Abstract
BACKGROUND
Hypercalciuria is the most common metabolic risk factor for calcium urolithiasis and is associated with bone loss in adult patients. Reduced bone mineral density (BMD) was already described in idiopathic hypercalciuria (IH) children, but the precise mechanisms of bone loss or inadequate bone mass gain remain unknown. Life-long hypercalciuria might be considered a risk to change bone structure and determine low bone mass throughout life. The peak of bone mass should occur without interferences. A beneficial effect of citrate formulations and thiazides on bone mass in adult and pediatric patients with IH have been shown.
AIM
To evaluate whether pharmacological therapy has a beneficial effect on bone mass in children and adolescents with IH.
METHODS
This retrospective cohort study evaluated 40 hypercalciuric children non-responsive to lifestyle and diet changes. After a 2-mo run-in period of citrate formulation (Kcitrate) usage, the first bone densitometry (DXA) was ordered. In patients with sustained hypercalciuria, a thiazide diuretic was prescribed. The second DXA was performed after 12 mo. Bone densitometry was performed by DXA at lumbar spine (L2-L4). A 24-h urine (calcium, citrate, creatinine) and blood samples (urea, creatinine, uric acid, calcium, phosphorus, magnesium, chloride, hemoglobin) were obtained. Clinical data included age, gender, weight, height and body mass index.
RESULTS
Forty IH children; median age 10.5 year and median time follow-up 6.0 year were evaluated. Nine patients were treated with Kcitrate (G1) and 31 with Kcitrate + thiazide (G2). There were no differences in age, gender, body mass index z-score and biochemical parameters between G1 and G2. There were no increases in total cholesterol, kalemia and magnesemia. Calciuria decreased in both groups after treatment. Lumbar spine BMD z-score increased after thiazide treatment in G2. There was no improvement in G1.
CONCLUSION
Results point to a beneficial effect of thiazide on lumbar spine BMD z-score in children with IH. Further studies are necessary to confirm the results of the present study.
Keywords: Children, Adolescent, Hypercalciuria, Bone mineral density, Thiazides, Citrate
Core Tip: Should pediatric idiopathic hypercalciuria be treated with hypocalciuric agents? Reduction in bone mass has already been described in hypercalciuric pediatric patients, and the precise mechanisms of bone loss or failure to achieve adequate bone mass remain unknown. Initial recommendations for the treatment of hypercalciuric pediatric patients consist of lifestyle changes and adequate diet. Children rarely comply with those recommendations and require potassium citrate supplementation or thiazides to reduce calciuria. However, anticalciuric therapy in children and adolescents is not based on strong clinical evidences. We speculate if the anticalciuric effect of pharmacologic therapy has a beneficial role on bone mass in pediatric idiopathic hypercalciuria and compromised bone mass in adulthood.
INTRODUCTION
Idiopathic hypercalciuria (IH) is the leading metabolic risk factor of pediatric urolithiasis (UL) and has high morbidity with or without UL. This morbidity is related to hematuria, renal colic, abdominal pain, urinary tract infection, voiding dysfunction symptoms and bone mass reduction[1]. Studies have showed a decrease in bone mineral density (BMD), especially at the lumbar spine, in idiopathic hypercalciuria children and adolescents[2-10]. García-Nieto et al[4] in 1997 showed the BMD reduction in 30% of their pediatric patients with IH. In sequence, Freundlich et al[10], Penido et al[2] and Schwaderer et al[9], demonstrated that 38%, 35% and 47% of their pediatric hypercalciuric patients also had significant reduction in BMD.
We all know that the highest accumulation of bone mass occurs during childhood and adolescence. The peak of bone mass is reached at the end of the second decade of life[11]. A current understanding is that the persistent hypercalciuria would be an important contributor to diminished bone mass. Thus, the peak bone mass should occur without interference in order to prevent the possibility of osteopenia, osteoporosis and fractures during adulthood[11,12]. The mechanisms of bone loss or failure of adequate bone mass gain is not completely understood. However, it is a consensus that bone mass acquired during childhood and adolescence is one of the major determinants of adult bone health[11,12]. The investigation on bone metabolism in pediatric IH is necessary to prevent the potential harmful consequences of this metabolic disorder.
In children with IH, the need for hypocalciuric therapy and its nature and duration remains to be determined. At present, anticalciuric therapy in pediatric hypercalciuria is not based on strong clinical evidence but more on clinical observation. This treatment may be required not only to protect against stone formation but also against low bone density.
