Abstract
The DSM provides distinct criteria for obsessive compulsive and various types of anxiety disorders, but phenomenological overlap, high rates of comorbidity, and early onset suggest common underlying mechanisms. This notion is further supported by use of the same treatments – cognitive behavioral therapy (CBT) and serotonin reuptake inhibitor medication – for managing both obsessive compulsive disorder (OCD) and non-OCD anxiety disorders in clinical settings. While early intervention with these gold standard treatments is recommended for pediatric OCD and anxiety disorders, young patients often remain symptomatic even after treatment. To guide the development of novel, mechanistically targeted treatments to better resolve OCD and anxiety symptoms, the identification of neural circuits underlying psychological constructs with relevance across disorders has been recommended. One construct that may be relevant for understanding pediatric OCD and anxiety disorders is cognitive control, given the difficulty that young patients experience in dismissing obsessions, compulsions and worry despite recognition that these symptoms are excessive and unreasonable. In this review, we examine findings from a growing body of literature implicating brain-behavioral markers of cognitive control in pediatric OCD and anxiety disorders, including before and after treatment. We conclude by suggesting that interventions designed to enhance the functioning of the task control circuits underlying cognitive control may facilitate brain maturation to help affected youth overcome symptoms.
Keywords: OCD, anxiety disorders, cognitive control, task control circuits, default mode network, triple network model, review
1. Introduction
1.1. Obsessive compulsive and anxiety symptoms: Relevance of cognitive control?
Distinctions between obsessive compulsive disorder (OCD) and non-OCD anxiety disorders are codified by categorical diagnostic systems, but common underlying mechanisms are suggested by repetitive, distressing thoughts (i.e., obsessions, worries and fears) and related avoidance behaviors across presentations. The obsessions of OCD tend to be more intrusive, bizarre and ego-dystonic than the worries and fears of anxiety disorders which are typically provoked by a triggering stimulus or situation and involve more ego-syntonic, everyday concerns (1). However, these distinctions fall along a continuum in real-life patients, particularly children, complicating differentiation between OCD and non-OCD in clinical settings (2). Across this continuum, patients typically recognize obsessions, fears and worries as unreasonable and/or excessive (1), even at young ages (3). Yet, despite this recognition, patients find the repetitive thoughts of both OCD and non-OCD anxiety difficult to dismiss, leading to illness-related avoidance behaviors. In OCD, avoidance takes the form of compulsions or mental ritualizing to reduce obsession-related distress (e.g., hand-washing to alleviate anxiety about contamination, thinking a ‘good’ thought to counteract a bad one). In anxiety disorders, avoidance can take the form of physically avoiding a feared stimulus as in specific or social phobias, or deliberately engaging in worry to attain a sense of control over possible negative outcomes in generalized anxiety (4–7). Avoidance is driven by a desire to alleviate anxiety but, over the long term, reinforces repetitive negative thinking and associated distress (8). To interrupt this cycle, patients must resist the urge to avoid until obsession-, fear- and worry-related distress naturally decreases (9). Here, we suggest that cognitive control – the ability to flexibly adapt thoughts and behaviors – is the key mechanism by which patients resist avoidance behaviors to break the vicious cycle of illness (see Figure 1).
Figure 1.
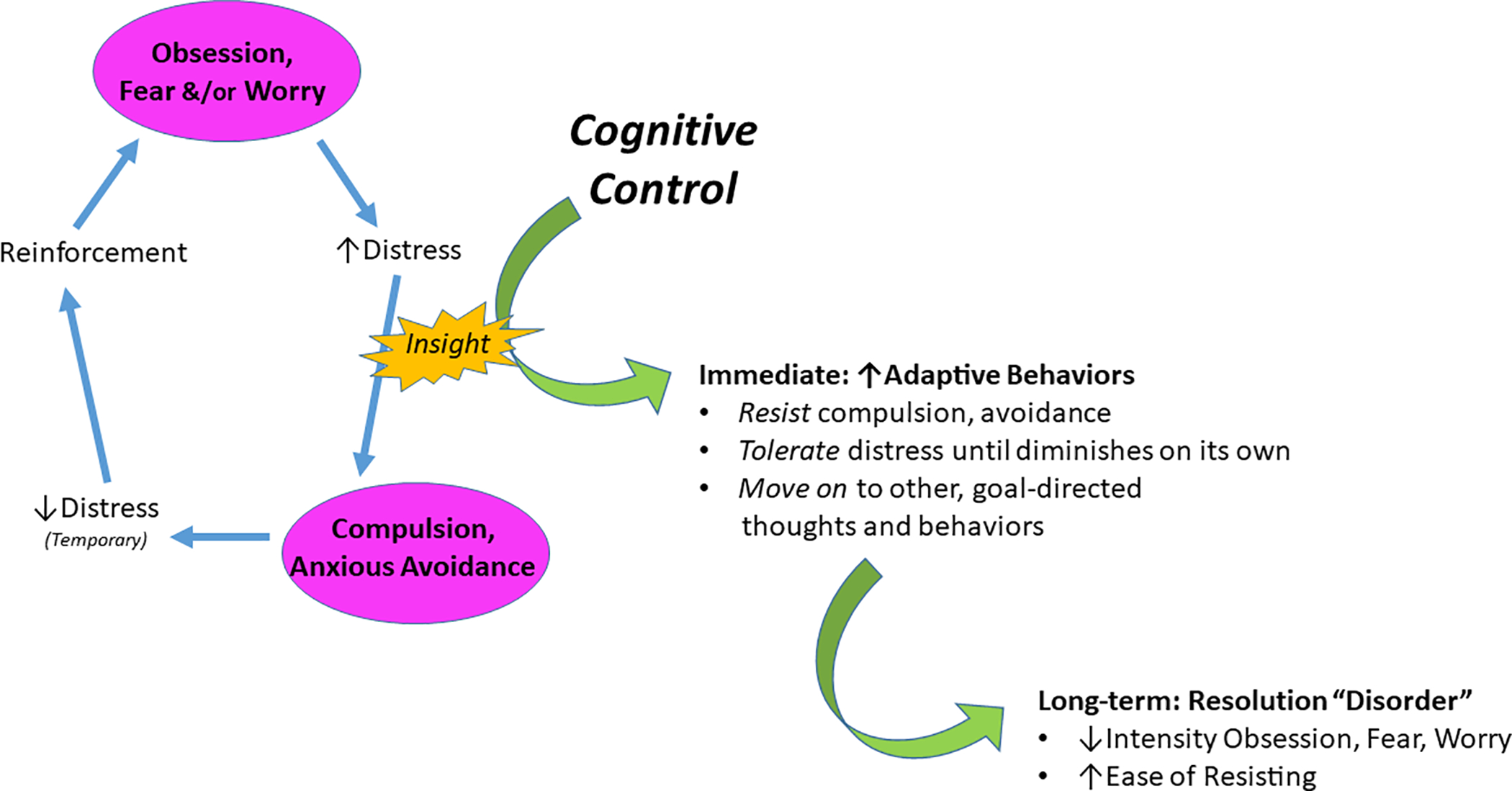
We posit that cognitive control is a key mechanism by which patients with obsessive-compulsive (OCD) and non-OCD anxiety disorders resist the urge to act on fear that they recognize as excessive and unreasonable. By effectively recruiting cognitive control, patients are able to resist the avoidance behaviors (e.g., compulsions in OCD; escape or over-preparation in phobias or generalized anxiety) that reinforce obsessions, worry and fear. Thus, cognitive control is the core process that enables patients to build from insight that obsessions, fears and worries “do not make sense” to resist the pathological urge to avoid, and thereby break the vicious cycle of illness.
