Key Points
Question
Is the implementation of a regional prehospital transport policy for comprehensive stroke center triage associated with increased rates of endovascular therapy (EVT)?
Findings
This preimplementation-postimplementation study of 663 patients who arrived at the hospital within 6 hours of onset of acute ischemic stroke found an association of increased EVT rates from the preimplementation period (4.8% [95% CI, 3.0%-7.8%]) to 13.6% [95% CI, 10.4%-17.6%) in the postimplementation period. No change in time to thrombolysis or rate of thrombolysis was observed.
Meaning
These findings suggest that implementation of a regional prehospital transport policy for comprehensive stroke center triage may result in a significant increase in EVT rates in patients with acute ischemic stroke and large vessel occlusion.
Abstract
Importance
Endovascular therapy (EVT) improves functional outcomes in acute ischemic stroke (AIS) with large vessel occlusion (LVO). Whether implementation of a regional prehospital transport policy for comprehensive stroke center triage increases use of EVT is uncertain.
Objective
To evaluate the association of a regional prehospital transport policy that directly triages patients with suspected LVO stroke to the nearest comprehensive stroke center with rates of EVT.
Design, Setting, and Participants
This retrospective, multicenter preimplementation-postimplementation study used an interrupted time series analysis to compare treatment rates before and after implementation in patients with AIS arriving at 15 primary stroke centers and 8 comprehensive stroke centers in Chicago, Illinois, via emergency medical services (EMS) transport from December 1, 2017, to May 31, 2019 (9 months before and after implementation in September 2018). Data were analyzed from December 1, 2017, to May 31, 2019.
Interventions
Prehospital EMS transport policy to triage patients with suspected LVO stroke, using a 3-item stroke scale, to comprehensive stroke centers.
Main Outcomes and Measures
Rates of EVT before and after implementation among EMS-transported patients within 6 hours of AIS onset.
Results
Among 7709 patients with stroke, 663 (mean [SD] age, 68.5 [14.9] years; 342 women [51.6%] and 321 men [48.4%]; and 348 Black individuals [52.5%]) with AIS arrived within 6 hours of stroke onset by EMS transport: 310 of 2603 (11.9%) in the preimplementation period and 353 of 2637 (13.4%) in the postimplementation period. The EVT rate increased overall among all patients with AIS (preimplementation, 4.9% [95% CI, 4.1%-5.8%]; postimplementation, 7.4% [95% CI, 7.5%-8.5%]; P < .001) and among EMS-transported patients with AIS within 6 hours of onset (preimplementation, 4.8% [95% CI, 3.0%-7.8%]; postimplementation, 13.6% [95% CI, 10.4%-17.6%]; P < .001). On interrupted time series analysis among EMS-transported patients, the level change within 1 month of implementation was 7.15% (P = .04) with no slope change before (0.16%; P = .71) or after (0.08%; P = .89), which indicates a step rather than gradual change. No change in time to thrombolysis or rate of thrombolysis was observed (step change, 1.42%; P = .82). There were no differences in EVT rates in patients not arriving by EMS in the 6- to 24-hour window or by interhospital transfer or walk-in, irrespective of time window.
Conclusions and Relevance
Implementation of a prehospital transport policy for comprehensive stroke center triage in Chicago was associated with a significant, rapid, and sustained increase in EVT rate for patients with AIS without deleterious associations with thrombolysis rates or times.
This preimplementation-postimplementation study evaluates the association of a regional prehospital transport policy for comprehensive stroke center triage for patients with suspected acute ischemic stroke and large vessel occlusion with rates of endovascular therapy.
Introduction
Time to reperfusion by endovascular therapy (EVT) is a major determinant of outcomes in acute large vessel occlusion (LVO) stroke, with delays in reperfusion leading to worse outcomes.1,2,3,4,5,6,7,8,9,10,11 However, 1 in 3 patients becomes ineligible for EVT because of unfavorable imaging characteristics caused in part by delays resulting from potentially eligible patients with LVO presenting at hospitals without EVT capability.12,13,14,15 Reminiscent of the paradigm shift in acute coronary artery disease therapy, EVT for acute ischemic stroke (AIS) with LVO has major implications for stroke systems of care. Similar to the CathPCI (diagnostic catheterization and percutaneous coronary intervention) Diverting Registry,16,17 LVO-specific scales may aid in identifying patients with AIS and suspected LVO in the prehospital setting to expedite direct transport to thrombectomy-capable centers and comprehensive stroke centers (CSCs).18,19,20
Limited data exist on the implementation of CSC triage protocols and their association with EVT rates.21,22,23,24,25,26 Therefore, we sought to evaluate the association of implementing a regional prehospital transport policy for patients with suspected LVO AIS to the nearest CSC with EVT rates in Chicago, Illinois.