The initial approach to pediatric IH consists in dietary modification with high fluid intake, low sodium and the Recommended Dietary Allowances[13] of protein and calcium. However, children do not comply with these recommendations, and frequently require anticalciuric therapy. If dietary recommendations fail, pharmacological therapy should be considered[12,13].
A beneficial effect of citrate formulations [particularly potassium citrate (Kcitrate)] on bone mass in adult patients with IH has been shown since the 1990s[14-19]. Recently, it has been described that supplementation with alkaline potassium salts leads to reduction in urinary calcium excretion and reduction in bone resorption[20]. Nevertheless, some authors pointed out that the current evidence is still heterogeneous[21].
In pediatric patients, Kcitrate has been shown to decrease the recurrence of new stones and the growth of residual stone fragments following lithotripsy[22,23]. However, until now there is no study confirming the beneficial effect of Kcitrate on bone mass in children and adolescents.
Studies in vitro have suggested that thiazides reduce osteoclastic activity[24] and stimulate osteoblast differentiation and bone mineral formation[25]. Bolland et al[26] demonstrated in 122 postmenopausal women that hydrochlorothiazide produces small positive benefits on cortical bone density. LaCroix et al[27] demonstrated in a randomized, double-blind, placebo controlled study that low-dose hydrochlorothiazide could preserve BMD in older adults. As for Kcitrate, up to now there is no study confirming the beneficial effect of thiazides on bone mass in pediatric patients. In this sense, the aim of this study was to examine whether pharmacological therapy has a beneficial effect on bone mass in children and adolescents with IH.
MATERIALS AND METHODS
Design
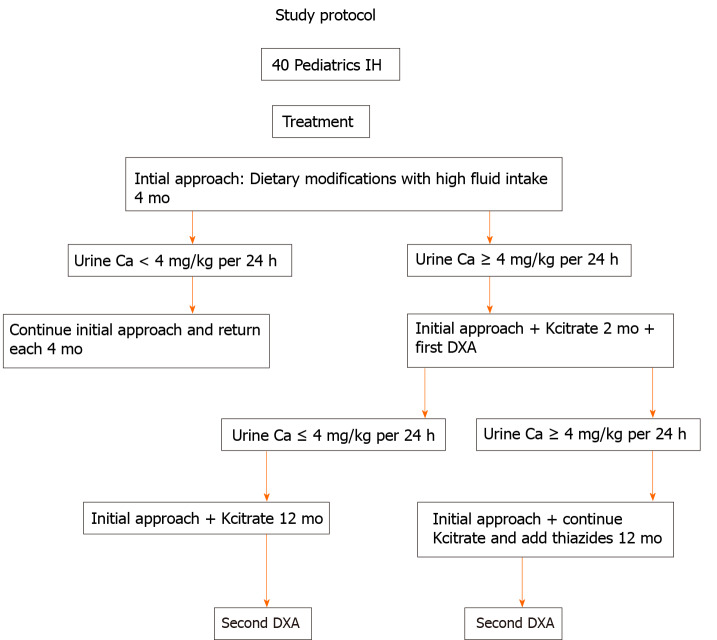
This is a retrospective cohort study with 40 children and adolescents diagnosed with IH. The study protocol is represented in Figure 1.
Figure 1.
Study protocol for children and adolescents with idiopathic hypercalciuria. IH: Idiopathic hypercalciuria.
Patients and treatment
All 40 pediatric patients were evaluated and regularly followed at the Pediatric Nephrology Unit of the Nephrology Center of Santa Casa de Belo Horizonte, Belo Horizonte and the Pediatric Nephrology Unit of Clinic Hospital, School of Medicine of Federal University of Minas Gerais, in State of Minas Gerais, Brazil, between 1990 and November 2018. Inclusion criteria included no previous treatment of IH, no history of drug use with possible actions on calcium metabolism during the preceding year before diagnosis, diurnal and nocturnal continency, symptomatic (defined as lumbar and/or abdominal pain, voiding dysfunction or gross hematuria) and urinary calcium excretion ≥ 4 mg/kg per 24 h or an increased calcium/creatinine ratios, according to age[28-31]. Exclusion criteria included: Patients older than 18 years of age at initial evaluation, patients with chronic immobilization, physically impaired, nephrocalcinosis, hypercalcemia, defects of the lumbar spine, history of malignancy, hyperuricemia, excessive oral ingestion of calcium or vitamin D, tubular defects, long-term steroid treatment and concurrent conditions associated with metabolic bone disease. Patients were kept on a usual diet, water intake, physical exercise and sun exposure. All parents/caregivers were asked in detail about the dietary habits. All 40 patients had a complete evaluation at 4-mo intervals during the entire follow-up period, and all clinical and laboratory data were recorded.
