Abstract
Objective
To evaluate contemporary patterns in prepregnancy cardiovascular health (CVH) in the United States (US).
Methods
We conducted a serial, cross-sectional study of National Center for Health Statistics Natality Data representing all live births in the US from 2011 to 2019. We assigned 1 point for each of four ideal prepregnancy metrics (nonsmoking and ideal body mass index [18.5–24.9 kg/m2] provided by maternal self-report, and absence of hypertension and diabetes ascertained by the healthcare professional at delivery) to construct a prepregnancy clinical CVH score ranging from 0 to 4. We described the distribution of prepregnancy CVH, overall and stratified by self-reported race/ethnicity, age, insurance status, and receipt of the Women, Infants, and Children program (WIC) for supplemental nutrition. We examined trends by calculating average annual percent changes (AAPCs) in optimal prepregnancy CVH (score of 4).
Results
Of 31,643,982 live births analyzed between 2011 and 2019, 53.6% were to non-Hispanic White, 14.5% non-Hispanic Black, 23.3% Hispanic, and 6.6% non-Hispanic Asian women. The mean age (SD) was 28.5 (5.8) years. The prevalence (per 100 live births) of optimal prepregnancy CVH score of 4 declined from 42.1 to 37.7 from 2011 to 2019, with an AAPC (95% CI) of -1.4% per year (-1.3,-1.5). While the relative decline was observed across all race/ethnicity, insurance, and WIC subgroups, significant disparities persisted by race, insurance status, and receipt of WIC. In 2019, non-Hispanic Black women (28.7 per 100 live births), those on Medicaid (30.4), and those receiving WIC (29.1) had the lowest prevalence of optimal CVH.
Conclusions
Overall, less than half of pregnant women had optimal prepregnancy CVH, and optimal prepregnancy CVH declined in each race/ethnicity, age, insurance, and WIC subgroup between 2011-2019 in the US. However, there were persistent disparities by race/ethnicity and socioeconomic status.
Keywords: cardiovascular health, Pregnancy
Cardiovascular risk factors present before pregnancy are associated with increased risk of adverse pregnancy outcomes and subsequent risk of cardiovascular disease (CVD) [1]. Given rising trends in maternal morbidity and mortality in the US due, in part, to CVD [2], defining contemporary patterns in prepregnancy cardiovascular health (CVH) is needed to inform strategies to optimize CVH before conception, when interventions may be most effective because there is a longer time period to modify cardiovascular risk than during pregnancy. Therefore, we sought to determine trends in a composite measure of prepregnancy CVH between 2011 and 2019.
1. Methods
We conducted a serial, cross-sectional study of National Center for Health Statistics birth certificate data representing all live births in the US. Birth certificates are recorded by the medical professional (e.g. physician, certified nurse midwife) present at delivery [3] and include data on four maternal metrics that are part of the American Heart Association CVH framework [4]: prepregnancy smoking, body mass index (BMI), hypertension, and diabetes. These metrics are based on a combination of maternal self-report (prepregnancy smoking, height, and weight) and health record data (prepregnancy hypertension and diabetes) [5]. We included women aged 15–44 years with data available for all 4 CVH metrics (representing 96.1% of eligible records). We created a clinical CVH score ranging from 0 to 4 by assigning 1 point for each optimal prepregnancy metric (non-smoking, normal BMI [18.5–24.9 kg/m2], no hypertension, and no diabetes). We described the distribution of prepregnancy CVH, overall and stratified by self-reported race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic Asian), age (15–24, 25–34, and 35–44 years), insurance status (Medicaid and private insurance), and receipt of the Women, Infants, and Children program (WIC) for supplemental nutrition (WIC and no WIC). We then calculated average annual percent changes (AAPCs) to describe relative changes in the prevalence of optimal prepregnancy CVH (score of 4) per 100 live births from 2011 to 2019. The data used in this study are publicly available at https://www.cdc.gov/nchs/nvss/births.htm, and this study was exempt from IRB review given the deidentified, publicly available data. We used Joinpoint Regression Program version 4.9 to compute AAPCs and Stata version 15.1 for all other analyses.