1.2. Cognitive Control: Definition, development, and focus on relevant subcomponents
Cognitive control is a set of capacities that support goal-directed behaviors in the face of difficulties (10). Common control processes include inhibitory control (withholding prepotent, but task-irrelevant responses), conflict processing (resolving competition between response options), task switching (flexibly adjusting between tasks), and working memory (holding information in mind while carrying out a task). In addition, error monitoring (detecting and adjusting to response errors) supports the adaptation of behavior to optimize performance across the various component processes that contribute to cognitive control. Factor analyses indicate these capacities are interrelated but separable (11). Among the sub-processes of cognitive control, inhibitory control and error-monitoring have been a focus of research into mechanisms underlying OCD (12) and have also received some attention in mechanistic studies of non-OCD anxiety disorders (13). Inhibitory control is invoked by tasks that require response inhibition or conflict processing (12), whereas error-monitoring occurs in tasks in which mistakes are made. Importantly, these processes interact to support cognitive control, with error-monitoring signaling for inhibitory control to optimize subsequent task performance (10).
Many neurofunctional models of cognitive control exist (10, 11, 14), and most implicate frontoparietal and cingulo-opercular networks (FPN, CON). These ‘task control (TC)’ networks have been identified and segregated through analysis of task-based and resting-state fMRI datasets from healthy individuals (15–18) (Figure 2). The FPN (sometimes referred to as the central executive network) consists of dorsolateral prefrontal cortex (dlPFC), intraparietal sulcus, midcingulate, and intraparietal lobe, whereas the CON (sometimes referred to as the salience network) consists of dorsal anterior cingulate cortex (dACC), anterior insula (frontal operculum), thalamus, and anterior prefrontal cortex (17). Both networks are engaged during cognitive control processes, including inhibitory control and error monitoring. However, these networks operate on dissociable timescales, with CON performing sustained monitoring of performance across the task, and FPN enlisted during specific instances of adjusting control (17, 19, 20).
Figure 2.
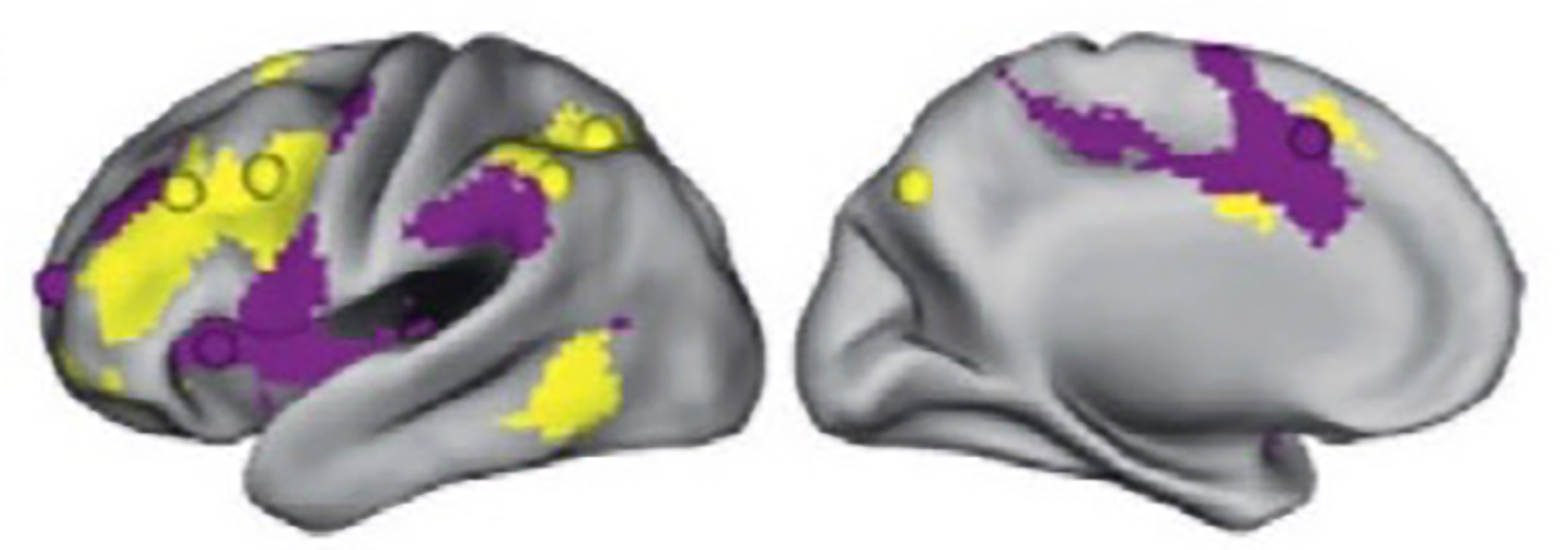
Task control circuits: Cingulo-opercular and frontoparietal networks, adapted from 20. The colors in the figure identify the CON (purple) and FPN (yellow).
Importantly, the CON and FPN interact reciprocally with the task-negative default mode network (DMN). DMN is comprised of posterior cingulate and ventromedial prefrontal cortices that are typically engaged during rest, mind wandering, and self-referential processes, and deactivated during tasks demanding cognitive control (21–24). Deficient recruitment of TC networks appears to couple with atypical engagement of DMN during cognitive control processing, leading to impaired performance (25).
Healthy development from early childhood through adolescence is characterized by improvements in cognitive control performance (faster reaction times, higher accuracy) that parallel the maturation of task control networks (26). Resting-state fMRI data indicate control networks are organized and stably integrated (i.e., functionally coupled) with other networks before adolescence but that integration involving the CON continues into adulthood (20, 27). Further, cross-sectional and longitudinal data suggest regions within the DMN become increasingly integrated (28–30) and segregated from TC regions over development (31, 32). Thus, the continued enhancement of CON integration as well as increasing segregation between TC and default mode regions may support the maturation of cognitive control processes over healthy development.
An electrophysiological marker of CON function sensitive to development is the error-related negativity (ERN, 33) an index of dACC-based error-monitoring functions (34, 35). The ERN is observed in children as young as 3 years old (36), and increases in amplitude with age, being largest among healthy adults (37, 38), consistent with the gradual development of neural substrates of cognitive control (26). Larger ERN is typically associated with better performance on cognitive control tasks (39, 40), consistent with improvements in behavioral performance with development (41). Longitudinal data suggest increasing dACC-indexed CON activation to errors mediates improvements in inhibitory control performance from 8 to 26 years (42) while activation of FPN regions remains unchanged or decreases with age, suggesting developmental changes in the control functions of the FPN and CON. Thus, age-related increases in ERN magnitude may track the increasing sophistication of CON performance-monitoring functions in relation to FPN capacity for adjusting control (17, 27). Indeed, the dACC – a critical hub within adult CON and one of the main generators of the ERN (33) – is more integrated in the FPN in young children, but gradually segregates from FPN and consolidates within CON by adulthood (29).