Initial approach was high fluid intake and dietary modifications: Calcium and protein according to Recommended Dietary Allowances[13], sodium restriction (2.0-2.4 g/d) and potassium supplementation (3.0-3.5 g/d) with fruits and vegetables. However, none achieved a normal calciuria level. Kcitrate (0.5-1.0 mEq/kg per day) was added to the treatment for 2 mo, and the first bone densitometry was done, according to the protocol study. If no normalization of calciuria occurred after 2 mo on Kcitrate or if signs and symptoms continued (chronic abdominal pain or prolonged gross hematuria)[12], a diuretic thiazide (0.5-1.0 mg/kg per day) was added. After 12 mo on Kcitrate alone or Kcitrate and thiazide treatment, the second bone densitometry was done. Patients took Kcitrate or Kcitrate in combination with thiazides for at least 1 year. Nine patients were treated with Kcitrate alone and were called Group 1 (G1). Thirty-one patients were treated with Kcitrate + thiazide and were called Group 2 (G2).
Bone densitometry
Bone densitometry was performed by dual-energy X-ray absorptiometry (DEXA) using a Lunar DPX-IQ 2516 device. The exposure to radiation was 0.96 mrem for the lumbar spine.
A 76-KeV X-ray source of energy was used and the accuracy of this test was 0.1%. BMD was measured in the lumbar spine between L1-L4 and was also corrected for volume of the vertebra, as described by Kröger[32] and Gordon et al[33]. Lumbar spine BMD Z scores were calculated in relation to individuals of the same gender and age range and corrected for height according to the literature[34,35].
Laboratory and clinical data
Two 24-h urine samples were obtained, followed by a two single fasting urine samples (were kept refrigerated). A venous blood sample was drawn after the collection of the urine samples. Blood samples were analyzed for creatinine, urea, uric acid, calcium, phosphorus, magnesium, chloride, sodium, potassium, pH, bicarbonate, parathyroid hormone, alkaline phosphatase, hematocrit and hemoglobin. The 24-h urine samples analysis included calcium and creatinine. A complete urinalysis was performed on the two single fasting urine samples, and the pH determined. Clinical data included patient age, gender, weight, height and body mass index (BMI).
Statistical analysis
Clinical, laboratory and bone densitometry data are expressed as median, minimum and maximum values. The normality of data distribution was analyzed using the Shapiro-Wilk’s test. In the case of non-normal distributions, non-parametric tests were chosen and data represented as medians, first and third quartiles and/or range. The following tests were used for comparison between groups: The Mann-Whitney U test and T test for unpaired samples when normality conditions were fulfilled and the Wilcoxon test when they were not. The statistical analysis was conducted with SPSS ver. 18.0 (SPSS, Chicago, IL, United States). Anthropometric data were analyzed with AnthroPlus software[36]. The significance level was set at 5%. The comparison between lumbar BMD z-scores before and after treatment were analyzed by the Wilcoxon paired test. The statistical review of the study was performed by a biomedical statistician.
Ethical aspects
The study was approved by the institutional review board of the Clinics Hospital of the Federal University of Minas Gerais, Brazil (ETIC 0479.0.203.000-10, December 01, 2010) and by the Research Ethics Committee of Santa Casa de Belo Horizonte. It was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The participants and/or their guardians were adequately informed about the study and signed informed consent forms.
RESULTS
Study group characteristics
The group of study contained 40 pediatric patients, of whom 22 (55%) were males and 18 (45%) were females (1.2:1.0, male/female ratio). The mean age of clinical presentation was 10.5 ± 3.5 (3.2-19.6) years. The children and adolescents were regularly followed for a median time of 6.0 (range 4.5-8.3) years. All patients had normal physical examination, including blood pressure evaluation. Blood levels of hematocrit, hemoglobin, bicarbonate, pH, creatinine, urea, phosphorus, calcium, parathyroid hormone, alkaline phosphatase, sodium, potassium, magnesium, uric acid and chloride were all within the normal range. In the same way, the 24-h urine sample was collected properly, and its volume evaluated according to Tiselius[37] and Hellerstein et al[38]. No patient showed clinical signs of malnutrition or had proteinuria and urinary infection during the sample collection. There were no differences in BMI z-score and age between G1 and G2 before and after treatment (Table 1). No increase in total cholesterol or low-density lipoprotein-cholesterol as well as no hypokalemia nor hypomagnesemia were observed with pharmacologic treatment (Kcitrate or thiazides).