2. Results
Of 31,643,982 live births analyzed between 2011 and 2019, 53.6% were to non-Hispanic White, 14.5% non-Hispanic Black, 23.3% Hispanic, and 6.6% non-Hispanic Asian women (Table 1). The age distribution was 27.2% aged 15–24 years, 56.5% aged 25-34 years, and 16.3% aged 35-44 years, with a mean age (SD) of 28.5 (5.8) years. 42.9% of women had Medicaid, 48.8% had private insurance, and 41.0% of women received WIC during pregnancy. Compared to women with suboptimal prepregnancy CVH (score of 0 to 3), those with optimal prepregnancy CVH (score of 4) had a greater proportion of non-Hispanic White and non-Hispanic Asian race/ethnicity, greater educational attainment, a greater proportion of private insurance, and a lesser proportion of receipt of WIC and multiparity.
Table 1.
Maternal characteristics of the analytic sample in the United States, 2011-2019.
| Characteristic | Overall | Optimal CVH (4) | Suboptimal CVH (0-3) |
|---|---|---|---|
| N | 31,643,982 | 12,717,518 | 18,926,464 |
| Age, mean (SD) | 28.5 (5.8) | 28.6 (5.9) | 28.4 (5.8) |
| Age category, N (%) | |||
| 15-24 years | 8,605,389 (27.2%) | 3,335,594 (26.2%) | 5,269,795 (27.8%) |
| 25-34 years | 17,869,267 (56.5%) | 7,269,198 (57.2%) | 10,600,069 (56.0%) |
| 35-44 years | 5,169,326 (16.3%) | 2,112,726 (16.6%) | 3,056,600 (16.1%) |
| Race/ethnicity, N (%) | |||
| Non-Hispanic White | 16,969,241 (53.6%) | 7,065,645 (55.6%) | 9,903,596 (52.3%) |
| Non-Hispanic Black | 4,600,710 (14.5%) | 1,418,417 (11.2%) | 3,182,293 (16.8%) |
| Hispanic | 7,388,754 (23.3%) | 2,775,995 (21.8%) | 4,612,759 (24.4%) |
| Non-Hispanic Asian | 2,101,849 (6.6%) | 1,252,042 (9.8%) | 849,807 (4.5%) |
| Other | 583,428 (1.8%) | 205,419 (1.6%) | 378,009 (2.0%) |
| Education, N (%) | |||
| Less than high school | 4,507,924 (14.4%) | 1,521,392 (12.1%) | 2,986,532 (15.9%) |
| High school graduate | 7,902,746 (25.2%) | 2,611,015 (20.8%) | 5,291,731 (28.2%) |
| Greater than high school | 18,899,430 (60.4%) | 8,438,262 (67.1%) | 10,461,168 (55.8%) |
| Insurance, N (%) | |||
| Medicaid | 13,479,003 (42.9%) | 4,379,619 (34.7%) | 9,099,384 (48.5%) |
| Private Insurance | 15,317,725 (48.8%) | 7,091,354 (56.2%) | 8,226,371 (43.8%) |
| Self Pay | 1,265,984 (4.0%) | 608,204 (4.8%) | 657,780 (3.5%) |
| Other | 1,326,799 (4.2%) | 536,237 (4.3%) | 790,562 (4.2%) |
| Received WIC, N (%) | 12,824,644 (41.0%) | 4,133,256 (32.9%) | 8,691,388 (46.4%) |
| Received prenatal care, N (%) | 30,462,564 (98.6%) | 12,417,885 (98.7%) | 18,486,348 (98.5%) |
| Multiparous, N (%) | 19,318,227 (61.2%) | 7,167,529 (56.5%) | 12,150,698 (64.4%) |
| Singleton, N (%) | 30,580,298 (96.6%) | 12,717,518 (96.8%) | 18,926,464 (96.5%) |
CVH, cardiovascular health; WIC, Women, Infants, and Children program
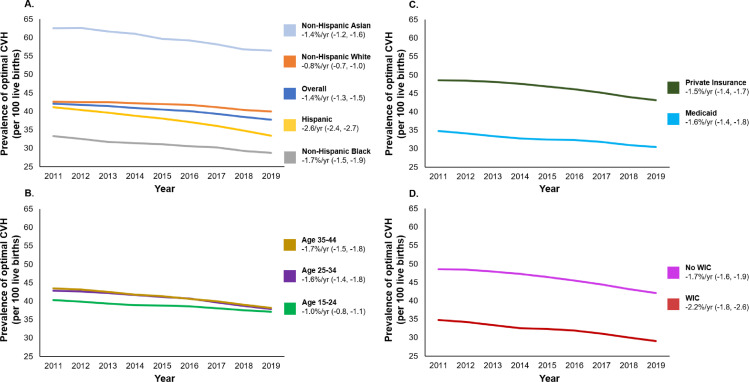
The prevalence (per 100 live births) of optimal prepregnancy CVH score of 4 declined from 42.1 to 37.7 from 2011 to 2019, with an AAPC (95% CI) of -1.4% per year (-1.3, -1.5) (Fig. 1). From 2011 to 2019, the overall prevalence (per 100 live births) of suboptimal prepregnancy CVH (score of 0 to 3) changed from 49.8 to 55.1 for CVH score of 3, 7.8 to 6.8 for CVH score of 2, 0.3 to 0.4 for CVH score of 1, and 0.02 to 0.02 for CVH score of 0.
