus; 25–30 g), from the vivarium of the Evandro Chagas Institute (IEC; Ananindeua-PA, Brazil), and kept in the vivarium of UFPA, were used. The present study was submitted to the Ethics Committee on the Use of Research Animals of the Federal University of Pará (CEUA/UFPA; Brazil) and approved under report no. 7464060618 (ID 001020). All procedures with animals followed the international guidelines of animal experimentation, according to the ethical principles and regulations of the Brazilian Society of Sciences in Laboratory Animals.
The animals were housed in cages (five mice per cage), under controlled conditions of temperature (25 ± 1 °C), and in an alternating cycle of 12 h of light/dark. Water and food were provided ad libitum during the experiments.
For acute toxicity tests, fixed dose procedures were used [37], as it uses much fewer animals than the lethal dose study (LD50) and provides adequate information on the toxicity of substances. Furthermore, if the high dose does not cause mortality in the treated groups, there is no need to administer lower doses. The animals (12 females and 12 males) were divided into 4 groups (3 females and 3 males each) and treated as follows: the control group was treated with aqueous solution of DMSO (99:1 v/v); EEEp treated with 2000 mg/kg (1 mL/100 g of weight) of extract through a 4 cm stainless-steel gastric tube (Becton & Dickinson Co™); FDMEp was submitted to similar treatment conditions, but the dichloromethane fraction was used; ISO was treated, similarly to the other groups with isoeleutherin. All samples were solubilized in an aqueous DMSO solution (99:1 v/v).
The animals were observed for 14 days, with a Hippocratic evaluation or screening being carried out, providing a general estimative of the pharmacological and toxicological nature of the tested substance. The monitoring of possible signs and symptoms associated with toxicity was observed at 0, 30, 60, 120, 180, and 240 min after administration of the substances. The animals were monitored twice a day for the next 14 days. Body weights were daily recorded. At the end of the observation period, the animals were euthanized by anesthesia with xylazine and ketamine, with subsequent collection of blood by cardiac puncture in an EDTA-coated tube. The blood was centrifuged at 3000 rpm. The separated plasma was frozen at −80 °C, and the serum immediately underwent biochemical analysis. The euthanized mice were subjected to gross necropsy [38,39].
For the assessment of acute toxicity of repeated doses of EEEp, FDMEp, and control, the methodology described in Guide 407 of the OECD guidelines [40] was applied, using the limit test with a dose of 1000 mg/kg of EEEp and FDMEp. The animals were divided into three groups (n = 5 females). The first group received an aqueous solution containing DMSO (99:1 v/v) for 28 days; the second group received a daily dose of EEEp (1 mL/100 g of weight; 1000 mg/kg) dissolved in water for 28 days; the third group received a daily dose of FDMEp (1 mL/100 g of weight; 1000 mg/kg) dissolved in an aqueous solution containing DMSO (99:1 v/v) for 28 days.
The monitoring of signs and symptoms associated with toxicity was observed at 0, 30, 60, 120, 180, and 240 min after substance administration, and the animals were monitored twice a day for the next 28 days. Body weights were daily recorded. At the end of the observation period, the animals were euthanized as previously described, with subsequent collection of blood by cardiac puncture in an EDTA-coated tube. The blood was centrifuged at 3000 rpm and the separated plasma was frozen at −80 °C, after biochemical analysis of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine and urea, and hematological parameters of blood count and leukogram. The euthanized mice were subjected to gross necropsy [38].
2.4. Molecular docking
First, re-docking simulations using the co-crystallized ligand (DARP) were performed in the Genetic Optimization for Ligand Docking (GOLD) program to validate our docking protocol. The redocking simulations obtained a small root mean square deviation (RMSD) value equal to 1.792 Å when compared with the reference crystallographic structure (PDB: 2Y1L). The RMSD values obtained from the redocking simulation validated our docking protocol.
The 3D structure of caspase-8 of the Homo sapiens complex with dopamine releasing protein (DARP) was recovered from the Protein Data Bank (PDB ID: 2Y1L, resolution: 1.80 Å), prepared by removing the water molecules, adding atoms of hydrogen, removing ions and other ligands from the active site.
The virtual screening was performed using the program GOLD version 5.1, which uses a genetic algorithm for flexible ligand docking experiments within protein binding sites [41,42].
Molecular docking is a methodology applied to study molecular behavior within protein binding sites, a tool widely used for the discovery of new drugs [43]. The virtual screening was performed by using the GOLD 2020.1 software, which performs docking simulations, using a Genetic Algorithm (GA) to generate and select conformers of flexible compounds that bind to the receptor site of a protein or DNA [41,42]. The GoldScore scoring function was used with the number of GA runs set at 100 % search efficiency [44].
The scoring function, the amino acid residue from which the interaction cavity is defined, and the radius of the interaction cavity for anchoring the ligands were chosen after redocking experiments, evaluating the deviation of the RSMD in relation to the position of the atoms of the inhibitor's crystallographic ligand and their conformation. Docking calculations were performed within a 10 Å radius sphere. The crystal orientation and redocking confirmation of the inhibitor were compared with those obtained from the Protein Data Bank, PDB ID: 2Y1L. A visual inspection of the results was performed to assess the positional representation generated by the Poseview Program [45].
2.5. Statistical analysis
The data obtained in the tests were compared using the Student t-test with the software Prisma 5.0, and a significance level of 5 % (p < 0.05) was considered in all tests. The variables were analyzed and expressed in mean ± standard deviation.