Methods
Ethics Approval
This preimplementation-postimplementation study was approved by the institutional review board at the University of Chicago, Chicago, Illinois. Participating sites were granted a waiver of informed consent under the common rule because the data are collected from medical records for local quality improvement purposes and aggregated in a deidentified manner for regional reporting. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Setting
The city of Chicago has more than 2.69 million residents living within its 227 square miles. The racial/ethnic makeup is 50.0% White, 29.6% Black, 6.6% Asian, and 28.8% Hispanic.27 The city is served by 1 emergency medical services (EMS) provider that responds to 911 calls, the Chicago Fire Department. In 2009, Illinois passed legislation recommending preferential transport of patients with suspected stroke to the nearest stroke center, resulting in improved EMS prenotification, a doubling of intravenous thrombolysis administered at primary stroke centers, and major reductions in door-to-needle times.28,29
In 2016, after state legislation that recognized CSCs and directed EMS systems to incorporate CSCs into destination policies for patients with suspected stroke and after the American Heart Association’s Mission Lifeline for Stroke recommendations, the Chicago Stroke Advisory Subcommittee worked collaboratively with the Chicago EMS system and the Chicago Fire Department to develop criteria for prehospital CSC transport of patients with severe stroke using a 3-item stroke scale (3I-SS) for prehospital LVO screening.30 The 3I-SS is a simple prehospital LVO screening tool that assesses 3 parameters: (1) level of consciousness, (2) gaze, and (3) motor function. Each item is graded 0, 1, or 2, where 0 indicates normal and 2 indicates severe findings. A 3I-SS score of greater than 3 estimates LVO in the anterior circulation with high specificity.31
Protocol
Patients with suspected stroke using the Cincinnati Prehospital Stroke Scale within 6 hours of symptom onset were subsequently assessed for LVO using the 3I-SS (range of scores, 0-6) and the finger-to-nose test. Patients with a 3I-SS score of at least 4 were then triaged as having suspected LVO and recommended for transport to the closest CSC if the additional transport time did not exceed 15 minutes compared with transport to the closest primary stroke center. Patients with a 3I-SS score of less than 4 were triaged as having suspected stroke without LVO and recommended for transport to the closest stroke center. At the time of implementation, the Chicago EMS system included 15 primary stroke centers and 8 CSCs30 (eFigure 1 in the Supplement).
The Chicago EMS System Transport of Stroke Patient Policy and Protocol with criteria for triage and transport to CSCs was approved by the Illinois Department of Public Health in May 2018 with a rollout of EMS education using both live and web-based training modules in September 2018 (the policy and protocol for which are presented in eFigure 2 in the Supplement), with an official go-live date of November 28, 2018. The selection criteria for EVT at the CSCs did not change before or after the CSC transport policy in Chicago. In addition, staff and physicians at all comprehensive stroke hospitals received education on emergency department protocols for vessel imaging in patients with suspected stroke presenting within 6 hours of onset, data collection requirements using the Get With The Guidelines–Stroke program32 (GWTG-Stroke; IQVIA, Inc), and the definition of LVO per GWTG-Stroke criteria in April 2018. Hospitals were required to abstract data into the GWTG-Stroke database as participants of the Chicago EMS system for quality improvement and monitoring; each hospital agreed to allow the American Heart Association/American Stroke Association Midwest Affiliate to aggregate, share, and report deidentified results with the Chicago Stroke Advisory Subcommittee and EMS leadership monthly.
Cohort Selection
For the primary analysis, we included patients in the regional GWTG-Stroke database (1) arriving by EMS to the hospital within 6 hours from the last time the patient was confirmed to be symptom free (last known normal time) and (2) with a final diagnosis of AIS. Patients with hemorrhagic stroke, transient ischemic attack, and stroke mimics were excluded. For the secondary analyses, we included all patients with a final diagnosis of AIS regardless of time window or mode of arrival.
Data
Available data in the GWTG-Stroke database for analysis included demographics (age, sex, and self-reported race as defined by the US Census Bureau); medical history (hypertension, dyslipidemia, diabetes, atrial fibrillation, coronary artery disease, chronic kidney disease, congestive heart failure, and current smoking); times of symptom onset, arrival, thrombolytic drug administration (if performed), and groin puncture (if performed); presence of LVO; EVT; and discharge disposition. All data were entered by local site coordinators without central adjudication, interpretation, or review, consistent with GWTG-Stroke data acquisition.
Statistical Analysis
Data were analyzed from December 1, 2017, to May 31, 2019. We used descriptive statistics including proportions (95% CIs), means (SDs), or medians (interquartile ranges [IQRs]) as appropriate. Using χ2 or Fisher exact tests for proportions, 2-tailed t tests for means, and Mann-Whitney tests for medians of continuous variables, we compared baseline demographics, risk factors, reperfusion treatment rates, and discharge outcomes in the 9 months after CSC triage protocol implementation with the 9 months before for the primary and secondary outcomes.
We compared data from December 1, 2017, to August 31, 2018 (9-month preimplementation period) and from September 1, 2018, to May 31, 2019 (9-month postimplementation period). The analyzed time frame was based on a prespecified power calculation, which indicated 90% power to detect an effect size of 2.0 or greater (translates to a 10% level change) with 18 monthly time points (9 before and 9 after implementation), assuming autocorrelation of 0.2 or greater for a level change in EVT rate from a simulation-based power calculation for designing interrupted time series (ITS) analyses.33 Because intensive education on the protocol, including the ability to consult with online medical control if suspicion of severe stroke was high, started in September 2018, we chose that month as the intersection between 2 periods. In primary analysis, we compared EVT rates among EMS-transported patients with AIS arriving within 6 hours of the last known normal time. In secondary analyses, we compared (1) EVT rates in patients with AIS stratified by mode of arrival (EMS, walk in, or interhospital transfer) and time window (<6 vs 6-24 hours from the last known normal time) and (2) door to groin puncture times among patients with AIS arriving by EMS.