1.3. Cognitive Control in youth OCD and anxiety disorders: A frame for reviewing the evidence
Here, we focus on the inhibitory control and error-monitoring subcomponents of cognitive control that have received the most attention in the broader literature and our own work on pediatric OCD and anxiety disorders. Further, we consider OCD across symptom clusters and the pediatric anxiety disorders across diagnostic categories (i.e., social, separation and generalized anxiety disorders), consistent with other clinical (43, 44) and neuroimaging research (45) and the commingling of OCD and anxiety symptoms in early development (i.e., 60% of children with OCD have a comorbid anxiety disorder; 2, 46–48). Finally, we focus our review on pediatric samples. Building from evidence linking brain-behavioral markers of cognitive control with pediatric OCD and non-OCD anxiety disorders, we suggest that altered development of TC networks may contribute to the emergence and early course of illness and could be targeted to help young patients overcome symptoms.
2. Brain-behavioral markers of cognitive control in pediatric OCD and anxiety disorders
2.1. Behavioral indices of cognitive control studies
Pediatric OCD.
Deficient response inhibition has long been posited in OCD, given patients’ inability to suppress inappropriate thoughts and compulsive behaviors (49). Indeed, longer reaction times and more errors on tasks requiring inhibitory control are noted in affected youth and adults (12). In pediatric OCD, deficient response inhibition is most consistently evident on anti-saccades tasks (50, 51). By contrast, a meta-analysis of performance across a wider variety of cognitive control sub-processes revealed largely equivalent performance between OCD and healthy children (52).
Pediatric anxiety disorders.
Problems with executive functions (distractibility, difficulties concentrating) are included in the symptom criteria of many anxiety disorders, suggesting deficits in cognitive control. However, findings from behavioral studies of “cold” cognitive tasks in pediatric anxiety disorders are mixed. In contrast to OCD, some theorists even propose that anxiety may be associated with enhanced response inhibition, consistent with the behavioral inhibition risk factor (53). Despite some evidence for enhanced response inhibition in pediatric anxiety disorders (54, 55), other work shows no behavioral differences compared to healthy children (56–60). On the other hand, performance deficits during other cognitive control sub-processes (e.g., working memory and set shifting) have been demonstrated (61). Methodological (e.g., sampling, task parameters), analytic (e.g., covariates such as ADHD symptoms), and developmental processes likely contribute to discrepant findings in the literature (62).
2.2. Task control network function
Accumulating research demonstrates alterations of CON and FPN function in adults with OCD (12, 63–67) and non-OCD anxiety disorders (13, 68, 69) that may couple with a failure to deactivate the DMN during cognitive control processes (68–73). Given the role of the DMN in self-referential, emotive processes, atypical engagement of this network could underlie the intrusive, distressing thoughts and worries experienced by patients, while failure to engage CON and FPN may associate with their inability to dismiss these thoughts and related avoidance behaviors as contextually inappropriate. Altered balance among these networks is consistent with the triple network model (74) whereby the CON interfaces between the DMN and FPN, acting like a switch modulating the attention and cognitive resources between self-referential thoughts, internal processes (i.e., DMN processes) and external goal-directed behavior (i.e., TC processes). While most of these findings come from studies of adult patients, research from our labs and others suggest the involvement of TC and default mode networks in pediatric patients. Acknowledging the need for longitudinal research directly comparing children with OCD and non-OCD anxiety disorders, we review cross-sectional evidence suggesting that the triple network model may be relevant for understanding the mechanisms underlying both illnesses from the earliest stages.
2.2.1. OCD
FMRI findings from adults with OCD demonstrate alterations in TC networks (64, 67, 75, 76), with meta-analysis providing strong evidence of CON hyperactivation during error-processing, and hypoactivation during inhibitory control (12). In pediatric OCD, varied findings across studies (77–80) may be due to the component control process studied, methodological choices (MRI acquisition parameters, data processing procedures) and samples (included ages, medication status, OCD severity, comorbidities). Indeed, we have demonstrated greater pMFC response to errors with older age (8–18 years, replicating prior report (77)) and lower OCD severity in pediatric patients, and with better performance in healthy, but not OCD-affected youth (78). Collectively, these findings raise the possibility that “hyperactive” CON response to errors may represent an adaptive response that normally facilitates task performance and, in pediatric OCD, develops with age to help patients control symptoms. Other fMRI data suggest atypical involvement of FPN and DMN during the engagement of control processes in pediatric OCD. In OCD-affected compared to healthy youth, evidence suggests FPN hypo-activation during error-processing (79, 81), cognitive conflict (77, 79), task switching (79, 82) and planning (83). Consistent with research in adult OCD, failure to deactivate the vmPFC region of the DMN during cognitive conflict is also reported in pediatric patients (80). Thus, pediatric patients seem to exhibit hypoactivation of FPN regions during cognitive control demands (both error processing and inhibitory control), and may also show DMN hyperactivation. By contrast, hypo- versus hyper-activation of CON during error-processing may relate to patient age, with hypoactivation more likely at younger ages and hyperactivation emerging from adolescence into adulthood.
2.2.2. Anxiety disorders
Deficient recruitment of prefrontal cortex during control processes may also relate to non-OCD anxiety in children and adults (13, 81, 84, 85) (Figure 2). Hypoactive dlPFC response to errors in pediatric patients with separation, social and/or generalized anxiety disorders, as well as those with OCD, suggests deficient recruitment of FPN for behavioral adaptation across these diagnostic boundaries (81). Other work in pediatric anxiety disorders suggests differential associations between age (8–18 years) and error-related activation of CON and FPN regions (positive for anxious, negative for healthy youth) (45). These findings follow the same pattern as those from pediatric OCD (78) and could thus reflect an age-associated compensatory mechanism by which maturing task control networks are enlisted to mitigate symptoms in older OCD- and anxiety-affected youth. By contrast, in younger children (8–12 years), greater “overcontrol”, an anxiety-related phenotype defined by cognitive inflexibility, perfectionism and an aversion to making mistakes, associates with less error-related dACC activation (86). Thus, less CON activation may relate to heightened levels of overcontrol in younger children, supporting a developmental model in which less error-related engagement of TC networks associates with greater propensity for anxiety at younger ages.
The bulk of neuroimaging research in pediatric anxiety disorders has examined the processing and regulation of response to emotional and/or threatening stimuli. While not designed to examine TC function, this work suggests an important role of TC networks in regulating threat responses. When studied categorically, patients with pediatric anxiety disorders, relative to healthy youth, exhibit altered recruitment of TC regions, such as vlPFC (87–91) and ACC (91–93). Reviewed elsewhere (94), this work suggests heightened amygdala reactivity to threat detection, reduced prefrontal cortical regulation of threat-related signals from amygdala and, depending on task, compensatory prefrontal cortical regulation of the amygdala when anxiety is less severe. Relatedly, when attention is directed away from threat, greater connectivity of task control regions such as vlPFC and ACC with limbic and/or subcortical regions (e.g., amygdala, parahippocampus/hippocampus, basal ganglia) associates with lower levels of anxiety (91, 93, 95). Collectively, these findings suggest that greater prefrontal-mediated regulatory control over threat processing may aid the suppression of anxiety symptoms.