3. Results
3.1. In vitro toxicity assessment
The cytotoxic evaluation test showed similar results for ethanol extract and dichloromethane fraction, while isoeleutherin displayed less toxicity. In all evaluations, the behavior of the results was identical, in which the 24 h results were more cytotoxic than at 48 h (Table1).
Table 1.
Inhibition Concentration 50 % (IC50) of extracts and isoeleutherin from E. plicata.
| Samples | HepG2 (IC50 μg/mL) |
|
|---|---|---|
| 24 h | 48 h | |
| EEEp | 19.61 + 0.78 | 48.96 + 1.266 |
| FDMEp | 19.04 + 1.15 | 25.00 + 1.843 |
| Isoeleutherin | 31.11 + 1.64 | 32.50 + 2.883 |
EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; Values are expressed as mean ± standard deviation.
In the antioxidant test, the results showed similar behavior for all samples: the higher the concentration of EEEp, FDMEp, and isoeleutherin, the lower the antioxidant potential of these samples, were inversely proportional. This means that at the highest concentration of EEEp, FDMEp, and isoeleutherin, the antioxidant capacity is lower (Table 2).
Table 2.
Antioxidant capacity of extracts and isoeleutherin from Eleutherine plicata.
| Samples | Concentration (μg/mL) | Antioxidant capacity (mM) |
|---|---|---|
| Negative Control | – | 0.142 + 0.006 |
| EEEp | 9.80 | 0.127 + 0.007 |
| 4.90 | 0.264 + 0.002 | |
| 2.45 | 0.461 + 0.005 | |
| FDMEp | 9.52 | 0.250 + 0.002 |
| 4.76 | 0.424 + 0.004 | |
| 2.38 | 0.495 + 0.006 | |
| Isoeleutherin | 15.55 | 0.159 + 0.006 |
| 7.77 | 0.278 + 0.002 | |
| 3.88 | 0.428 + 0.004 |
EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata. Values are expressed as mean ± standard deviation.
From the 50 % inhibitory concentrations determined in the MTT assay, it was possible to determine the concentrations to be used in the comet assay, which were performed in three concentrations for EEEp, FDMEp, and isoeleuterin. From them, we obtained the DNA damage index of the samples and we concluded that the higher the concentration of the samples, the higher this index will be, both for EEEp, FDMEp, and isoeleutherin fraction (Table 3). Isoeleutherin and FDMEP, at the highest concentrations, were responsible for a high level of DNA damage.
Table 3.
Damage Index found by the Comet Assay for extracts and isoeleutherin from Eleutherine plicata.
| Samples | Concentration (μg/mL) | Damage index (DI) |
|---|---|---|
| Negative Control | – | 0.77 + 0.13 |
| Doxorubicin | 0.02 | 2.22 + 0.04 |
| EEEp | 9.80 | 1.48 + 0.05 |
| 4.90 | 1.41 + 0.01 | |
| 2.45 | 1.10 + 0.08 | |
| FDMEp | 9.52 | 1.96 + 0.06 |
| 4.76 | 1.78 + 0.02 | |
| 2.38 | 1.69 + 0.02 | |
| Isoeleutherin | 15.55 | 2.07 + 0.02 |
| 7.77 | 2.05 + 0.02 | |
| 3.88 | 1.84 + 0.03 |
EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; Values are expressed as mean ± standard deviation.
3.2. In vivo toxicity assessment
During the acute toxicity test period, all animals (males and females) in the groups treated with EEEp (2000 mg/Kg), FDMEp (2000 mg/Kg), isoeleutherin (2000 mg/Kg), and the control group (water) did not show any signs of overt toxicity, and none died during the fourteen-day follow-up period. Furthermore, the treatment of animals of both sexes did not interfere with the animals' weight (Table 4).
Table 4.
Weight assessment of mice treated with extracts and isoeleutherin from Eleutherine plicata.
| Days | Groups (males) |
P* | |||
|---|---|---|---|---|---|
| EEEp | FDMEp | Isoeleutherin | GC | ||
| 1 | 27.23 ± 0.52 | 26.5 ± 0.3 | 27.3 ± 0.65 | 26.6 ± 0.20 | 0.283 |
| 7 | 28.9 ± 1.56 | 28.6 ± 0.52 | 28.68 ± 0.42 | 27.8 ± 0.45 | 0.385 |
| 14 | 29.35 ± 0.34 | 29.1 ± 0.20 | 30.42 ± 0.54 | 29.3 ± 0.15 | 0.214 |
| Days | Groups (females) |
P* | |||
|---|---|---|---|---|---|
| EEEp | FDMEp | Isoeleutherin | GC | ||
| 1 | 26.2 ± 0.65 | 25.5 ± 0.82 | 25.6 ± 0.42 | 25.9 ± 0.40 | 0.225 |
| 7 | 28.75 ± 0.74 | 27.65 ± 0.56 | 28.8 ± 0.20 | 28.3 ± 0.27 | 0.348 |
| 14 | 29.58 ± 0.58 | 29.1 ± 0.20 | 30.24 ± 0.30 | 29.4 ± 0.53 | 0.256 |
Days = period of treatment. EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; CG: Control group (99 % water + 1 % DMSO). Values expressed as mean ± standard deviation (n = 3).
p was calculated using the T Student test.
During the subacute toxicity test period, the females treated with EEEp (1000 mg/Kg), FDMEp (1000 mg/Kg), and the control group did not show any signs of evident toxicity, and there was no death of the animals during the twenty- eight days of experiment. Furthermore, the treatment in both groups did not interfere with the animals' weight (Table 5).
Table 5.