Fig. 1.
Trends in the prevalence of optimal clinical prepregnancy cardiovascular health score, 2011–2019.
The decline in optimal prepregnancy CVH was observed across all demographic subgroups (race/ethnicity, age) from 2011–2019. Persistent racial disparities were observed, and the prevalence (per 100 live births) of optimal prepregnancy CVH (score of 4) varied 2-fold from non-Hispanic Black (28.7) to non-Hispanic Asian women (56.5) in 2019. From 2011 to 2019, the relative decline (95% CI) in optimal CVH ranged from -0.8% per year (-0.7, -1.0) in non-Hispanic White women to -2.6% per year (-2.4, -2.7) in Hispanic women. Across age subgroups, trends in optimal CVH were similar; in 2019, prevalence of CVH score of 4 was between 37 and 38 for all age ranges, and rates of decline ranged from -1.0% per year (-0.8, -1.1) in women aged 15–24 years to -1.7% per year (-1.5, -1.8) in women aged 35-44 years.
Optimal prepregnancy CVH also declined across groups reflecting socioeconomic status (insurance and WIC status). Populations with greater socioeconomic advantage had a greater prevalence of optimal prepregnancy CVH; in 2019, this prevalence (per 100 live births) was 43.1 in women with private insurance compared with 30.4 in women with Medicaid, and 42.1 in women not receiving WIC compared with 29.1 in women receiving WIC. AAPC ranged from -1.5% per year (-1.4, -1.7) in women with private insurance to -2.2% per year (-1.8, -2.6) in women receiving WIC.
3. Discussion
This analysis of maternal data from all live births in the US between 2011 and 2019 demonstrates that less than half of women had optimal prepregnancy CVH, and the prevalence of optimal CVH declined by 10.4% over the 8-year study period. Optimal prepregnancy CVH declined in each race/ethnicity, age, and socioeconomic subgroup as assessed by insurance and WIC status. However, there were persistent disparities by race, insurance, and report of WIC. Non-Hispanic Black women had the lowest prevalence and Hispanic women had the fastest rate of decline of optimal clinical CVH. There was approximately a 13 per 100 live birth difference in optimal CVH in the socioeconomic strata (Medicaid vs. private insurance, WIC vs. no WIC). An important limitation of this analysis is the potential for misclassification due to self-reported body mass index and smoking, and ascertainment bias of prepregnancy diabetes and hypertension. Validation studies have shown that items on birth certificates generally have variable sensitivity and high specificity [6]; for example, the sensitivity of birth certificate data for prepregnancy diabetes is approximately 50%, while the specificity is >98% [7]. Therefore, our analysis focuses on trends and relative differences over time and between race/ethnicity, age, and socioeconomic groups, but likely underestimates the true prevalence of suboptimal prepregnancy CVH. Additional limitations include the lack of objective risk factor levels and no measure of lifestyle factors (e.g., diet, physical activity).
Although data on prepregnancy CVH are sparse, our results are consistent with and complement prior findings from Perak et al. that identified a high prevalence of poor CVH when assessed during pregnancy [8]. Our analysis extends prior findings by shifting the focus upstream and suggesting that poor CVH in pregnancy begins before conception. We also include key differences over time and between subgroups to highlight persistent disparities over time and provide a greater context of the high prevalence of suboptimal prepregnancy CVH. In particular, of all subgroups, prepregnancy CVH in Hispanic women declined the fastest. While subgroup findings should be interpreted cautiously [9], the trend occurs in the context of Hispanic adults having a disproportionately high and rising prevalence of obesity [10] and diabetes [11], and the overall decline in CVH may conceal important heterogeneity among Hispanic/Latina subgroups [12]. The trend of declining prepregnancy CVH across all subgroups over the last decade is also consistent with secular increases in the prevalences of obesity [13], diabetes [14], and hypertension awareness (although the prevalence of hypertension has been stable) [15] among all young adults, which more than offset continuing decreases in smoking prevalence [16]. Future studies should seek to characterize comprehensive CVH, including all seven metrics, in the prepregnancy period, and to contextualize key social determinants of health, including structural racism [17], as upstream drivers of the observed disparities in CVH.