2.2.3. Error monitoring and the ERN in OCD and anxiety disorders
Larger ERN is demonstrated in adults with OCD and anxiety disorders compared to healthy individuals (96–98); however, the direction of ERN-anxiety associations may shift at younger ages. When studied dimensionally, a smaller ERN was marginally (trend-level) associated with higher levels of anxiety in younger children (8–10 years) whereas the more adult-like pattern of larger ERN was demonstrated in older anxious children (11–13 years; 99). This pattern is consistent with evidence from a community sample of 5–7 year-olds in which a smaller ERN was associated with greater separation anxiety symptoms (58). Other work found a smaller ERN with more anxiety at 4–7 years of age, and a larger ERN with more anxiety (i.e., adult-like pattern) at 7–9 years of age, but only among girls (57). The possible shift of ERN-anxiety relations in childhood is further supported by longitudinal work showing more fearful temperament at age 3 to associate with smaller ERN among 6-year-old children, but with larger ERN when children were re-assessed at aged 9 (40). By contrast, when anxiety was examined categorically, six-year-olds who met criteria for one or more anxiety disorders exhibited larger ERN compared to healthy children (i.e., adult-like pattern; 99).
The functional significance of developmentally-sensitive ERN-anxiety associations remains poorly understood. We hypothesize that low levels of ERN-indexed cognitive control in younger children may leave early anxiety symptoms unchecked, whereas the reversal of this relationship at older ages may reflect a compensatory process by which increasing neural capacity for cognitive control is leveraged to maintain adequate performance on task (100) and/or reduce anxiety symptom severity (101). The latter possibility aligns with the insight that is characteristic in patients with pediatric OCD and anxiety disorders, and tends to increase with age (102). That is, patients may correctly detect obsessions, worries and fears as “thinking errors” but, due to immature neural substrate for cognitive control, are unable to dismiss these to move on to more adaptive, contextually appropriate behaviors. As patients age, maturation of CON-based error-signaling could help to mitigate symptoms, as suggested by fMRI research in pediatric OCD (78), but not fully resolve illness due to residual deficits of neural substrate for inhibitory control (12). Alternatively, given evidence that worry/anxious apprehension rather than fear symptoms drive associations of larger ERN with greater anxiety in adults (97), the reversal of ERN-anxiety associations in children may stem from the greater prevalence of fear symptoms (e.g., phobias) at younger ages (103). Thus, extant literature raises the possibility that ERN-anxiety associations may shift with age, gender, symptom severity and symptom type.
2.2.4. Atypical TC development in OCD and anxiety disorders?
In general, children with less mature task control networks seem to have less capacity for cognitive control and are therefore more likely to find a given task more difficult (lower accuracy, slower reaction times at younger ages). Moreover, clinically affected patients may be more likely to exhibit TC network hypoactivation at younger ages, when TC networks are less developed, possibly in association with the emergence of symptoms. By contrast, adolescent patients may be more likely to show a different pattern of CON and FPN alterations relative to healthy youth. Based on the literature reviewed, we suggest that brain maturation enables compensatory error-related CON engagement relative to persistent FPN hypoactvation during inhibitory control in the presence of persistent illness. We thus recommend that future, longitudinal fMRI research assess both error- and inhibitory control-related functioning of TC networks in youth with OCD and anxiety over time, considering main effects and interactions between age, performance and symptom severity on these brain functions. Ultimately, such work may pave the way for developmentally sensitive therapies to modulate TC circuits and reduce or prevent childhood onset illness.
2.2.5. TC networks as predictors of treatment response
Brain-behavioral deficits in cognitive control in pediatric OCD and anxiety disorders raise the possibility that clinical trial research incorporating measures of TC function may generate clinically useful predictors of treatment response and elucidate mechanisms underlying treatment-induced symptom reduction. Much of this work has been conducted in adult patients, with some evidence of increased activity after CBT and pharmacological treatment in TC networks during cognitive processing in OCD (104) and during the regulation of threat response in non-OCD anxiety disorders (105). Below, we focus on task-based fMRI, resting-state connectivity and structural findings from treatment studies that implicate TC networks in pediatric patients.. Collectively, the findings reviewed suggest that, in young patients, more ‘mature’ TC function may predict better treatment response and that these circuits might become more efficient when treatment is most effective.
OCD.
In pediatric OCD, better CBT response associates with increased pre- to post-CBT activation of dlPFC, dACC/pre-supplementary motor area and premotor cortex during cognitive conflict (77) and dlPFC and parietal cortex during planning (83), suggesting that CBT may reduce symptoms by increasing TC capacity for cognitive control. In adolescent and adult patients, pre-CBT engagement of the CON (ACC node) during cognitive conflict predicted better CBT outcomes across age groups (106). Other findings from adults with OCD show that greater conflict-related CON and DMN activation during the resolution of cognitive conflict on a Simon task predicts better response to CBT (107). Collectively, these data suggest greater TC network responsivity to cognitive control demands may identify patients who are able to benefit from CBT. Moreover, given that TC activation typically couples with DMN deactivation during cognitive control (21–24), patients who are better able to engage the CON and FPN may require less DMN suppression to meet the cognitive control demands required by CBT (i.e., resisting performing rituals when exposed to OCD-triggering stimuli; 107). Further, reduced FPN-DMN resting-state connectivity was detected in OCD-affected compared to healthy youth, with less altered (i.e., less reduced) connectivity predicting better CBT response in patients (108). In the same sample, reduced cortical thickness in FPN regions and fewer diffusion-weighted MRI streamline counts (less anatomical connectivity) between CON regions predicted CBT response (107). Given the synaptic pruning of these regions with advancing age (109), these data suggest that more advanced structural maturation of TC networks may underlie the capacity of young patients to benefit from CBT.
Anxiety.
A growing body of work suggests that treatment outcomes in the pediatric anxiety disorders also depend on the engagement of TC networks, albeit during tasks designed to test the regulation of emotion. For example, when directly attending to threatening faces (explicit threat processing), greater activation in dlPFC and vlPFC predicted greater response to CBT and SSRI treatment in patients with mixed pediatric anxiety disorders (110). By contrast, when diverting attention from threatening faces to attend to other, non-emotional stimuli (implicit threat processing), less activation of the dorsal ACC and dorsomedial PFC predicted better treatment outcomes (111). Other work has shown pre- to post-treatment with CBT and/or SSRI increases of activation in the rostral ACC (112) and vlPFC during explicit threat processing in anxiety-affected adolescents (113). These converging lines of evidence in youth with clinically-significant anxiety suggest that those with greater TC engagement during direct appraisal of threat may be most likely to benefit from treatment, whereas those with pre-existing functional deficits of TC networks during implicit fear processing may have more to gain from treatment, presumably via increased treatment-related TC engagement to regulate response to indirect threat.