Weight assessment of female Balb/c mice treated orally with repeated doses of EEEp, FDMEp, and control.
| Day | Weight (g) |
||
|---|---|---|---|
| GC | EEEp | FDMEp | |
| 1 | 28.42 ± 1.13 | 27.54 ± 2.12 | 26.36 ± 1.27 |
| 7 | 29.56 ± 1.12 | 28.85 ± 1.52 | 28.56 ± 1.95 |
| 14 | 29.62 ± 1.41 | 29.39 ± 1.32 | 30.93 ± 1.48 |
| 21 | 31.27 ± 1.51 | 32.28 ± 0.87 | 31.82 ± 1.38 |
Days = period of treatment. EEEp: ethanolic extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; CG: Control group (99 % water + 1 % DMSO). Values expressed as mean ± standard deviation (n = 5).
Regarding the results of hematological parameters for the groups treated with EEEp (1000 mg/Kg), FDMEp (1000 mg/Kg), and control, all parameters are within the reference values and, despite small changes between groups, there was no statistical significance (Table 6).
Table 6.
Hematological parameters of female Balb/c mice orally treated with repeated doses of EEEp, FDMEp, and control.
| Parameters | Groups |
Reference | ||
|---|---|---|---|---|
| GC | EEEp | FDMEp | ||
| Red Cells (106/mm3) | 6.25 ± 1.23 | 6.0 ± 0.34 | 6.90 ± 0.21 | 7.2–11.2 |
| Hematocrit (%) | 38.6 ± 0.45 | 32.36 ± 2.45 | 33.36 ± 2.54 | 33.1–52.0 |
| Hemoglobin (g/dL) | 12.23 ± 2.66 | 12.8 ± 0.38 | 13.1 ± 0.26 | 10.3–16.6 |
| VCM | 51.5 ± 0.52 | 51.5 ± 1.65 | 49.5 ± 1.72 | 45.0–47.0 |
| HCM | 17.8 ± 1.45 | 18.4 ± 0.86 | 17.86 ± 0.71 | 13.9–15.5 |
| CHCM | 34.8 ± 2.74 | 35.1 ± 1.47 | 36.1 ± 1.3 | 30.3–33.7 |
| Platelets (103/mm3) | 682.7 ± 85.8 | 668.33 ± 28.3 | 724.33 ± 29.02 | 439.0–957.0 |
| Leukocytes (103/mm3) | 2.40 ± 0.15 | 2.185 ± 0.02 | 2.263 ± 0.20 | 1.0–5.5 |
EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; CG: Control group (99 % water + 1 % DMSO). Values expressed as mean ± standard deviation (n = 5), considering (p < 0.05) for the Student T test. Reference: [61].
Therefore, for the subacute toxicity test, the samples obtained from E. plicata displayed very low toxicity. In addition, no significant changes were observed for the biochemical parameters analyzed (Table 7). Regarding microscopy, no changes were observed in the cardiac tissue, kidneys, lungs, or liver in both treated and control groups.
Table 7.
Biochemical parameters of female Balb/c mice orally treated with repeated doses of EEEp, FDMEp, and control.
| Parameters | Groups |
Reference | ||
|---|---|---|---|---|
| GC | EEEp | FDMEp | ||
| AST | 89.23 ± 2.85 | 87.00 ± 3.25 | 91.00 ± 6.24 | 64.0–258.0 |
| ALT | 46.00 ± 2.36 | 42.54 ± 2.14 | 51.22 ± 4.72 | 32.0–178.0 |
| UREA | 44.61 ± 3.26 | 46.38 ± 3.32 | 45.65 ± 2.28 | 27.0–70.0 |
| CREATININE | 0.28 ± 0.04 | 0.31 ± 0.03 | 0.36 ± 0.05 | 0.2–0.9 |
EEEp: ethanol extract of Eleutherine plicata; FDMEp: dichloromethane fraction of Eleutherine plicata; CG: Control group (99 % water + 1 % DMSO). AST: aspartate aminotransferase; ALT: alanine aminotransferase; Values expressed as mean ± standard deviation (n = 5). Reference: [61].
3.3. Molecular docking
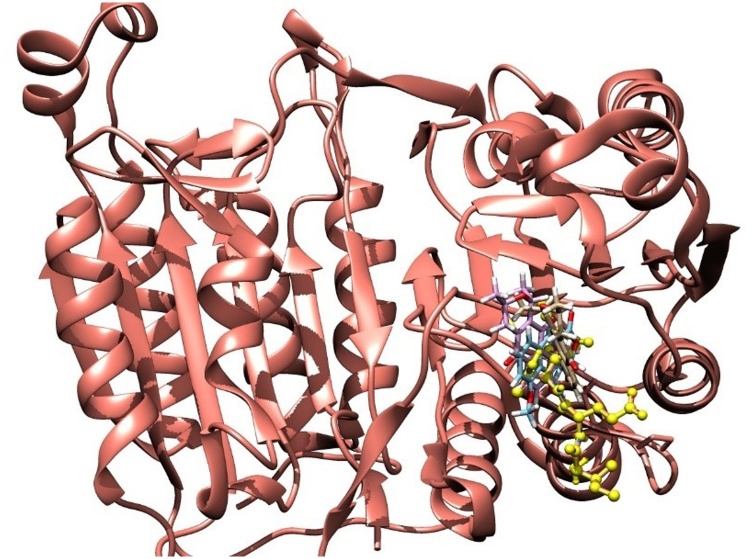
The molecular docking simulations of the three naphthoquinones with the target enzyme that showed the best GoldScore values obtained for the compounds were 41.93, 41.03, and 39.06 for eleutherin, isoeleutherin and eleutherol, respectively. Fig. 1 shows that the structures of naphthoquinones are close to the co-crystallized ligand (DARP) in the active site of the enzyme.