For the primary analysis, we further analyzed trends using an ITS analysis based on segmented linear regression, which divides a time series into preimplementation and postimplementation periods. The rigorous quasi-experimental analysis using ITS rather than crude before-and-after comparison will prevent missed temporal trends unrelated to the implementation. The aggregate rates of EVT and tissue plasminogen activator use as well as median times from onset to thrombolysis for each month were calculated. Segmented regression analysis was used to model the ITS data to estimate the change in level and trend using September 2018 as the change point. A change in level between the preimplementation and postimplementation periods indicates step change, whereas a change in slope indicates a change in trend over time.
The regression model used the following equation:
| Yt = β0 + β1 × Timet + β2 × Implementationt + β3 × Time After Implementationt + et |
where β0 estimates the baseline rate of EVT use at the beginning of the time series; β1 estimates the preimplementation trend where Time is a continuous variable indicating the time in months at time t from the start of the study period; β2 estimates the change in level after implementation where Implementationt is 0 before and 1 after the implementation; β3 estimates the change in postimplementation trend where Time After Implementationt is a continuous variable indicating the number of months after the start of the implementation and is coded zero before the implementation; and et includes random error and autocorrelation. In addition, we included a term in the regression model for the lagged residuals to correct for autocorrelation effects. Sensitivity analyses for missing mode of arrival were also performed. All P values were 2-sided, and P < .05 indicated statistical significance. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
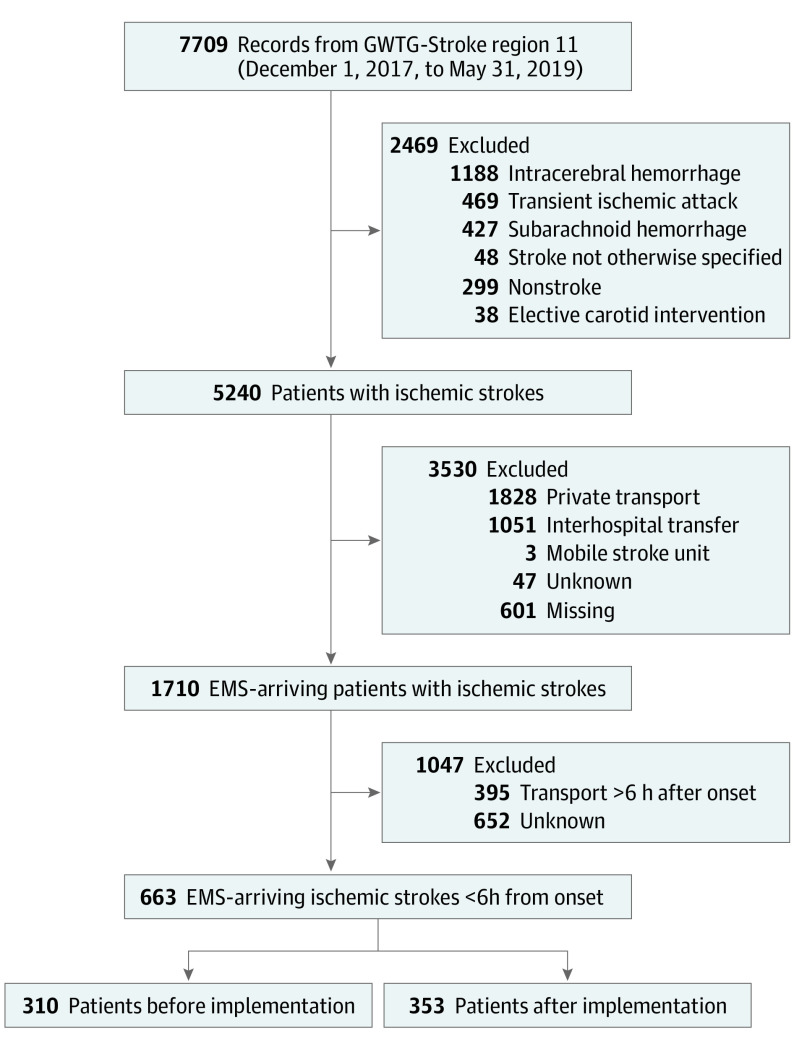
After reviewing 7709 records from 24 Chicago stroke centers using the GWTG-Stroke database from December 1, 2017, to May 31, 2019, there were 2603 patients with AIS in the preimplementation period and 2637 in the postimplementation period, of whom 797 and 913, respectively, were transported by EMS (Figure 1). The mean (SD) age of the patients was 68.5 [14.9] years; there were 342 women (51.6%) and 321 men (48.4%). In the primary analysis of 310 preimplementation and 353 postimplementation EMS-transported patients with AIS less than 6 hours from the last known normal time, age, sex, race, and medical history were not different between the preimplementation and postimplementation periods (Table 1). Mean times from stroke onset to hospital arrival were not different between the 2 periods. Intravenous tissue plasminogen activator was administered to 124 patients (40.4%) in the preimplementation period vs 136 patients (38.5%) in the postimplementation period (P = .63). Time from onset to tissue plasminogen activator administration was also not different between periods (mean [SD], 135.1 [53.4] vs 131.4 [55.5] minutes; P = .59). Distribution of discharge disposition was similar between periods (eg, discharge to home, 111 [35.8%] vs 136 [38.5%]; overall P = .77).
Figure 1. Study Cohort Assembly Flowchart.
EMS indicates emergency medical services; GWTG-Stroke, Get With The Guidelines–Stroke.