3. Future Directions
3.1. Opportunities for clinical translation
Collectively, the extant literature suggests that TC network alterations in pediatric OCD and anxiety could be targeted to enhance cognitive control and thereby help young patients to overcome symptoms. Given that TC networks are still developing during childhood and adolescence (20, 27, 31, 32), an early intervention such as cognitive control training (CCT) that is targeted to more plastic TC networks may facilitate the maturation of these systems. Indeed, younger age in pediatric OCD patients associates with better response to CBT (114) --- a therapy linked to TC network function (106, 108). In studies testing CCT effects in healthy children, younger age predicts greater transfer to distal cognitive domains (i.e., improved performance on non-trained cognitive tasks; 115).We posit that CCT could serve to increase both CON-mediated error signaling and FPN-based inhibitory control function in youth with OCD and anxiety disorders, thereby helping resolve symptoms.
Brain indices of cognitive control have not been specifically targeted for the treatment of pediatric OCD or anxiety disorders; however, prior work suggests that CCT can enhance activation of the TC networks in response to increasing cognitive demands (116–119) and resolve neurocognitive insufficiencies observed in other patient groups (118, 120, 121). For example, four weeks of a computer-delivered cognitive training “game” has been shown to improve behavioral capacity for cognitive control, increase TC network activity and ameliorate cognitive decline in older adults (118). Further, an adaptation of the same intervention, styled as a fun and engaging video game, improves performance on non-trained cognitive tests and reduces attention deficit hyperactivity symptoms in children, suggesting transfer to functionally meaningful behaviors in the “real world” (120–122). Interestingly, in a clinical trial of adolescents with anxiety disorders, CCT had similar benefits as CBT in reducing anxiety severity (123).
Extending from this work, we hypothesize that CCT-induced increases in brain-behavioral capacity for cognitive control may help young patients resolve interference between distressing, repetitive thoughts (i.e., obsessions, fears and worries) and dismiss prepotent, but contextually inappropriate avoidance responses in favor of more adaptive behaviors. CCT could serve to augment CBT effect by “priming” the TC-based cognitive control capacity that the reviewed literature suggests is necessary for patients to successfully engage in, and thus benefit from currently available treatments (e.g., CBT). Alternatively, it is possible that CCT could serve as a stand-alone treatment for pediatric OCD and anxiety disorders if, as we suggest, cognitive control is needed by patients to break the vicious cycle of illness (i.e., resist avoidance behaviors to engage with fears).
3.2. Limitations
Discrepancies across the existing literature are likely due, in part, to methodological differences, thus we encourage efforts to employ consistent techniques across future studies. For example, task-based, resting-state and structural MRI studies often use regions of interest or parcellations to examine specific areas of the brain. No clear cut “best” parcellation of the brain exists, and different atlases include, for example, the CON with the salience and ventral attention network as one network (e.g., the 7-network solution from 124) while others separate these three networks into distinct entities (e.g., 15, 125). Some atlases describe putative functional brain areas (126) while others describe larger scale functional networks based on connectivity patterns (15, 124). Future studies might apply several of these parcellation atlases (124, 125), reporting results from one as primary while also including results for others as supplemental material to facilitate comparison with other studies. Other important technical considerations are choice of fMRI tasks used, MRI pulse sequences, and data processing methods; development of standard methodology is likely to improve replicability of findings. Critically, poor test-retest reliability in both fMRI and ERP research limits reproducibility across studies (127, 131), presenting a challenge that must be overcome if neuroimaging metrics are to guide treatment development or inform clinical management (e.g., treatment selection).
In addition, discrepancies between studies may relate to sample variation in age, task performance, medication status, comorbidities, and symptom severity --- factors shown to impact TC circuit function (26, 78, 128, 129). This is exacerbated by small sample sizes, which bias fMRI estimates (130), and is common among pediatric samples which have more motion artifact and data loss.. Future work could benefit from increased sample size and more data collected from each participant, potentially with novel tasks designed to capture both within- and between-subject effects (131). Large, diverse samples will be especially important for testing the premise that mechanisms of psychopathology can be revealed by understanding how brain-behavioral indices of constructs like cognitive control associate with the normal-to-abnormal range of psychopathology (132). Future work will also need to consider that different psychological constructs (e.g., cognitive control, threat sensitivity), each with unique underlying neurobiology, may combine in different ways in relation to patient-specific presentations (133). Finally, we must consider the implications inherent in using cross-sectional data to inform developmental changes in control processes (42, 128). Because the extant literature in of pediatric OCD and anxiety disorders is limited to cross-sectional studies, developmental changes in TC network functions should be tested in future longitudinal studies assessing error-monitoring, inhibitory control in relation to other relevant processes (e.g., threat responding) in the same patients over time, as OCD and non-OCD anxiety symptoms emerge and progress.
4. Conclusion
In sum, altered cognitive control and/or TC network function has been demonstrated in youth with OCD and anxiety disorders, suggesting TC-based cognitive control alterations may underlie expression of symptoms. However, extant cross-sectional correlational data are insufficient to establish casual roles of TC networks in disease pathophysiology. Yet, data suggesting that TC networks predict and change with treatment (107, 108, 111) implicates these circuits in the expression of OCD and anxiety disorder symptoms. To translate these lines of research into the treatment and prevention of illness, research must now establish interventions that modulate TC network function in the service of reducing symptoms, particularly at young ages when these circuits are more plastic (29, 134) and likely receptive to modulations that could alter symptom expression. Studies of CCT in pediatric OCD and anxiety disorders may be especially promising to assess whether changes in TC function drive symptom reduction, potentially resolving the imbalance between TC networks and those responsible for introspective and emotion processing (DMN and amygdala). Moving forward, we envision an iterative process in which neuroscience-guided interventions, such as CCT, will be used to refine understanding of mechanisms underlying pediatric OCD and anxiety disorders, and then be adapted to treat and/or prevent the development of these disorders in young children.
Figure 3.
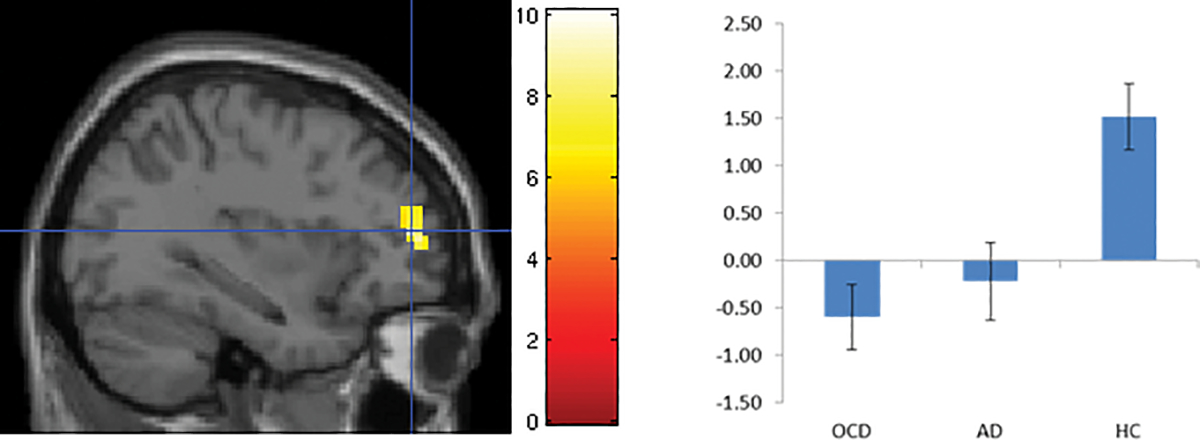
Error-related hypoactivation of left dorsolateral prefrontal cortex in pediatric patients with obsessive compulsive (OCD) and anxiety disorders (AD) compared to healthy controls (HC), originally published in 81.