Fig. 1.
Molecular docking results with three naphthoquinones compounds and co-crystallized ligand (yellow).
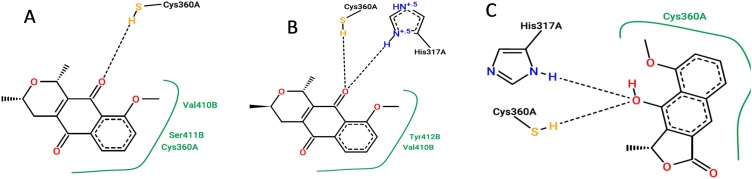
The interactions of the three compounds were analyzed in the PoseView online server. For eleutherin, two hydrogen bonds were observed with amino acid residues Hys317 and Cys360, and one hydrophobic interaction with Arg413 (Fig. 2A). For isoeleutherin, there is one hydrogen bond with Cys360 and two hydrophobic interactions with Cys360 and Ser411 (Fig. 2B). From Fig. 2C, for eleutherol, two hydrophobic interactions with Cys360 and Val410, and two hydrogen bonds with Arg260 and Arg413 amino acid residues were observed. Interestingly, the three compounds studied made an important interaction with the Cys360 residue (Fig. 2), and it was determined as the key residue for activity.
Fig. 2.
Interactions between ligands and caspase-8. Legend: A- eleutherin, B- isoeleutherin, C- eleutherol.
4. Discussion
The present study evaluated the cytotoxicity of EEEp, FDMEp, and isoeleutherin from E. plicata against human hepatoma cells (HepG2). It was observed that, after 24 h of exposure, the fractionation reduced cytotoxicity, and isoeleutherin was the least toxic sample. These results suggest the acute cytotoxicity of EEEp and FDMEp results from the synergism between the compounds eleutherin and isoeleutherin, which when administered together are more active and can increase toxicity [25]. Many chemical compounds bind weakly to their receptor, bringing an unsustainable effect, since in a short time they disconnect from the receptor, interfering with the biological activity [46].
After 48 h of treatment, isoeleutherin and FDMEp were more cytotoxic than the ethanol extract. The biological activities of E plicata have been attributed to quinonic compounds [26,27,47]. It is believed that it is an intrinsic property of these compounds associated with other structural factors, such as resonance effects, that may contribute to cytotoxicity. Quinonoid carbonyls are susceptible to reduction, generating alkylating intermediates [48], which may be involved in DNA alkylation and consequently, cell death [49]. FDMEp has a higher content of naphthoquinones than EEEp [25]. Moreover, it has at least two associated naphthoquinones (eleutherin and isoeleutherin) and this may explain its greater cytotoxicity at 48 h [27]. An in vitro study on different cell lines, including U-251 (glioma), MCF-7 (breast), NCI/ADR-RES (ovary expressing multidrug resistance phenotype), 786-0 (kidney), NCI-H460 (lung, non-small cells), HT-29 (colon), and K562 (leukemia), showed that eleutherin was more cytotoxic in all tested strains, when compared to its isoeleutherin isomer [50].
The delayed effect is probably due to the slowness in the formation of covalent bonds by DNA alkylation. There is initially an attraction between the reduced agent and the DNA, then a covalent bond is formed, an effect that can easily occur in naphthoquinones, such as atovaquone [51].
When the damage to DNA was analyzed, the FDMEp damage rate was higher than that of EEEp. In another study, the result was similar, in which the dichloromethane fraction was more genotoxic than the ethanol extract [52]. This fact can be explained by the higher content of naphthoquinones in the fraction, which in addition to alkylating, can also stabilize the topoisomerase II-DNA complex, what is clarified under in silico studies by docking and molecular modeling [28].
Isoeleutherin, especially at the highest concentration, was responsible for a high level of DNA damage, as assessed by the comet assay. Another study using micronucleus assay, demonstrated relatively low genotoxic potential [28]. The comet assay assesses the rate of DNA damage before repair, while the micronucleus assesses the maintenance of damage after repair [53]. Therefore, the damage to DNA caused by isoeleutherin can be repaired, while the damage caused by the dichloromethane fraction does not seem to be repaired. This fact reaffirms the hypothesis that toxicity must be related to another compound, such as eleutherin.
In a recent study with a naphthoquinone (1,4-naphthoquinone derivative- 2- (4-methoxyphenylthio)-5,8-dimethoxy-1,4-naphthoquinone), the results demonstrated that it has the ability to induce apoptosis in nine gastric cancer cell lines with accumulation of reactive oxygen species, decreased effect when pre-treated with an antioxidant (N-acetyl-l-cysteine), thus the apoptotic effect of naphthoquinone seems to be mediated by oxidative stress, and the use of N-acetyl-l-cysteine significantly decreased levels of p-JNK, p-p38, and cleaved caspase-3, while increasing levels of p-ERK1/2 and pSTAT3 [54].
To assess whether eleutherin, eleutherol, or isoeleutherin can bind directly to proteins involved in apoptosis, molecular docking was performed using caspase-8. The results demonstrated the activation of caspase-8 by these molecules, due to the interaction with the Cys360 residue of this enzyme, being determined as key residues for the activity. Caspase-8 is involved in the extrinsic pathway of apoptosis, and activation of caspase-8 leads to the activation of caspase-3 directly, or through cleavage of proapoptotic members of the Bcl-2 family (B cell CLL/lymphoma 2), promoting the release of cytochrome C from mitochondria, which has pro- and anti-apoptotic proteins [55]. In the cytoplasm, cytochrome c binds to apoptotic protease activating factor 1 (Apaf-1) and pro-caspase-9, forming the apoptosome complex and activating caspase-9, which in turn activates caspase-3, resulting in morphological and biochemical aspects of cell death [55,56]. Therefore, the cell death mechanism by eleutherin and isoeleutherin may involve the direct protein activation in the extrinsic pathway of apoptosis.