Table 1.
Demographic variables in G1 and G2
|
|
Age (yr), mean ± SD
|
Gender, M/F
|
BMIz-score before, mean ± SD |
BMIz-score after, mean ± SD |
| G1 (n = 9) | 9.11 ± 2.57 | 4/5 | -0.74 ± 0.76 | -0.80 ± 0.76 |
| G2 (n = 31) | 10.09 ± 3.65 | 18/13 | -0.82 ± 2.06 | -0.63 ± 1.15 |
| P value | NS | NS | 10.72 | 10.80 |
T test. There were no differences in age, gender, body mass index z-score, and biochemical parameters between G1 and G2. There were no increases in total cholesterol and in low-density lipoprotein-cholesterol as well as hypokalemia and hypomagnesemia. BMI: Body mass index; F: Female; M: Male.
As already mentioned, the study sample was divided into two groups: G1 composed of 9 patients treated with Kcitrate alone and G2 composed of 31 patients treated with Kcitrate + thiazide. The mean urinary calcium excretion in G1 before and after the treatment were 5.12 ± 0.84 and 2.57 ± 0.82 mg/kg per 24 h, respectively (P = 0.0004). The median urinary calcium excretion in G2 before and after the treatment were 5.2 (4.3-7.0) and 2.9 (2.0-4.3) mg/kg per 24 h, respectively (0.001) (Table 2).
Table 2.
Biochemical variables in G1 and G2
|
|
Calciuria before (mg/kg per 24 h)
|
Calciuria after (mg/kg per 24 h)
|
P
value
|
| G1 (n = 9), mean ± SD | 5.12 ± 0.84 | 2.57 ± 0.82 | 10.0004 |
| G2 (n = 31), median (Min-Max) | 5.2 (4.3-7.0) | 2.9 (2.0-4.3) | 20.001 |
T test (normal distribution).
Wilcoxon test (abnormal distribution). Calciuria was significantly lower after treatment in G1 as well as in G2.
BMD
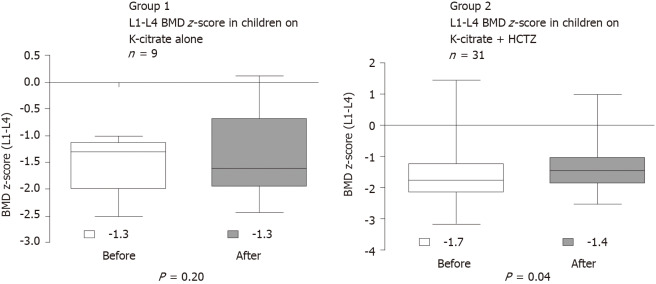
All patients underwent bone densitometry, and all had BMD z-score of the lumbar spine (L1-L4) < -1. The interval between BMD measurements was 1 year. BMD z-score of the lumbar spine (L1-L4) increased significantly after the treatment in G2 (from -1.7 to -1.4; P = 0.04; U-Test Mann-Whitney), and there was no improvement in G1 (from -1.3 to -1.6; P = 0.16; U-Test Mann-Whitney) (Figure 2).
Figure 2.
Comparison of the lumbar spine bone mineral density z-score between Group 1 and Group 2 before and after treatment. Test: U-test Mann-Whitney. BMD: Bone mineral density; HCTZ: Hydrochlorothiazide.
DISCUSSION
The acquisition of bone mass occurs during childhood and adolescence. The maximum peak of bone mass is reached at the end of the second decade of life. It is known that bone mass acquired during childhood and adolescence is the major determinant of adult bone health. Several studies have already demonstrated reduced BMD in hypercalciuric pediatric patients[1-6,9,10]. If reduced BMD in childhood can compromise adult peak bone mass, therapeutic strategies to optimize bone mass in pediatric IH should be considered. Considering that the chronic loss of calcium in the urine is a risk factor for the reduction of BMD and may therefore compromise the final bone mass in adulthood, there is a question that is not yet answered: “should pediatric idiopathic hypercalciuria be treated with hypocalciuric agents?” This study evaluated the BMD of pediatric patients with IH before and after treatment with Kcitrate and thiazides in order to examine whether pharmacological therapy has a beneficial effect on its bone mass.