The high and rising prevalence of suboptimal prepregnancy CVH underscores the need to optimize CVH before conception when interventions may have the greatest benefit. While each component of CVH has been individually associated with higher risk of adverse maternal and offspring outcomes [1], the composite CVH profile has been additionally associated with adverse pregnancy outcomes [18] as well as long-term maternal [19] and offspring CVH [20]. Given these implications, targeted clinical and public health efforts beginning before pregnancy are needed. One potential strategy that has been recommended by the American Heart Association and American College of Obstetricians and Gynecologists [21] is comprehensive assessment of CVH before pregnancy in women of reproductive age to achieve consistent health messaging across the life course and optimize maternal and childhood outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
Supported by grants from the Nationtal Institutes of Health (P30AG059988; P30DK092939) and the American Heart Association (#19TPA34890060) to SSK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
The funding sponsor did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication. Dr. Khan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
None.
Contributions
MCW and SSK designed the study. MCW conducted the statistical analysis. All authors contributed to interpretation of data. MCW and SSK drafted the manuscript. All authors revised the manuscript for important intellectual content. SSK provided supervision of the research.
References
- 1.Virani SS, Alonso A, Aparicio HJ. Heart disease and stroke statistics–2021 update: a report from the American heart association. Circulation. 2021;0(0) doi: 10.1161/CIR.0000000000000950. CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Creanga AA, Berg CJ, Ko JY. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt) 2014;23(1):3–9. doi: 10.1089/jwh.2013.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics . 2012. Birth edit specifications for the 2003 proposed revision of the US standard certificate of birth. https://www.cdc.gov/nchs/data/dvs/birth_edit_specifications.pdf PublishedAccessed 17 June, 2021. [Google Scholar]
- 4.Lloyd-Jones DM, Hong Y, Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics . National Center for Health Statistics, Centers for Disease Control and Prevention; 2019. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision) https://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf PublishedUpdated September 2019. Accessed 17 June, 2021. [Google Scholar]
- 6.Dietz P, Bombard J, Mulready-Ward C. Validation of selected items on the 2003 US standard certificate of live birth: New York City and Vermont. Public Health Rep. 2015;130(1):60–70. doi: 10.1177/003335491513000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2009;13(5):660–666. doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- 8.Perak AM, Ning H, Khan SS, Horn LVV, Grobman WA, DM Lloyd-Jones. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. Journal of the American Heart Association. 2020;9(4) doi: 10.1161/JAHA.119.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang M. Trends in the prevalence of diabetes among US adults: 1999-2016. Am J Prev Med. 2018;55(4):497–505. doi: 10.1016/j.amepre.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Diaz CL, Shah NS, Lloyd-Jones DM, Khan SS. State of the nation's cardiovascular health and targeting health equity in the United States: a narrative review. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Du Y, Wu Y, Snetselaar LG, Wallace RB, Bao W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011-18: population based study. BMJ. 2021;372:n365. doi: 10.1136/bmj.n365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Li X, Wang Z. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. 2021 doi: 10.1001/jama.2021.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70(4):736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025–1035. doi: 10.1161/CIRCULATIONAHA.118.035550. [DOI] [PubMed] [Google Scholar]
- 17.Churchwell K, Elkind MSV, Benjamin RM. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American heart association. Circulation. 2020;142(24):e454. doi: 10.1161/CIR.0000000000000936. e468. [DOI] [PubMed] [Google Scholar]
- 18.Wang MC, Freaney PM, Perak AM. Association of pre-pregnancy cardiovascular risk factor burden with adverse maternal and offspring outcomes. Eur J Prev Cardiol. 2021 doi: 10.1093/eurjpc/zwab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q, Cogswell ME, Flanders WD. Trends in cardiovascular health metrics and associations with all-cause and cvd mortality among US adults. JAMA. 2012;307(12):1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perak AM, Lancki N, Kuang A. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325(7):658–668. doi: 10.1001/jama.2021.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown HL, Warner JJ, Gianos E. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American heart association and the american college of obstetricians and gynecologists. Circulation. 2018;137(24):e843–e852. doi: 10.1161/CIR.0000000000000582. [DOI] [PubMed] [Google Scholar]