Table 1. Demographic and Clinical Characteristics Before and After Implementation of the Prehospital Stroke Triage and Transport Policy for the Primary Analysis Cohort.
| Characteristic | Study perioda | P value | |
|---|---|---|---|
| Preimplementation (n = 310) | Postimplementation (n = 353) | ||
| Age, mean (SD), y | 68.9 (15.1) | 68.2 (14.8) | .55 |
| Sex | |||
| Female | 165 (53.2) | 177 (50.1) | .44 |
| Male | 145 (46.8) | 176 (49.9) | |
| Race/ethnicity | |||
| White | 95 (30.6) | 112 (31.7) | .28 |
| Black | 157 (50.7) | 191 (54.1) | |
| Otherb | 58 (18.7) | 50 (14.2) | |
| Hypertension | 224 (73.0) | 248 (70.3) | .49 |
| Diabetes | 95 (30.9) | 115 (32.6) | .68 |
| Dyslipidemia | 89 (29.0) | 93 (26.3) | .49 |
| Current smoker | 41 (13.4) | 53 (15.0) | .58 |
| Atrial fibrillation | 55 (17.9) | 50 (14.2) | .20 |
| Coronary artery disease | 50 (16.3) | 59 (16.7) | .92 |
| Congestive heart failure | 29 (9.1) | 27 (7.6) | .48 |
| Chronic kidney disease | 14 (4.6) | 17 (4.8) | >.99 |
| Last known normal to arrival times, mean (SD), min | 118.3 (98.3) | 126.6 (103.3) | .30 |
| Thrombolysis rate | 124 (40.4) | 136 (38.5) | .63 |
| Onset to thrombolysis time, mean (SD), min | 135.1 (53.4) | 131.4 (55.5) | .59 |
| Discharge disposition | |||
| Home | 111 (35.8) | 136 (38.5) | .77 |
| Inpatient rehabilitation facility | 68 (21.9) | 86 (24.4) | |
| Acute care facility | 53 (17.1) | 55 (15.6) | |
| Skilled nursing facility | 36 (11.6) | 40 (11.3) | |
| Death or comfort care | 22 (7.1) | 19 (5.4) | |
| Other | 20 (6.5) | 17 (4.8) | |
Unless otherwise indicated, data are expressed as number (%) of patients. Owing to missing data, denominators may not be the total numbers in column headings.
Includes Hispanic, Asian, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, or unable to determine.
In the postimplementation compared with the preimplementation periods, the rate of EVT increased 2.8-fold (13.6% [95% CI, 10.4%-17.6%] vs 4.8% [95% CI, 3.0%-7.8%]; P < .001). On ITS analysis, there was a step (level) increase in EVT use by 7.15% in September 2018 (P = .04) with no slope change before (0.16%; P = .71) or after (0.08%; P = .89) implementation. No change was noted in thrombolysis rate (step change, 1.42%; P = .82) (Figure 2 and Table 2).
Figure 2. Monthly Rates of Intravenous Tissue Plasminogen Activator (tPA) Therapy and Endovascular Therapy (EVT) Before and After Implementation in Chicago .
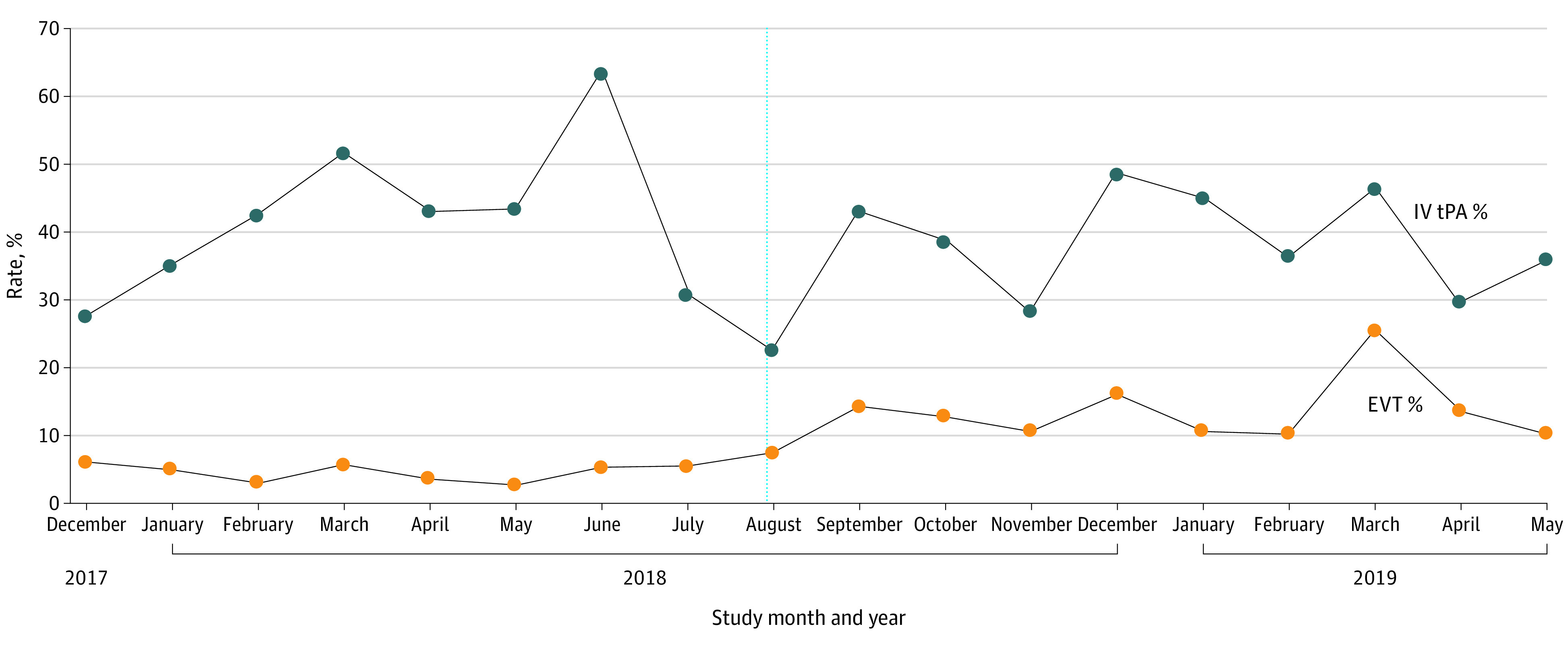
Participants include patients with acute ischemic stroke arriving by emergency medical services transport within 6 hours of the last time the patient was confirmed to be symptom free.