Quinones can generate reactive oxygen species because of their reduction products (semiquinones and hydroquinones), which can generate superoxide radical anions (O2−), hydroxyl radicals (OH•), hydrogen peroxide (H2O2), and singlet oxygen (*O2) that can induce cell damage [57]. Many quinones also exhibit biological activity against cancer cells, due to this capacity that can interact with cell membrane structures, proteins in the cytoplasm, or even the nucleus, interacting with DNA. In the present study, however, the antioxidant capacity of these compounds was evaluated, but there were no significant differences in the antioxidant capacity among the samples, suggesting that oxidative stress is probably not the main route related to the cytotoxicity displayed by the naphthoquinones present in E. plicata. Perhaps, other mechanisms, such as stabilization with the topoisomerase II-DNA complex [28], and the induction of apoptosis, are responsible for the cytotoxicity of these compounds.
In summary, the results suggest that the dichloromethane fraction is the most toxic, whereas the ethanol extract is the least toxic of the samples studied. Because of these results, it was decided to assess the safety of acute and repeated doses of oral administration of these samples through in vivo studies. In these results, clinical changes, weight gain, and repeated doses were also evaluated for hematological changes and tested for kidney and liver function parameters.
The results showed that both the use of one dose or repeated doses did not cause clinical or weight changes, and there were no significant differences between the hematological and biochemical data of the treated animals and the control group.
To obtain information to certify the safety of these candidates to medicine development, toxicity studies on animals were carried out [58]. Especially by the study of repeated doses, it is possible to characterize the toxicological profile and determine the adverse effects of a drug candidate. In addition, this test allows to identify changes in physiological, hematological, and biochemical parameters [59]. In the present study, no physiological, hematological, or biochemical changes were observed, suggesting that the use of EEEp and FDMEp in mice is safe.
Animal models can be good indicators of toxicity for humans, despite differences between species. The main differences are in toxicokinetics, with lab animals tending to metabolize toxic agents faster than humans; the perfusion rate of hepatocytes is higher, and the enzymatic activity of mammals increases with decreasing body weight. Other anatomical and physiological differences exist between Humans and animals [60], therefore, before conducting clinical studies, it is essential to demonstrate the safety of drug candidates in animal models.
When analyzing the results of in vitro and in vivo studies, EEEp is the one with the least toxic potential. Another study showed that this extract displays antimalarial potential, however, the fraction containing naphthoquinones was a little more active. Nevertheless, when antimalarial activity is linked to toxicity data, perhaps EEEp is the more promising fraction.
5. Conclusion
In vitro cytotoxicity study suggests that FDMEp, EEEp, and isoeleutherin display similar toxicity in treatment times. Isoeleutherin and FDMEp, especially at the highest concentrations, were responsible for a high level of DNA damage, as assessed by the comet assay. We believed that E. plicata cytotoxicity is related to naphthoquinones and it involves different signaling pathways, such as oxidative stress, inducing apoptosis, DNA alkylation, and stabilization of the topoisomerase II-DNA complex. This study demonstrated through molecular docking, for the first time, that naphthoquinones (eleutherin, eleutherol, and isoeleutherin) can activate caspase-8 resulting in possible apoptosis, due to the interaction of these compounds with the enzyme.
The animals treated with EEEp and FDMEp, with single and repeated doses, did not display any clinical, hematological, or biochemical alterations, suggesting they are safe. Our results showed that E. plicata has low toxic potential, being safe to use and, therefore, its compounds could be promising candidates for the development of antimalarials.
Author statement
Antonio Rafael Quadros Gomes: Investigation, Data curation, Writing - original draft Natasha Costa da Rocha Galucio: Data curation Kelly Cristina Oliveira de Albuquerque: Formal analysis Heliton Patrick Cordovil Brígido: Formal analysis Everton Luiz Pompeu Varela: Formal analysis Ana Laura Gadelha Castro: Formal analysis Valdicley Vieira Vale: Validation, Formal analysis Marcelo Oliveira Bahia: Methodology Rommel Mario Rodriguez Burbano: Methodology Fábio Alberto de Molfeta: Methodology Liliane Almeida Carneiro: Methodology Sandro Percario: Conceptualization, Supervision, Writing - review & editing Maria Fâni Dolabela: Conceptualization, Funding acquisition, Project administration.
Ethics approval
The project followed the international guidelines for research with experimental animals and the procedures were reviewed and approved by the Ethics Committee in Research with Experimental Animals of the Federal University of Pará - CEPAE/UFPA (Report No. 7464060618 (ID 001020).
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This study was financed in part by the Coordination of Superior Level Staff Improvement (CAPES)National Council for Scientific and Technological Development (CNPq, Universal-432458/2018-2). Publication expenses were funded by PROPESP/UFPA.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
Antonio Rafael Quadros Gomes, Email: rafaelquadros13@hotmail.com.
Natasha Costa da Rocha Galucio, Email: natashagalucio@gmail.com.
Kelly Cristina Oliveira de Albuquerque, Email: kellyoalbuquerque@hotmail.com.
Heliton Patrick Cordovil Brígido, Email: helitom2009@hotmail.com.
Everton Luiz Pompeu Varela, Email: evertonlpvarela@gmail.com.