Acknowledgments
This work was supported by R01 MH114958 (KDF) and R01 MH115024 (RM). The authors would like to thank Lana Khamash and Jenna Patterson for assistance formatting this manuscript.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Association AP (2013): Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- 2.Gillett CB, Bilek EL, Hanna GL, Fitzgerald KD (2018): Intolerance of uncertainty in youth with obsessive-compulsive disorder and generalized anxiety disorder: A transdiagnostic construct with implications for phenomenology and treatment. Clinical psychology review. 60:100–108. [DOI] [PubMed] [Google Scholar]
- 3.Selles RR, Højgaard DR, Ivarsson T, Thomsen PH, McBride N, Storch EA, et al. (2018): Symptom insight in pediatric obsessive-compulsive disorder: Outcomes of an international aggregated cross-sectional sample. Journal of the American Academy of Child and Adolescent Psychiatry. 57:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comer JS, Kendall PC, Franklin ME, Hudson JL, Pimentel SS (2004): Obsessing/worrying about the overlap between obsessive-compulsive disorder and generalized anxiety disorder in youth. Clin Psychol Rev. 24:663–683. [DOI] [PubMed] [Google Scholar]
- 5.Borkovec T, Hu S (1990): The effect of worry on cardiovascular response to phobic imagery. Behaviour Research and Therapy. 28:69–73. [DOI] [PubMed] [Google Scholar]
- 6.Borkovec T, Roemer L (1995): Perceived functions of worry among generalized anxiety disorder subjects: Distraction from more emotionally distressing topics? Journal of behavior therapy and experimental psychiatry. 26:25–30. [DOI] [PubMed] [Google Scholar]
- 7.Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R (1994): Why do people worry? Personality and individual differences. 17:791–802. [Google Scholar]
- 8.Krypotos A-M, Effting M, Kindt M, Beckers T (2015): Avoidance learning: a review of theoretical models and recent developments. Frontiers in behavioral neuroscience. 9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramowitz JS, Deacon BJ, Whiteside SP (2019): Exposure therapy for anxiety: Principles and practice. Guilford Publications. [Google Scholar]
- 10.Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annual review of neuroscience. 24:167–202. [DOI] [PubMed] [Google Scholar]
- 11.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology. 41:49–100. [DOI] [PubMed] [Google Scholar]
- 12.Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. (2019): Error processing and inhibitory control in obsessive-compulsive disorder: A meta-analysis using statistical parametric maps. Biological Psychiatry. 85:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop SJ (2007): Neurocognitive mechanisms of anxiety: an integrative account. Trends in cognitive sciences. 11:307–316. [DOI] [PubMed] [Google Scholar]
- 14.Braver TS (2012): The variable nature of cognitive control: a dual mechanisms framework. Trends in cognitive sciences. 16:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. (2011): Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. (2007): Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power JD, Petersen SE (2013): Control-related systems in the human brain. Current opinion in neurobiology. 23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marek S, Tervo-Clemmens B, Nielsen AN, Wheelock MD, Miller RL, Laumann TO, et al. (2019): Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Developmental cognitive neuroscience. 40:100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marek S, Hwang K, Foran W, Hallquist MN, Luna B (2015): The contribution of network organization and integration to the development of cognitive control. PLoS biology. 13:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: the default network and stimulus-independent thought. Science. 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A. 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH (2012): The role of default network deactivation in cognition and disease. Trends in cognitive sciences. 16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R (2015): An integrative model of the maturation of cognitive control. Annual review of neuroscience. 38:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marek S, Dosenbach NU (2019): Control networks of the frontal lobes. Handbook of clinical neurology: Elsevier, pp 333–347. [DOI] [PubMed] [Google Scholar]
- 28.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V (2010): Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 52:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 104:13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin LQ, Supekar K, Menon V (2010): Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Frontiers in systems neuroscience. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M (2014): Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental cognitive neuroscience. 10:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S (2014): Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of cognitive neuroscience. 26:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gehring WJ, Liu Y, Orr JM, Carp J (2012): The error-related negativity (ERN/Ne). In: LSaK E, editor. Handbook of Event-Related Potential Components. New York: Oxford University Press., pp 231–291. [Google Scholar]
- 34.Holroyd CB, Coles MG (2002): The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological review. 109:679. [DOI] [PubMed] [Google Scholar]
- 35.Yeung N, Botvinick MM, Cohen JD (2004): The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological review. 111:931. [DOI] [PubMed] [Google Scholar]
- 36.Grammer JK, Carrasco M, Gehring WJ, Morrison FJ (2014): Age-related changes in error processing in young children: A school-based investigation. Developmental cognitive neuroscience. 9:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies PL, Segalowitz SJ, Gavin WJ (2004): Development of response-monitoring ERPs in 7-to 25-year-olds. Developmental neuropsychology. 25:355–376. [DOI] [PubMed] [Google Scholar]
- 38.Lo SL (2018): A meta-analytic review of the event-related potentials (ERN and N2) in childhood and adolescence: Providing a developmental perspective on the conflict monitoring theory. Developmental Review. 48:82–112. [Google Scholar]
- 39.Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E (1993): A neural system for error detection and compensation. Psychological science. 4:385–390. [Google Scholar]
- 40.Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, et al. (2013): Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 54:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R (1999): Development of inhibitory control across the life span. Developmental psychology. 35:205. [DOI] [PubMed] [Google Scholar]
- 42.Ordaz SJ, Foran W, Velanova K, Luna B (2013): Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience. 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pediatric O (2004): Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. Jama. 292:1969. [DOI] [PubMed] [Google Scholar]
- 44.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. (2008): Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 359:2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AR, White LK, Leibenluft E, McGlade AL, Heckelman AC, Haller SP, et al. (2020): The heterogeneity of anxious phenotypes: neural responses to errors in treatment-seeking anxious and behaviorally inhibited youths. Journal of the American Academy of Child & Adolescent Psychiatry. 59:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanidir C, Adaletli H, Gunes H, Kilicoglu AG, Mutlu C, Bahali MK, et al. (2015): Impact of gender, age at onset, and lifetime tic disorders on the clinical presentation and comorbidity pattern of obsessive-compulsive disorder in children and adolescents. Journal of child and adolescent psychopharmacology. 25:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beesdo K, Knappe S, Pine DS (2009): Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics. 32:483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. (2011): Development of lifetime comorbidity in the World Health Organization world mental health surveys. Archives of general psychiatry. 68:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ (2005): The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience & Biobehavioral Reviews. 29:399–419. [DOI] [PubMed] [Google Scholar]