Table 2. ITS Analysis of the Association of Policy Implementation With EVT and Thrombolysis Rates Before and After Implementation of the Prehospital Triage and Transport Policy for the Primary Analysis Cohort.
| Rates by implementation timing | Parameter estimate (SE), % | P value |
|---|---|---|
| EVT rate | ||
| β0: Baseline (December 2017) | 4.07 (2.38) | .11 |
| β1: Preimplementation trend | 0.16 (0.43) | .71 |
| β2: Level change immediately after implementation | 7.15 (3.19) | .04 |
| β3: Postimplementation trend | 0.08 (0.59) | .89 |
| Intravenous thrombolysis rate | ||
| β0: Baseline (December 2017) | 39.82 (8.37) | <.001 |
| β1: Preimplementation trend | 0.003 (1.52) | >.99 |
| β2: Level change immediately after implementation | 1.42 (11.78) | .82 |
| β3: Postimplementation trend | −0.49 (2.06) | .97 |
Abbreviations: EVT, endovascular therapy; ITS, interrupted time series.
In secondary analyses in all patients with AIS, there was an association with increased EVT overall (4.9% [95% CI, 4.1%-5.8%] vs 7.4% [95% CI, 7.5%-8.5%]; P < .001) and in the 6-hour window (11.2% [95% CI, 9.6%-14.4%] vs 17.0% [95% CI, 14.4%-20.0%]; P = .01) but not in the 6- to 24-hour window (5.6% [95% CI, 4.1%-7.7%] vs 6.1% [95% CI, 4.6%-8.2%]; P = .77). In stratified analysis by time window and mode of arrival, the association with increased EVT rates was only observed in EMS-transported patients less than 6 hours from the last known normal time (4.8% [95% CI, 3.0%-7.8%] vs 13.6% [95% CI, 10.4%-17.6%]; P < .001). There were no differences in rates of EVT in those not arriving by EMS in the 6- to 24-hour window or by interhospital transfer or walk-in irrespective of time window (Table 3). Door to groin puncture times were captured in 12 of 22 patients receiving EVT and arriving by EMS in the preimplementation period and in 64 of 74 in the postimplementation period and were not different (median, 123.0 [IQR, 51.0-150.0] vs 123.5 [IQR, 61.5-181.0] minutes; P = .91).
Table 3. Rates of EVT in Patients With AIS Stratified by Implementation Period, Mode of Arrival, and Time Window for Secondary Analyses.
| Arrival mode by time window | Study period | P value | |||
|---|---|---|---|---|---|
| Preimplementation | Postimplentation | ||||
| No. | EVT rate (95% CI), % | No. | EVT rate (95% CI), % | ||
| EMS | |||||
| <6 h From onset | 310 | 4.8 (3.0-7.8) | 353 | 13.6 (10.4-17.6) | <.001 |
| 6-24 h From onset | 150 | 4.0 (1.9-8.5) | 183 | 4.4 (2.2-8.4) | .59 |
| Transfer | |||||
| <6 h From onset | 137 | 43.8 (35.8-52.2) | 136 | 47.8 (39.6-56.1) | .59 |
| 6-24 h From onset | 100 | 24.0 (16.7-33.2) | 101 | 27.7 (19.9-37.1) | .66 |
| Walk-in | |||||
| <6 h From onset | 189 | 3.7 (1.8-7.4) | 204 | 2.4 (1.1-5.6) | .56 |
| 6-24 h From onset | 271 | 0.0 (0-1.4) | 281 | 1.8 (0.1-4.1) | .06 |
Abbreviations: AIS, acute ischemic stroke; EMS, emergency medical services; EVT, endovascular therapy.
In sensitivity analysis to assess the arrival mode missingness, we considered patients with missing mode of arrival who arrived within 6 hours from onset as EMS transported. In an ITS model including these patients, we found no change in main associations (eTable in the Supplement).
Discussion
After the implementation of a regional, prehospital CSC transport policy, there was an association with increased EVT overall among patients with AIS but markedly among EMS-transported patients presenting within 6 hours from the last known normal time. The association was noted immediately after stroke protocol and policy training in September 2018, even before the official go-live date on November 28, 2018, and sustained in the 9 months afterwards. Moreover, the EVT rate increase was not associated with any change in the rate of intravenous thrombolysis or the onset-to-arrival or treatment times. Despite a lack of association with discharge outcomes, such a policy is likely to introduce important public health benefits given the known beneficial association of EVT with long-term functional outcomes after AIS with LVO.