Ana Laura Gadelha Castro, Email: lauracastro.farmacia@gmail.com.
Valdicley Vieira Vale, Email: valdicleyvale@gmail.com.
Marcelo Oliveira Bahia, Email: mobahia1970@yahoo.com.br.
Rommel Mario Rodriguez Burbano, Email: rommel@ufpa.br.
Fábio Alberto de Molfeta, Email: proffabioufpa@gmail.com.
Liliane Almeida Carneiro, Email: liliane.carneiro@cenp.gov.br.
Sandro Percario, Email: percario@ufpa.br.
Maria Fâni Dolabela, Email: fani@ufpa.br.
References
- 1.Couto C.L.L., Moraes D.F.C., Cartagenes M.S.S., Amaral F.M.M., Guerra R.N. Eleutherine bulbous (Mill.) Urb.: a review study. J. Med. Plants Res. 2016;10:286–297. doi: 10.5897/JMPR2016.6106. [DOI] [Google Scholar]
- 2.Pereira M.G.S., Coelho-Ferreira M. Uso e diversidade de plantas medicinais em uma comunidade quilombola na Amazônia Oriental, Abaetetuba, Pará. Biota Amaz. 2017;7:57–68. [Google Scholar]
- 3.Gois M.A.F., Lucas F.C.A., Costa J.C.M., Moura P.H.B., Lobato G.J.M. Etnobotânica de espécies vegetais medicinais no tratamento de transtornos do sistema gastrointestinal. Rev. Bras. Plantas Med. 2016;18:547–557. doi: 10.1590/1983-084X/15_15.170. [DOI] [Google Scholar]
- 4.Oliveira V.B., Rocha M.C.A. Levantamento das plantas utilizadas como medicinais na cidade de Caxias-MA: uma perspectiva etnofarmacológica. Rev. Interdisc. 2016;9:43–52. [Google Scholar]
- 5.Luziatelli G., Sonresen M., Theilade I., Molgaard P. Asháninka medicinal plants: a case study from the native community of Bajo Quimiriki, Junín, Peru. J. Ethnobiol. Ethnomed. 2010;6:1–23. doi: 10.1186/1746-4269-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vásquez S.P.F., Mendonça M.S., Noda S.N. Etnobotânica de plantas medicinais em comunidades ribeirinhas do município de Manacapuru, Amazonas, Brasil. Acta Amazôn. 2014;44:457–472. doi: 10.1155/2019/6087509. [DOI] [Google Scholar]
- 7.Lans C. A review of the plant-based traditions of the Cocoa Panyols of Trinidad. GeoJournal. 2018;83:1425–1454. doi: 10.1007/s10708-017-9835-2. [DOI] [Google Scholar]
- 8.Sarquis R.S.F.R., Sarquis I.R., Sarquis I.R., Fernandes C.P., Silva G.A., Silva R.B.L., Jardim M.A.G., Sanchez-Ortiz B.L., Carvalho J.C.T. The use of medicinal plants in the Riverside Community of the Mazagão River in the Brazilian Amazon, Amapá, Brazil: Ethnobotanical and ethnopharmacological studies. Evid. Based Complement. Altern. Med. 2019;2019:1–25. doi: 10.1155/2019/6087509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odonne G., Valadeau C., Alban-Castillo J., Stien D., Sauvain M., Bourdy G. Medical ethnobotany of the Chayahuita of the Paranapura basin (Peruvian Amazon) J. Ethnopharmacol. 2013;146:127–153. doi: 10.1016/j.jep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Malheiros L.C.S., Mello J.C.P., Barbosa W.L.R. Phytochemicals – Isolation, Characterization and Role in Human Health. INTECH; 2015. Eleutherine plicata – quinones and antioxidant activity; pp. 323–338. Chapter 14. [Google Scholar]
- 11.Hara H., Maruyama N., Yamashita S., Hayashi Y., Lee K.H., Bastow K.F., Chairul, Marumoto R., Imakura Y. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem. Pharm. Bull. 1997;45:1714–1716. [Google Scholar]
- 12.Zhengxiong C., Huizhu H., Chengrui W., Yuhui L., Jianimi D., Sankawa U., Noguchi H., Iitaka Y. Hongconin, a new naphthalene derivative from Hong-Cong, the rizome of Eleutherine americana Merr. et Heyne (Iridaceae) Chem. Pharm. Bull. 1986;37:2743–2746. [Google Scholar]
- 13.Weniger B., Haag-Berrurier M., Anton R. Plants of Haiti used as antifertility agents. J. Ethnopharmacol. 1982;6:67–84. doi: 10.1016/0378-8741(82)90072-1. [DOI] [PubMed] [Google Scholar]
- 14.Paramapojn S., Ganzera M., Gritsanapan W., Stuppner H. Analysis of naphthoquinone derivatives in the Asian medicinal plant Eleutherine americana by RP-HPLC and LC-MS. J. Pharm. Biomed. Anal. 2008;47:990–993. doi: 10.1016/j.jpba.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Omar S., Lemonnier B., Jones N., Ficker C., Smith M.L., Neema C., Towers G.H., Goel K., Arnason J.T. Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. J. Ethnopharmacol. 2000;73:161–170. doi: 10.1016/s0378-8741(00)00294-4. [DOI] [PubMed] [Google Scholar]
- 16.Xu H.L., Yu X.F., Qu S.C., Zhang R., Qu X.R., Chen Y.P., Ma X.Y., Sui D.Y. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondrial dependent pathway. Eur. J. Pharmacol. 2010;645:14–22. doi: 10.1016/j.ejphar.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 17.Xu H.L., Yu X.F., Qu S.C., Qu X.R., Jiang Y.F., Sui D.Y. Juglone, from Juglans mandshruica Maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 through a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012;50:590–596. doi: 10.1016/j.fct.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Martindale J.L., Holbrook N.J. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 19.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.F., Zhang X., Groopman J.E. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J. Biol. Chem. 2004;279:27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 21.Igney F.H., Krammer P.H. Death and anti-death: tumor resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara N., Inoue J., Kawano T., Tanimoto K., Kozaki K., Inazawa J. miR-634 activates the mitochondrial apoptosis pathway and enhances chemotherapy-induced cytotoxicity. Cancer Res. 2015;75:3890–3901. doi: 10.1158/0008-5472.CAN-15-0257. [DOI] [PubMed] [Google Scholar]