Study group characteristics
The male/female ratio of our patients was 1.2:1.0, and the mean age at onset of clinical presentation was 10.5 years, in accordance with other studies[9,39]. As shown in Table 1, there were no differences in BMI z-score and age between G1 and G2 before and after treatment. It should be noted that BMI must not be different in the two groups evaluated (G1 and G2). According to García-Nieto et al[40], there is a correlation between the improvements in the BMI and BMD values, indicating that the BMI is a decisive factor to increase the BMD. Schwaderer et al[9] found a negative correlation between BMD and BMI. However, there are some studies reporting the opposite. Both weight gain[41] and exercise[42-45] lead to an increase in BMD. A Brazilian study showed that the lean body mass is the main predictor of bone mass at the end of adolescence in healthy individuals[43]. Inomoto[44] evaluated the effects of physical exercise (moderate exercise activities for 2 h) in Japanese children and concluded that the observed increase in BMD was associated with an increase in overall muscle quantity. In the same way, Sardinha et al[45] reported that daily vigorous physical activity for 25 min a day seems to improve femoral neck bone health in children. These recommendations should be given to all children and adolescents.
Laboratorial data and treatment
No increase in total cholesterol, low-density lipoprotein-cholesterol, hypokalemia and hypomagnesemia were observed with thiazide use. It is known that diuretics, especially thiazides, have a significant effect on lipid profiles. However, Akhtar et al[46] in a meta-analysis of randomized controlled trials concluded that further research is needed because this conclusion is supported by a low number of studies. Hollifield[47] showed that the undesirable effects of hypokalemia and hypomagnesemia are associated with increasing doses of hydrochlorothiazide. In the present study, the thiazide dose was low (0.5-1.0 mg/kg per day), and no changes were seen in cholesterol, potassium and magnesium levels.
In the two studied groups (G1 and G2), a significant reduction in urinary calcium excretion after treatment with Kcitrate and/or thiazides was observed, in accordance with García-Nieto et al[40] and Moreira Guimarães Penido et al[3]. There were no differences in biochemical and mineral parameters between G1 and G2 before and after treatment, as previously demonstrated[3,40].
BMD
BMD z-score of the lumbar spine (L1-L4) increased significantly after the treatment in G2, and there was no improvement in G1 (Figure 2). G1 was treated with Kcitrate alone and G2 was treated with Kcitrate and thiazides. Our results continued pointing to a beneficial effect of thiazide on BMD z-score in children with IH. However, pharmacological treatment of children and adolescents with IH is not yet based on strong scientific evidence. Usually, this treatment is initiated in patients who do not improve calciuria or maintain signs and symptoms with non-pharmacological treatment, which includes modification of dietary habits with high water intake.
In IH adult patients a beneficial effect of Kcitrate on bone mass was observed. Pak et al[15] treated their adult patients with Kcitrate and observed that spinal bone density increased in most of them. Marangella et al[17] suggested that treatment with an alkaline salt, such as potassium citrate, can reduce bone resorption. Vescini et al[18] showed that long-term treatment with Kcitrate increases forearm BMD in idiopathic calcium stone formers. Recently, Lambert et al[20], based on the role of acid-base homeostasis as a determinant of bone health and the contribution of supplemental alkali in promoting skeletal integrity, performed a meta-analysis study. The authors confirmed that supplementation with alkaline potassium salts determined a significant reduction in urinary calcium excretion and reduction in bone resorption. Granchi et al[21] highlighted the main functions of citrate, focusing especially on its role in the pathophysiology of metabolic bone diseases. However, the authors believe that current evidence is still very heterogeneous. In a model of hypercalciuria, Krieger et al[48] demonstrated that Kcitrate significantly raises urine citrate levels and lowers urinary calcium. However, the increases in phosphate, oxalate, and pH levels would lead to increase calcium oxalate and calcium phosphate supersaturation. The authors pointed that Kcitrate may not be beneficial in preventing calcium phosphate stone formation because it can induce complex changes in urinary chemical constitution[48].