The association of increased EVT rates was noted with the initiation of online training and EMS dissemination of the policy in September 2018, 2 months in advance of formal implementation in November 2018. This finding parallels the observations from primary stroke center certification, wherein thrombolysis rates increased even before full certification, and from EMS education initiatives, wherein stroke recognition and hospital prenotification improved immediately after paramedic training.34,35 We suspect that informal implementation began in September 2018 after paramedic training, with paramedics assessing patients for severe stroke and consulting with online medical control as outlined in the Chicago EMS system policies.
Awareness of extended window EVT trial results11,36 may have also contributed to the association with increased EVT rates in the region. However, the rate of EVT among patients arriving in the 6- to 24-hour window was unchanged in the preimplementation and postimplementation periods. It is also possible that training on advanced vessel imaging and implementation of computed tomographic angiography protocols for patients with suspected AIS in primary stroke centers and CSCs, which occurred in advance of our protocol implementation, increased LVO detection and therefore EVT rates. However, prehospital CSC transport policies should be linked to emergency department protocols for rapid neurovascular evaluation, including advanced imaging, to have their intended association with treatment rates.
Few LVO scales have been applied by the EMS prospectively in a prehospital setting within the US.37,38 Implementation of an EMS-based, prehospital AIS triage protocol using an LVO scale was feasible and showed that approximately 1 in 30 patients with stroke will be eligible for EVT, and specific LVO scale cutoffs could identify as many as 77% of EVT-eligible patients.38 Besides these real-world data, several simulation studies39,40 have noted differential effects in urban vs rural areas; in urban areas, conditional probability modeling suggests that a direct-to-mothership (ie, CSC transport) paradigm is more efficient, whereas in rural areas, a drip-and-ship (ie, transfer from primary stroke center to CSC) model is usually more beneficial. Although such mathematical models are more generalizable and flexible than clinical trials, real-world data, such as our experience, is necessary to validate simulation studies.39,40
Our study also reinforces the feasibility and effectiveness of regionalization of stroke care on a large scale, covering a populous city such as Chicago. Although Chicago has multiple CSCs, the delay due to interfacility transport from non-CSCs to CSCs for patients eligible for EVT prolongs time to EVT and may even disqualify some patients from receiving EVT, which would be expected to result in worse outcomes.41 As EMS systems and stroke systems of care mature to ensure transport of all patients with suspected stroke to certified stroke centers,29 our study further supports regionalization of stroke systems of care with preferential transport of patients with suspected LVO stroke directly to a CSC. Moreover, because more recent data showed significant decreases in disability in patients with stroke receiving care in mobile stroke units,42,43,44 such ambulances, equipped with computed tomographic and computed tomographic angiography capability, could rapidly identify LVO strokes not only for thrombolysis but also for rapid transport to the closest CSC.
The choice of 3I-SS as our prehospital LVO screening tool was based on its simplicity, its reported high specificity, and acceptance by EMS professionals. Although the Rapid Arterial Occlusion Evaluation has been the most studied scale nationally, it is more time consuming and requires significant education of EMS professionals before and after implementation.38 Other proposed scales have similar performance and weaknesses.21,37,38,45,46,47,48,49,50
Strengths and Limitations
The strengths of our study include the planned implementation of a prehospital CSC triage and transport policy within a large urban area with multiple tiers of stroke centers, training of a single EMS agency’s paramedics, and rigorous quasi-experimental analysis using ITS rather than crude before-and-after comparisons that could miss temporal trends unrelated to the implementation. Indirect associations, including public education on stroke warning signs and calling 911 for suspected stroke, and an increasing willingness to perform EVT for select patients previously excluded could have influenced our results. However, the association of increased EVT rates with a step change rather than a gradual increase argues against these indirect or unmeasured temporal associations.
There are several other limitations to our study. First, our findings may not be generalizable to nonurban settings or areas with fewer or geographically distant stroke centers. Second, although we included data from 24 stroke centers in the Chicago region, we did not have data from the remaining 9 nonstroke centers and therefore may have omitted patients with an eventual diagnosis of stroke who were cared for at a nonstroke center. Third, because we do not have access to linked EMS-hospital data, we do not know the intervals from the last known normal time to EMS dispatch, the number or the proportion of patients who bypassed non-CSCs to be transported to a CSC, or how many patients with LVO stroke were transported to primary stroke centers or non–primary stroke centers. Fourth, because we only analyzed data for patients with a final diagnosis of AIS, we do not know how many patients with stroke mimics were triaged to CSCs, the accuracy of LVO screening, the rates of EVT in patients with true-positive and false-negative findings, or the association of the policy with hemorrhagic stroke care. Because of the potential of LVO scales to detect hemorrhagic stroke, patients with hemorrhagic stroke may have had an abnormal 3I-SS but were excluded from this analysis, possibly underestimating the impact of the intervention. Fifth, our data might not be generalizable to other EMS agencies in a different setting or that may not have similar rigorous, centralized training and feedback processes. In a related issue, because no thrombectomy-capable centers are part of the Chicago EMS system, we were unable to determine the effect of direct transport to thrombectomy-capable centers. Last, the GWTG-Stroke registry depends on the accuracy and completeness of clinical documentation and medical record abstraction from local sites, which might be prone to selection bias and retrospective data collection errors. However, regional and external requirements for data reporting without sampling mitigate these issues.