- 50.Bey K, Meyhöfer I, Lennertz L, Grützmann R, Heinzel S, Kaufmann C, et al. (2019): Schizotypy and smooth pursuit eye movements as potential endophenotypes of obsessive-compulsive disorder. European archives of psychiatry and clinical neuroscience. 269:235–243. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg DR, Averbach DH, O’Hearn KM, Seymour AB, Birmaher B, Sweeney JA (1997): Oculomotor response inhibition abnormalities in pediatric obsessive-compulsive disorder. Arch Gen Psychiatry. 54:831–838. [DOI] [PubMed] [Google Scholar]
- 52.Abramovitch A, Abramowitz JS, Mittelman A, Stark A, Ramsey K, Geller DA (2015): Research Review: Neuropsychological test performance in pediatric obsessive–compulsive disorder–a meta-analysis. Journal of Child Psychology and Psychiatry. 56:837–847. [DOI] [PubMed] [Google Scholar]
- 53.Henderson HA, Pine DS, Fox NA (2015): Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 40:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardinale EM, Subar AR, Brotman MA, Leibenluft E, Kircanski K, Pine DS (2019): Inhibitory control and emotion dysregulation: A framework for research on anxiety. Development and psychopathology. 31:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy YE, Luke A, Brennan E, Francazio S, Christopher I, Flessner CA (2018): An investigation of executive functioning in pediatric anxiety. Behavior modification. 42:885–913. [DOI] [PubMed] [Google Scholar]
- 56.Baving L, Rellum T, Laucht M, Schmidt M (2004): Attentional enhancement to NoGo stimuli in anxious children. Journal of Neural Transmission. 111:985–999. [DOI] [PubMed] [Google Scholar]
- 57.Ip KI, Liu Y, Moser J, Mannella K, Hruschak J, Bilek E, et al. (2019): Moderation of the relationship between the error-related negativity and anxiety by age and gender in young children: A preliminary investigation. Developmental cognitive neuroscience. 39:100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo SL, Schroder HS, Fisher ME, Durbin CE, Fitzgerald KD, Danovitch JH, et al. (2017): Associations between Disorder-Specific Symptoms of Anxiety and Error-Monitoring Brain Activity in Young Children. J Abnorm Child Psychol. 45:1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, et al. (2013): Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of abnormal child psychology. 41:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oosterlaan J, Logan GD, Sergeant JA (1998): Response inhibition in AD/HD, CD, comorbid AD/HD+ CD, anxious, and control children: A meta-analysis of studies with the stop task. Journal of child psychology and psychiatry. 39:411–425. [PubMed] [Google Scholar]
- 61.Toren P, Sadeh M, Wolmer L, Eldar S, Koren S, Weizman R, et al. (2000): Neurocognitive correlates of anxiety disorders in children:: A preliminary report. Journal of anxiety disorders. 14:239–247. [DOI] [PubMed] [Google Scholar]
- 62.Korenblum CB, Chen SX, Manassis K, Schachar RJ (2007): Performance monitoring and response inhibition in anxiety disorders with and without comorbid ADHD. Depression and Anxiety. 24:227–232. [DOI] [PubMed] [Google Scholar]
- 63.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. (2016): Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 73:815–825. [DOI] [PubMed] [Google Scholar]
- 64.Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB (2014): Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biological psychiatry. 75:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Juhás M, Greenshaw AJ, Hu Q, Meng X, Cui H, et al. (2016): Abnormal resting-state functional connectivity of the left caudate nucleus in obsessive-compulsive disorder. Neuroscience letters. 623:57–62. [DOI] [PubMed] [Google Scholar]
- 66.Stern ER, Welsh RC, Gonzalez R, Fitzgerald KD, Abelson JL, Taylor SF (2012): Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Human brain mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, et al. (2012): Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Human brain mapping. 33:1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon C-M, Sundaram T, Choi N-G, Jeong G-W (2016): Working memory dysfunction associated with brain functional deficits and cellular metabolic changes in patients with generalized anxiety disorder. Psychiatry Research: Neuroimaging. 254:137–144. [DOI] [PubMed] [Google Scholar]
- 69.Balderston NL, Vytal KE, O’Connell K, Torrisi S, Letkiewicz A, Ernst M, et al. (2017): Anxiety patients show reduced working memory related dlPFC activation during safety and threat. Depression and anxiety. 34:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, et al. (2011): Hyperactive Error Responses and Altered Connectivity in Ventromedial and Frontoinsular Cortices in Obsessive-Compulsive Disorder. Biol Psychiatry. 69:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rasgon A, Lee W, Leibu E, Laird A, Glahn D, Goodman W, et al. (2017): Neural correlates of affective and non-affective cognition in obsessive compulsive disorder: A meta-analysis of functional imaging studies. European Psychiatry. 46:25–32. [DOI] [PubMed] [Google Scholar]
- 72.Ottaviani C, Watson DR, Meeten F, Makovac E, Garfinkel SN, Critchley HD (2016): Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biological Psychology. 119:31–41. [DOI] [PubMed] [Google Scholar]
- 73.Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, et al. (2009): Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain research bulletin. 79:409–413. [DOI] [PubMed] [Google Scholar]
- 74.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 75.Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF (2012): Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PloS one. 7:e36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu B-M, Park J-Y, Kang D-H, Lee SJ, Yoo SY, Jo HJ, et al. (2008): Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 131:155–164. [DOI] [PubMed] [Google Scholar]
- 77.Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F (2011): Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive–compulsive disorder: a fMRI study with a Flanker task before and after CBT. Journal of Child Psychology and Psychiatry. 52:1251–1260. [DOI] [PubMed] [Google Scholar]
- 78.Fitzgerald KD, Liu Y, Johnson TD, Moser JS, Marsh R, Hanna GL, et al. (2018): Development of Posterior Medial Frontal Cortex Function in Pediatric Obsessive-Compulsive Disorder. J Am Acad Child Adolesc Psychiatry. 57:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K (2008): Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br J Psychiatry. 192:25–31. [DOI] [PubMed] [Google Scholar]
- 80.Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, et al. (2010): Altered Function and Connectivity of the Medial Frontal Cortex in Pediatric Obsessive-Compulsive Disorder. Biol Psychiatry. 68:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fitzgerald KD, Liu Y, Stern ER, Welsh RC, Hanna GL, Monk CS, et al. (2013): Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 52:1183–1191. e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Britton JC, Rauch SL, Rosso IM, Killgore WD, Price LM, Ragan J, et al. (2010): Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 49:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F (2010): Functional magnetic resonance imaging during planning before and after cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 49:1238–1248, 1248 e1231–1235. [DOI] [PubMed] [Google Scholar]
- 84.van den Heuvel OA, Mataix-Cols D, Zwitser G, Cath DC, van der Werf YD, Groenewegen HJ, et al. (2011): Common limbic and frontal-striatal disturbances in patients with obsessive compulsive disorder, panic disorder and hypochondriasis. Psychol Med. 41:2399–2410. [DOI] [PubMed] [Google Scholar]
- 85.Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. (2016): Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. American journal of psychiatry. 173:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilbert K, Perino MT, Myers MJ, Sylvester CM (2020): Overcontrol and neural response to errors in pediatric anxiety disorders. Journal of Anxiety Disorders.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. (2006): Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 163:1091–1097. [DOI] [PubMed] [Google Scholar]
- 88.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. (2008a): Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 65:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. (2007): Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 64:97–106. [DOI] [PubMed] [Google Scholar]