In pediatric patients, there is no study confirming the beneficial effect of Kcitrate on bone mass. The mechanism of the association between the use of Kcitrate and interruption of bone mass loss can occur through the reduction of endogenous acid production with a consequent decrease in urinary calcium excretion[49]. In a study involving dietary load of Kcitrate in prepubescent girls, there was no change in urinary calcium excretion, although net acid excretion decreased[50]. In the same way, rats also did not decrease urinary calcium excretion after a load of alkaline potassium[51]. The differences observed in bone metabolism between children and adults (bone modeling vs remodeling) may be involved in the difference in calciuric response to alkaline potassium in these two groups. Jones et al[49] concluded in a cross-sectional study of 330 boys and girls that urinary potassium was associated with BMD independently of lean body mass in these well-nourished, calcium-replete children. The authors also suggested that these findings should be confirmed in longitudinal studies. According to Perez-Suarez et al[8] and others, the indiscriminate use of Kcitrate and thiazides indefinitely is not advisable[3,8,52-54]. Pharmacological treatment should be reserved in three situations: When there are marked clinical complaints as sustained dysuria, frequent macroscopic hematuria, or recurrent renal colic; when ultrasonography shows stones or nephrocalcinosis or in case of repeated fractures[3,9,52].
The effect of diuretic thiazides on the bone may extend beyond its anticalciuric actions and probably is related to the stimulation of osteoblastic bone formation[55]. Studies have shown that the mitogenic action of the thiazide diuretic may be partly due to the increased proliferation of osteoblastic cells associated with the inhibition of osteoclastic bone resorption due to the reduction in osteoclastic cell differentiation, which is mediated by the direct inhibition of hematopoietic precursors[55,56]. Data in the literature suggest a protective effect of thiazide diuretic treatment on bone health by increasing BMD. In fact, thiazides increase renal calcium reabsorption by inhibiting the sodium chloride cotransporter in the distal tubule. This action favors an increase in sodium urinary excretion and decrease in urinary calcium excretion[57]. Spivacow et al[58] evaluated retrospectively BMD and biochemical markers of bone turnover in response to thiazide therapy in 52 adult female patients with IH and nephrolithiasis (25 were pre-menopausal G1, and 27 were postmenopausal G2). The authors concluded that correction of hypercalciuria with long term treatment with low-dose hydrochlorothiazide/amiloride prevented bone loss. Patients with osteoporosis had a significant increase in BMD at the lumbar spine[58]. A systematic review and meta-analysis evaluated the effectiveness of thiazides on serum and urinary calcium levels and BMD in adult patients with osteoporosis. The authors concluded that thiazides might play a role in preserving bone mass and be effective in the prevention and treatment of osteoporosis[59]. Recently, van der Burgh et al[60] in a cross-sectional analysis of the Rotterdam Study concluded that the decrease in fracture risk in patients who used thiazide diuretics is explained by the increase in bone mass and not by the improvement in bone microarchitecture[60]. The Rotterdam Study is an ongoing prospective population-based cohort study with Dutch citizens and was designed to investigate chronic diseases in the elderly.
The administration of thiazides is considered to be an appropriate treatment for hypercalciuria in adult patients with IH, however, only a few published articles have reported on children with IH treated with thiazides. According to García-Nieto et al[40], these studies have been cross-sectional and were controversial in relation to the used technology to measured bone mass. Although there is evidence showing the ability of thiazide to control calciuria, little is known on its impact on BMD, especially in children.
Reusz et al[61] showed in their 18 pediatric patients with IH a positive effect of thiazides on BMD and an important reduction in urinary calcium excretion. Schwaderer et al[9] recommended the pharmacological treatment (Kcitrate and/or thiazide) if a decreased in BMD is observed, even if modifications in life habits have improved the clinical presentation of the metabolic change. Srivastava and Schwaderer[12] believe that the use of drug therapy should be reserved for children with symptomatic IH and/or rare monogenic disorders. Moreira Guimarães Penido et al[3] showed that the BMD of the lumbar spine (L1-L4) and its respective z-scores increased significantly after treatment with Kcitrate or Kcitrate and thiazides. The authors suggested a possible beneficial effect of this treatment on bone mass and pointed out that a normal calciuria with a persistent positive calcium balance could impact and contribute to an ideal peak in bone mass and/or an adequate bone mass acquisition over the years. García-Nieto et al[40] studied 22 children with IH and bone mass reduction who had received thiazides for 2.4 years and compared them with a group of 32 IH children also with bone mass reduction, who had not received thiazide treatment. The authors concluded that thiazide treatment does not improve the BMD z-score in children with IH. They also concluded that more than half of the hypercalciuric children showed a spontaneous improvement in their bone mass, suggesting that there is a tendency for the spontaneous improvement of BMD, which is associated with increased body mass[40]. The same Spanish group (2020) evaluate BMD evolution in 34 IH patients through three bone densitometry studies during over 20 years. They concluded that improved BMD may be related to female sex, increment of body mass, and reduction in bone resorption. No relationship with the use of thiazide diuretics was mentioned by the authors[8].