Conclusions
In this preimplementation-postimplementation study, we observed that the implementation of a regional prehospital protocol directing patients with suspected AIS and LVO to CSCs that included EMS training on LVO screening was associated with an increase in the rate of EVT for eligible patients with AIS. Our results provide further evidence to support the development of regional strategies to direct patients with suspected LVO to CSCs and thrombectomy-capable centers when feasible.
eFigure 1. Receiving Hospitals of the Chicago EMS System Showing Primary Stroke Centers and Comprehensive Stroke Centers
eFigure 2. EMS Policy and Protocol
eTable. Inclusion of Patients With Missing Mode of Arrival in Primary Analysis Using Interrupted Time Series to Evaluate the Association of Policy Implementation With Endovascular Therapy Rate and Thrombolysis Rate Before and After Implementation
References
- 1.Saver JL. Time is brain-quantified. Stroke. 2006;37(1):263-266. doi: 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Donnan G, Fieschi C, et al. ; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators . Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi: 10.1016/S0140-6736(04)15692-4 [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Arora S, Broderick JP, et al. ; Paul Coverdell Prototype Registries Writing Group . Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36(6):1232-1240. doi: 10.1161/01.STR.0000165902.18021.5b [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758. doi: 10.1161/CIRCULATIONAHA.110.974675 [DOI] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial: Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003-2011. doi: 10.1001/jama.282.21.2003 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 8.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 10.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Almekhlafi MA, Fan L, et al. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the Interventional Management of Stroke III trial. Circulation. 2014;130(3):265-272. doi: 10.1161/CIRCULATIONAHA.113.007826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froehler MT, Saver JL, Zaidat OO, et al. ; STRATIS Investigators . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokin M, Gupta R, Guerrero WR, Rose DZ, Burgin WS, Sivakanthan S. ASPECTS decay during inter-facility transfer in patients with large vessel occlusion strokes. J Neurointerv Surg. 2017;9(5):442-444. doi: 10.1136/neurintsurg-2016-012331 [DOI] [PubMed] [Google Scholar]
- 15.Smith EE, Saver JL, Cox M, et al. Increase in endovascular therapy in Get With the Guidelines–Stroke after the publication of pivotal trials. Circulation. 2017;136(24):2303-2310. doi: 10.1161/CIRCULATIONAHA.117.031097 [DOI] [PubMed] [Google Scholar]
- 16.Rathore SS, Curtis JP, Chen J, et al. ; National Cardiovascular Data Registry . Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. doi: 10.1136/bmj.b1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley EH, Nallamothu BK, Herrin J, et al. National efforts to improve door-to-balloon time results from the Door-to-Balloon Alliance. J Am Coll Cardiol. 2009;54(25):2423-2429. doi: 10.1016/j.jacc.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 20.Chartrain AG, Kellner CP, Mocco J. Pre-hospital detection of acute ischemic stroke secondary to emergent large vessel occlusion: lessons learned from electrocardiogram and acute myocardial infarction. J Neurointerv Surg. 2018;10(6):549-553. doi: 10.1136/neurintsurg-2017-013428 [DOI] [PubMed] [Google Scholar]
- 21.Smith EE, Kent DM, Bulsara KR, et al. ; American Heart Association Stroke Council . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49(3):e111-e122. doi: 10.1161/STR.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 22.Krebs W, Sharkey-Toppen TP, Cheek F, et al. Prehospital stroke assessment for large vessel occlusions: a systematic review. Prehosp Emerg Care. 2018;22(2):180-188. doi: 10.1080/10903127.2017.1371263 [DOI] [PubMed] [Google Scholar]
- 23.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 24.Carrera D, Gorchs M, Querol M, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Revalidation of the RACE scale after its regional implementation in Catalonia: a triage tool for large vessel occlusion. J Neurointerv Surg. 2019;11(8):751-756. doi: 10.1136/neurintsurg-2018-014519 [DOI] [PubMed] [Google Scholar]
- 25.Zaidi SF, Shawver J, Espinosa Morales A, et al. Stroke care: initial data from a county-based bypass protocol for patients with acute stroke. J Neurointerv Surg. 2017;9(7):631-635. doi: 10.1136/neurintsurg-2016-012476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson RL, Crowe RP, Patrick C, et al. Performance of the RACE score for the prehospital identification of large vessel occlusion stroke in a suburban/rural EMS service. Prehosp Emerg Care. 2019;23(5):612-618. doi: 10.1080/10903127.2019.1573281 [DOI] [PubMed] [Google Scholar]
- 27.US Census Bureau. US Census Bureau website. Accessed December 31, 2020. https://www.census.gov
- 28.Prabhakaran S, O’Neill K, Stein-Spencer L, Walter J, Alberts MJ. Prehospital triage to primary stroke centers and rate of stroke thrombolysis. JAMA Neurol. 2013;70(9):1126-1132. doi: 10.1001/jamaneurol.2013.293 [DOI] [PubMed] [Google Scholar]
- 29.Prabhakaran S, Lee J, O’Neill K. Regional learning collaboratives produce rapid and sustainable improvements in stroke thrombolysis times. Circ Cardiovasc Qual Outcomes. 2016;9(5):585-592. doi: 10.1161/CIRCOUTCOMES.116.003222 [DOI] [PubMed] [Google Scholar]