- 23.Maelfait J., Vercammen E., Janssens S., Schotte P., Haegman M., Magez S., Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin 1 beta maturation by caspase-8. J. Exp. Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriwaki K., Bertin J., Gough P.J., Orlowski G.M., Chan F.K.M. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vale V.V., Cruz J.N., Viana G.M.R., Póvoa M.M., Brasil D.S.B., Dolabela M.F. Naphthoquinones isolated from Eleutherine plicata herb: in vitro antimalarial activity and molecular modeling to investigate their binding modes. Med. Chem. Res. 2020;29:487–498. doi: 10.1007/s00044-019-02498-z. [DOI] [Google Scholar]
- 26.Borges E.S., Galucio N.C.da R., Veiga A.S.S., Busman D.V., Lins A.L.F.A., Bahia M.O., Rissino J.D., Correa R.M.S., Burbano R.M.R., Marinho A.M.R., Casique J.V., Percário S., Dolabela M.F. Botanical studies, antimicrobial activity and cytotoxity of Eleutherine bulbosa (Mill.) Urb. Res. Soc. Dev. 2020;9 doi: 10.33448/rsd-v9i11.9992. [S. l.] [DOI] [Google Scholar]
- 27.Castro A.L.G., Correa-Barbosa J., Campos P.S., Matte B.F., Lamers M.L., Siqueira J.E.S., Marinho A.M.R., Monteiro M.C., Vale V.V., Dolabela M.F. Antitumoral activity of Eleutherine plicata Herb. and its compounds. Int. J. Dev. Res. 2021;11:44673–44678. doi: 10.37118/ijdr.21154.02.2021. [DOI] [Google Scholar]
- 28.Castro A.L.G., Cruz J.N., Sodré D.F., Correa- Barbosa J., Azonsivo R., Oliveira M.S., Siqueira J.E.S., Galucio N.C.R., Bahia M.O., Burbano R.M.R., Marinho A.M.R., Percario S., Dolabela M.F., Vale V.V. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated fromEleutherine plicata Herb. using bioassays and in silico approaches. Arab. J. Chem. 2021;14 doi: 10.1016/j.arabjc.2021.103084. [DOI] [Google Scholar]
- 29.Reeves G., Chase M.W., Goldblatt P., Rudall P., Fay M.F., Cox A.V., Lejeune B., Chies T.S. Molecular systematics of Iridaceae: evidence from four plastid DNA regions. Am. J. Bot. 2001;88(11):2074–2087. PMID: 21669639. [PubMed] [Google Scholar]
- 30.Goldblatt P., Rodriguez A., Powel M.P., Davies T.J., Manning J.C., Van Der Ban K.M., Savolainen V. Iridaceae “out of Australasia”? Phylogeny, biogeography, and divergence time based on plastid DNA sequences. Syst. Bot. 2008;33(3):495–508. doi: 10.1600/036364408785679806. [DOI] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Laohavechvanich P., Muangnoi C., Butryee C., Kriengsinyos W. Protective effect of makrut lime leaf (Citrus hystrix) in HepG2 cells: implications for oxidative stress. Science Asia. 2010;36:112–117. doi: 10.2306/scienceasia1513-1874.2010.36.112. [DOI] [Google Scholar]
- 33.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 34.Garcia E.J., Oldoni T.L.C., Alencar S.M., Reis A., Loguercio A.D., Grande R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012;23:22–27. doi: 10.1590/S0103-64402012000100004. [online] [DOI] [PubMed] [Google Scholar]
- 35.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 36.Mota T.C., Cardoso P.C., Gomes L.M., Vieira P.C., Corrêa R.M., Santana P.D., Miranda M.S., Burbano R.M., Bahia M.O. In vitro evaluation of the genotoxic and cytotoxic effects of artesunate, an antimalarial drug, in human lymphocytes. Environ. Mol. Mutagen. 2011;52:590–594. doi: 10.1002/em.20659. [DOI] [PubMed] [Google Scholar]
- 37.OECD- Organization for Economic Co-Operation and Development . OECD Guideline for Testing of Chemicals. 2001. Acute oral toxicity – fixed dose procedure. n. 420. [Google Scholar]
- 38.Brito A.S. Manual de ensaios toxicológicos in vivo. Univ. Camp. 1994;1:122. [Google Scholar]
- 39.Vale V.V., Vilhena T.C., Trindade R.C.S., Coelho-Ferreira M.R., Percário S., Soares L.F., Pereira W.L.A., Brandão G.C., Oliveira A.B., Dolabela M.F., Vasconcelos F. Anti-malarial activity and toxicity assessment of Himatanthus articulatus, a plant used to treat malaria in the Brazilian Amazon. Malar. J. 2015;14:132. doi: 10.1186/s12936-015-0643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.OECD- Organization for Economic Cooperation and Development . Head of Publications Service; Paris: 1995. Guideline 407: Repeated Dose 28-Day Oral Toxicity Study in Rodent. [Google Scholar]