The weaknesses of our study were the fact of being retrospective, the small number of patients, especially in the K-citrate only group, the lack of long-term follow-up, and the fact that the thiazide treated patients received also K-citrate, which may have protected from hypokalemia and hypomagnesemia. Further prospective longitudinal studies with two subgroups of symptomatic hypercalciuric children (thiazides group vs no treatment group) are necessary.
CONCLUSION
BMD should be considered in the management of pediatric patients with IH. The diagnosis and prevention of bone mass reduction should be detected in these patients because they are considered to be at risk of reduced bone mass. Low BMD with subsequent osteopenia, osteoporosis, and increased fracture risk can have a considerable impact on the health system and in patient quality of life.
Some authors believe that hypercalciuria is not a disease but the physiology of people who occupy one extreme of a continuous spectrum of urine calcium excretion rates[52]. However, if this spectrum of high calcium excretion can cause harmful consequences and diseases in adulthood, it could be considered as a disease in pediatric patients. In fact, in most cases, it is simply a benign metabolic abnormality and as such should be conducted. However, it is known that reduced BMD is reported in pediatric hypercalciuria and can impact the health of adult bone.
Studies with IH pediatric patients indicated that citrate or thiazide diuretic treatment may improve BMD[3,61]. However, the treatment with these medications has very well-defined indications for use. As aforementioned, the initial approach of IH consists of dietary modifications and high fluid intake. In case of no normalization of calcium excretion with the dietary modifications and high fluid intake, hypocalciuric agents are considered. The first line of hypocalciuric agents is Kcitrate. Thiazide should be initiated combined with potassium citrate only if there is low BMD and/or fractures, and no improvement of calciuria levels and symptoms was obtained[3,4,6,12,13]. Controlled studies in the future will be able to define if children and adolescents with IH should or should not be treated.
In conclusion, we continue to speculate if thiazide has a role in bone mass and if steady positive calcium balance could impact on the achievement of optimal peak bone mass. However, we do believe that prospective randomized controlled studies are the key to test the effectiveness of pharmacological therapy in children with IH and if it prevents impairment of bone mass in adulthood.
ARTICLE HIGHLIGHTS
Research background
Idiopathic hypercalciuria (IH) is the leading metabolic risk factor for pediatric urolithiasis. The reduction in bone mass has already been described in hypercalciuric children. Life-long hypercalciuria might be considered a risk to change bone structure and determine low bone mass throughout life. A beneficial effect of citrate formulations and thiazides on bone mass in adult and pediatric patients with IH have been shown.
Research motivation
Considering that HI can cause a reduction in mineral bone density in children and adolescents and lead to osteopenia, osteoporosis, and an increased risk of fractures in adulthood, it would be important to know how to diagnose and treat this metabolic disorder.
Research objectives
Evaluated whether pharmacological therapy has a beneficial effect on bone mass in children and adolescents with IH.
Research methods
This is a retrospective cohort study that evaluated hypercalciuric children non-responsive to lifestyle and diet changes. They were treated with potassium Kcitrate or with Kcitrate combined to thiazides. Before and after treatment they underwent bone densitometry.
Research results
Forty IH children, median age 10.5 years and median time follow-up 6.0 years, were evaluated. Nine patients were treated with Kcitrate (G1) and 31 with Kcitrate + thiazide (G2). Calciuria decreased in both groups after treatment. Lumbar spine bone mineral density z-score increased after thiazide treatment in G2. There was no improvement in G1.
Research conclusions
Results point to a beneficial effect of thiazide on lumbar spine bone mineral density z-score in children with IH.
Research perspectives
Future perspectives are to understand better the pathogenesis of HI in order to treat children and adolescents, preventing bone mass reduction.
ACKNOWLEDGEMENTS
We thank the special collaboration of all children, adolescents, and their families involved in this study.