- 30.Illinois Department of Public Health. Stroke Center listing. Updated May 26, 2021. Accessed January 2, 2021. https://www.dph.illinois.gov/topics-services/emergency-preparedness-response/ems/stroke-program/stroke-centers
- 31.Singer OC, Dvorak F, du Mesnil de Rochemont R, Lanfermann H, Sitzer M, Neumann-Haefelin T. A simple 3-item stroke scale: comparison with the National Institutes of Health Stroke Scale and prediction of middle cerebral artery occlusion. Stroke. 2005;36(4):773-776. doi: 10.1161/01.STR.0000157591.61322.df [DOI] [PubMed] [Google Scholar]
- 32.American Heart Association. Get With The Guidelines®–Stroke registry tool. Updated May 25, 2021. Accessed June 30, 2021. https://www.heart.org/en/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-stroke/get-with-the-guidelines-stroke-registry-tool
- 33.Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol. 2011;64(11):1252-1261. doi: 10.1016/j.jclinepi.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 34.Oostema JA, Chassee T, Baer W, Edberg A, Reeves MJ. Brief educational intervention improves emergency medical services stroke recognition. Stroke. 2019;50(5):1193-1200. doi: 10.1161/STROKEAHA.118.023885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhakaran S, McNulty M, O’Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012;43(3):875-877. doi: 10.1161/STROKEAHA.111.640060 [DOI] [PubMed] [Google Scholar]
- 36.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teleb MS, Ver Hage A, Carter J, Jayaraman MV, McTaggart RA. Stroke vision, aphasia, neglect (VAN) assessment—a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. J Neurointerv Surg. 2017;9(2):122-126. doi: 10.1136/neurintsurg-2015-012131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jumaa MA, Castonguay AC, Salahuddin H, et al. Long-term implementation of a prehospital severity scale for EMS triage of acute stroke: a real-world experience. J Neurointerv Surg. 2020;12(1):19-24. doi: 10.1136/neurintsurg-2019-014997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holodinsky JK, Almekhlafi MA, Goyal M, Kamal N. Mathematical modeling for decision-making in the field for acute stroke patients with suspected large vessel occlusion. Stroke. 2019;50(1):212-217. doi: 10.1161/STROKEAHA.118.021381 [DOI] [PubMed] [Google Scholar]
- 40.Holodinsky JK, Williamson TS, Demchuk AM, et al. Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol. 2018;75(12):1477-1486. doi: 10.1001/jamaneurol.2018.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabhakaran S, Ward E, John S, et al. Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke. 2011;42(6):1626-1630. doi: 10.1161/STROKEAHA.110.609750 [DOI] [PubMed] [Google Scholar]
- 42.Yamal JM, Rajan SS, Parker SA, et al. Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. 2018;13(3):321-327. doi: 10.1177/1747493017711950 [DOI] [PubMed] [Google Scholar]
- 43.Ebinger M, Siegerink B, Kunz A, et al. ; Berlin_PRehospital Or Usual Delivery in Stroke Care (B_PROUD) Study Group . Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. 2021;325(5):454-466. doi: 10.1001/jama.2020.26345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grotta JC. BEST-MSU Study—benefits of stroke treatment delivered by a mobile stroke unit compared to standard management by emergency medical services. International Stroke Conference 2021; American Heart Association; February 10-12, 2021. Accessed May 15, 2021. https://professional.heart.org/-/media/phd-files/meetings/isc/2021/science-news/isc21_best_msu_summary_slide.pdf?la=en
- 45.Purrucker JC, Hametner C, Engelbrecht A, Bruckner T, Popp E, Poli S. Comparison of stroke recognition and stroke severity scores for stroke detection in a single cohort. J Neurol Neurosurg Psychiatry. 2015;86(9):1021-1028. doi: 10.1136/jnnp-2014-309260 [DOI] [PubMed] [Google Scholar]
- 46.Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke. 2008;39(8):2264-2267. doi: 10.1161/STROKEAHA.107.508127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llanes JN, Kidwell CS, Starkman S, Leary MC, Eckstein M, Saver JL. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8(1):46-50. doi: 10.1080/312703002806 [DOI] [PubMed] [Google Scholar]
- 48.Gupta R, Manuel M, Owada K, et al. Severe hemiparesis as a prehospital tool to triage stroke severity: a pilot study to assess diagnostic accuracy and treatment times. J Neurointerv Surg. 2016;8(8):775-777. doi: 10.1136/neurintsurg-2015-011940 [DOI] [PubMed] [Google Scholar]
- 49.Noorian AR, Sanossian N, Shkirkova K, et al. ; FAST-MAG Trial Investigators and Coordinators . Los Angeles Motor Scale to identify large vessel occlusion: prehospital validation and comparison with other screens. Stroke. 2018;49(3):565-572. doi: 10.1161/STROKEAHA.117.019228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duvekot MHC, Venema E, Rozeman AD, et al. ; PRESTO Investigators . Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol. 2021;20(3):213-221. doi: 10.1016/S1474-4422(20)30439-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Receiving Hospitals of the Chicago EMS System Showing Primary Stroke Centers and Comprehensive Stroke Centers
eFigure 2. EMS Policy and Protocol
eTable. Inclusion of Patients With Missing Mode of Arrival in Primary Analysis Using Interrupted Time Series to Evaluate the Association of Policy Implementation With Endovascular Therapy Rate and Thrombolysis Rate Before and After Implementation