- 90.Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. (2008): Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 65:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kircanski K, White LK, Tseng W-L, Wiggins JL, Frank HR, Sequeira S, et al. (2018): A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry. 75:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swartz JR, Phan KL, Angstadt M, Klumpp H, Fitzgerald KD, Monk CS (2014): Altered activation of the rostral anterior cingulate cortex in the context of emotional face distractors in children and adolescents with anxiety disorders. Depression and anxiety. 31:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. (2014): Looking under the hood of the dot-probe task: An fMRI study in anxious youth. Depression and anxiety. 31:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swartz JR, Monk CS (2013): The role of corticolimbic circuitry in the development of anxiety disorders in children and adolescents. The neurobiology of childhood: Springer, pp 133–148. [DOI] [PubMed] [Google Scholar]
- 95.Stoddard J, Tseng W-L, Kim P, Chen G, Yi J, Donahue L, et al. (2017): Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry. 74:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hajcak G (2012): What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science. 21:101–106. [Google Scholar]
- 97.Moser J, Moran T, Schroder H, Donnellan B, Yeung N (2013): On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in human neuroscience. 7:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinberg A, Dieterich R, Riesel A (2015): Error-related brain activity in the age of RDoC: A review of the literature. International Journal of Psychophysiology. 98:276–299. [DOI] [PubMed] [Google Scholar]
- 99.Meyer A, Weinberg A, Klein DN, Hajcak G (2012): The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moser JS (2017): The nature of the relationship between anxiety and the error-related negativity across development. Current Behavioral Neuroscience Reports. 4:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzgerald KD, Taylor SF (2015): Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS spectrums. 20:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geller DA, Biederman J, Faraone S, Agranat A, Cradock K, Hagermoser L, et al. (2001): Developmental aspects of obsessive compulsive disorder: findings in children, adolescents, and adults. The Journal of nervous and mental disease. 189:471–477. [DOI] [PubMed] [Google Scholar]
- 103.Meyer A, Hajcak G, Torpey-Newman D, Kujawa A, Olino TM, Dyson M, et al. (2018): Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Developmental psychobiology. 60:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thorsen AL, van den Heuvel OA, Hansen B, Kvale G (2015): Neuroimaging of psychotherapy for obsessive–compulsive disorder: a systematic review. Psychiatry Research: Neuroimaging. 233:306–313. [DOI] [PubMed] [Google Scholar]
- 105.Brooks SJ, Stein DJ (2015): A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues in clinical neuroscience. 17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Norman LJ, Mannella KA, Yang H, Angstadt M, Abelson JL, Himle JA, et al. (2020): Treatment-Specific Associations Between Brain Activation and Symptom Reduction in OCD Following CBT: A Randomized fMRI Trial. American Journal of Psychiatry.appi. ajp. 2020.19080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pagliaccio D, Middleton R, Hezel D, Steinman S, Snorrason I, Gershkovich M, et al. (2019): Task-based fMRI predicts response and remission to exposure therapy in obsessive-compulsive disorder. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cyr M, Pagliaccio D, Yanes-Lukin P, Fontaine M, Rynn M, Marsh R (2020): Altered network connectivity predicts response to Cognitive-Behavioral Therapy in pediatric Obsessive-Compulsive Disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sowell ER, Thompson PM, Tessner KD, Toga AW (2001): Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 21:8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, et al. (2016): Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology. 41:1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, Phan KL (2017): Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. Journal of Child Psychology and Psychiatry. 58:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, et al. (2018): Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 84:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, et al. (2010): A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 20:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Selles RR, Højgaard DR, Ivarsson T, Thomsen PH, McBride NM, Storch EA, et al. (2020): Avoidance, Insight, Impairment Recognition Concordance, and Cognitive-Behavioral Therapy Outcomes in Pediatric Obsessive-Compulsive Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 59:650–659. e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wass S, Scerif G, Johnson MH (2012): Training attentional control and working memory–Is younger, better? Developmental Review. 32:360–387. [Google Scholar]
- 116.Olesen PJ, Westerberg H, Klingberg T (2004): Increased prefrontal and parietal activity after training of working memory. Nature neuroscience. 7:75–79. [DOI] [PubMed] [Google Scholar]
- 117.Westerberg H, Klingberg T (2007): Changes in cortical activity after training of working memory—a single-subject analysis. Physiology & Behavior. 92:186–192. [DOI] [PubMed] [Google Scholar]
- 118.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. (2013): Video game training enhances cognitive control in older adults. Nature. 501:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stevens MC, Gaynor A, Bessette KL, Pearlson GD (2016): A preliminary study of the effects of working memory training on brain function. Brain imaging and behavior. 10:387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis NO, Bower J, Kollins SH (2018): Proof-of-concept study of an at-home, engaging, digital intervention for pediatric ADHD. PLoS One. 13:e0189749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yerys BE, Bertollo JR, Kenworthy L, Dawson G, Marco EJ, Schultz RT, et al. (2019): Brief Report: Pilot Study of a Novel Interactive Digital Treatment to Improve Cognitive Control in Children with Autism Spectrum Disorder and Co-occurring ADHD Symptoms. J Autism Dev Disord. 49:1727–1737. [DOI] [PubMed] [Google Scholar]
- 122.Wexler BE, Vitulano LA, Moore C, Katsovich L, Smith SD, Rush C, et al. (2020): An integrated program of computer-presented and physical cognitive training exercises for children with attention-deficit/hyperactivity disorder. Psychological Medicine.1–12. [DOI] [PubMed] [Google Scholar]
- 123.Hadwin JA, Richards HJ (2016): Working memory training and CBT reduces anxiety symptoms and attentional biases to threat: A preliminary study. Frontiers in psychology. 7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE (2016): Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral cortex. 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. (2016): A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clayson PE (2020): Moderators of the internal consistency of error-related negativity scores: A meta-analysis of internal consistency estimates. Psychophysiology.e13583. [DOI] [PubMed] [Google Scholar]
- 128.Koolschijn PCM, Schel MA, de Rooij M, Rombouts SA, Crone EA (2011): A three-year longitudinal functional magnetic resonance imaging study of performance monitoring and test-retest reliability from childhood to early adulthood. Journal of Neuroscience. 31:4204–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, et al. (2012): Error-related negativity and tic history in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 51:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yarkoni T (2009): Big correlations in little studies: Inflated fMRI correlations reflect low statistical power—Commentary on Vul et al.(2009). Perspectives on Psychological Science. 4:294–298. [DOI] [PubMed] [Google Scholar]
- 131.Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, et al. (2020): What Is the Test-Retest Reliability of Common Task-Functional MRI Measures? New Empirical Evidence and a Meta-Analysis. Psychological Science.0956797620916786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010): Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am Psychiatric Assoc. [DOI] [PubMed] [Google Scholar]
- 133.Sylvester CM, Corbetta M, Raichle M, Rodebaugh T, Schlaggar B, Sheline Y, et al. (2012): Functional network dysfunction in anxiety and anxiety disorders. Trends in neurosciences. 35:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL (2015): Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 40:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