- 41.Kroemer R.T. Structure-based drug design: docking and scoring. Curr. Protein Pept. Sci. 2007;8:312–328. doi: 10.2174/138920307781369382. [DOI] [PubMed] [Google Scholar]
- 42.Meng X.Y., Zhang H.X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A.N., Baruah M.M., Sharma N. Structure based docking studies towards exploring potential antiandrogen activity of selected phytochemicals against prostate cancer. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-02023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones G., Willett P., Glen R.C., Leach A.R., Taylor R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 45.Stierand K., Rarey M. Drawing the PDB: protein-ligand complexes in two dimensions. ACS Med. Chem. Lett. 2010;1:540–545. doi: 10.1021/ml100164p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marc J. Pharmacogenetics of drug receptors. EJIFCC. 2008;19:48–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Alves T.M.A., Kloos H., Zani C.L. Eleutherinone, a Novel Fungitoxic Naphthoquinone from Eleutherine bulbosa (Iridaceae) Memórias do Instituto Oswaldo Cruz. 2003;98:709–712. doi: 10.1590/S0074-02762003000500021. [DOI] [PubMed] [Google Scholar]
- 48.Silva M.N., Ferreira V.F., Souza M.C.B.V. Um panorama atual da química e da farmacologia de naftoquinonas com ênfase na β-lapachona e derivados. Quim. Nova. 2003;25:407–416. doi: 10.1590/S0100-40422003000300019. [DOI] [Google Scholar]
- 49.Verga D., Nadai M., Doria F., Percivalle C., Antonio M.D., Palumbo M., Richter S.N., Freccero M. Photogeneration and reactivity of naphthoquinone methides as purine selective DNA alkylating agents. J. Am. Chem. Soc. 2010;132:14625–14637. doi: 10.1021/ja1063857. [DOI] [PubMed] [Google Scholar]
- 50.Campos A., Vendramini-Costa D.B., Fiorito F.G., Ruiz L.T.G.A., Carvalho J.E., De Souza M.R.G., Delle-Monache F., Cechinel Filho V. Antiproliferative effect of extracts and pyranonaphthoquinones obtained from Cipura paludosa bulbs. Pharm. Biol. 2016;54:1022–1026. doi: 10.3109/13880209.2015.1091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutiérrez-Bonet A., Remeur C., Matsui J.K., Molander Gary A. Late-stage C-H alkylation of heterocycles and 1,4-quinones via oxidative homolysis of 1,4-dihydropyridines. J. Am. Chem. Soc. 2017;139:12251–12258. doi: 10.1021/jacs.7b05899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galucio N.C.R. Instituto de Ciências da Saúde, Universidade Federal do Pará; Belém, Pará - Brazil: 2014. Estudos fitoquímicos, citotoxicidade e genotoxicidade de Eleutherine plicata Herb. 90f. Dissertation (Master in Pharmaceutical Sciences) [Google Scholar]
- 53.Verri A.M., Moura A.A., Moura V.M. Testes Citogenéticos na Avaliação da Genotoxicidade de Produtos Naturais Provindos de Plantas Medicinais. Revista UNINGÁ Review. 2017;30:55–61. [Google Scholar]
- 54.Wang J.-R., Shen G.-N., Luo Y.-H., Piao X.-J., Zhang Y., Wang H., Li J.Q., Xu W.T., Zang Y., Wang S.-N., Zang T., Xue H., Cao L.-K., Jin C.-H. 2-(4-methoxyphenylthio)-5,8-dimethoxy-1,4-naphthoquinone induces apoptosis via ROS-mediated MAPK and STAT3 signaling pathway in human gastric cancer cells. J. Chemother. 2019;31:214–226. doi: 10.1080/1120009X.2019.1610832. [DOI] [PubMed] [Google Scholar]
- 55.Luchs A., Pantaleao C. Apoptose e modelos in vivo para estudo das moléculas relacionadas a este fenômeno. Einstein. 2010;8:495–497. doi: 10.1590/s1679-45082010rb1685. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann K.C., Bonzon C., Green D.R. The machinery of programmed cell death. Pharmacol. Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 57.Sousa E.T., Lopes W.A., Andrade J.B. Fontes, formação, reatividade e determinação de quinonas na atmosfera. Química Nova. 2016;39:486–495. doi: 10.5935/0100-4042.20160034. [DOI] [Google Scholar]
- 58.Faqi A.S. Introduction. In: Faqi A.S., editor. A Comprehensive Guide To Toxicology In Preclinical Drug Development. 1st ed. Elsevier; Amsterdan: 2013. p. 1. [Google Scholar]
- 59.EMA, European Medicines Agency . 2010. Guideline on Repeated Dose Toxicity.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500079536.pdf Available at. [Google Scholar]
- 60.Dybing E., Doe J., Groten J., Kleiner J., O’Brien J., Renwick A.G., Schlatter J., Steinberg P., Tritscher A., Walker R., Younes M. Hazard characterization of chemicals in food and diet: dose response, mechanism and extrapolation issue. Food Chem. Toxicol. 2002;40:247–261. doi: 10.1016/s0278-6915(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 61.Araujo F.T.M. 2012. Estabelecimento de valores de referência para parâmetros hematológicos e bioquímicos e avaliação do perfil imunológico de linhagens de camundongos produzidas nos biotérios do Centro de Pesquisas René Rachou/FIOCRUZ-Minas e do Centro de Criação de Animais de Laboratório/FIOCRUZ. (Doctoral dissertation) [Google Scholar]