Footnotes
Institutional review board statement: The study was approved by the institutional review board of the Clinics Hospital of the Federal University of Minas Gerais, Brazil (ETIC 0479.0.203.000-10, December 01, 2010) and by the Research Ethics Committee of Santa Casa de Belo Horizonte. It was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Invited manuscript
Peer-review started: April 1, 2021
First decision: July 8, 2021
Article in press: July 22, 2021
Specialty type: Urology and nephrology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bilic Curcic I S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Maria Goretti Moreira Guimarães Penido, Pediatric Nephrology Unit, Nephrology Center of Santa Casa de Belo Horizonte, Belo Horizonte 30150320, Minas Gerais, Brazil. mariagorettipenido@yahoo.com.br; Federal University of Minas Gerais, Faculty of Medicine, Department of Pediatrics, Pediatric Nephrology Unit, Belo Horizonte 30130100, Minas Gerais, Brazil.
Marcelo de Sousa Tavares, Pediatric Nephrology Unit, Nephrology Center of Santa Casa de Belo Horizonte, Belo Horizonte 30150320, Minas Gerais, Brazil.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at: mariagorettipenido@yahoo.com.br. Participants gave informed consent for data sharing. Data are anonymized and there were no risk of identification.
References
- 1.Pavlou M, Giapros V, Challa A, Chaliasos N, Siomou E. Does idiopathic hypercalciuria affect bone metabolism during childhood? Pediatr Nephrol. 2018;33:2321–2328. doi: 10.1007/s00467-018-4027-y. [DOI] [PubMed] [Google Scholar]
- 2.Penido MG, Lima EM, Marino VS, Tupinambá AL, França A, Souto MF. Bone alterations in children with idiopathic hypercalciuria at the time of diagnosis. Pediatr Nephrol. 2003;18:133–139. doi: 10.1007/s00467-002-1036-6. [DOI] [PubMed] [Google Scholar]
- 3.Moreira Guimarães Penido MG, de Sousa Tavares M, Campos Linhares M, Silva Barbosa AC, Cunha M. Longitudinal study of bone mineral density in children with idiopathic hypercalciuria. Pediatr Nephrol. 2012;27:123–130. doi: 10.1007/s00467-011-1952-4. [DOI] [PubMed] [Google Scholar]
- 4.García-Nieto V, Ferrández C, Monge M, de Sequera M, Rodrigo MD. Bone mineral density in pediatric patients with idiopathic hypercalciuria. Pediatr Nephrol. 1997;11:578–583. doi: 10.1007/s004670050341. [DOI] [PubMed] [Google Scholar]
- 5.Skalova S, Palicka V, Kutilek S. Bone mineral density and urinary N-acetyl-beta-D-glucosaminidase activity in paediatric patients with idiopathic hypercalciuria. Nephrology (Carlton) 2005;10:99–102. doi: 10.1111/j.1440-1797.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 6.García-Nieto V, Sánchez Almeida E, Monge M, Luis Yanes MI, Hernández González MJ, Ibáñez A. Longitudinal study, bone mineral density in children diagnosed with idiopathic hipercalciuria (IH) Pediatr Nephrol. 2009;24:2083. [Google Scholar]
- 7.Kusumi K, Schwaderer AL, Clark C, Budge K, Hussein N, Raina R, Denburg M, Safadi F. Bone mineral density in adolescent urinary stone formers: is sex important? Urolithiasis. 2020;48:329–335. doi: 10.1007/s00240-020-01183-w. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Suarez G, Yanes MIL, de Basoa MCMF, Almeida ES, García Nieto VM. Evolution of bone mineral density in patients with idiopathic hypercalciuria: a 20-year longitudinal study. Pediatr Nephrol. 2021;36:661–667. doi: 10.1007/s00467-020-04754-6. [DOI] [PubMed] [Google Scholar]
- 9.Schwaderer AL, Cronin R, Mahan JD, Bates CM. Low bone density in children with hypercalciuria and/or nephrolithiasis. Pediatr Nephrol. 2008;23:2209–2214. doi: 10.1007/s00467-008-0929-4. [DOI] [PubMed] [Google Scholar]
- 10.Freundlich M, Alonzo E, Bellorin-Font E, Weisinger JR. Reduced bone mass in children with idiopathic hypercalciuria and in their asymptomatic mothers. Nephrol Dial Transplant. 2002;17:1396–1401. doi: 10.1093/ndt/17.8.1396. [DOI] [PubMed] [Google Scholar]
- 11.Carrié Fässler AL, Bonjour JP. Osteoporosis as a pediatric problem. Pediatr Clin North Am. 1995;42:811–824. doi: 10.1016/s0031-3955(16)39018-6. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava T, Schwaderer A. Diagnosis and management of hypercalciuria in children. Curr Opin Pediatr. 2009;21:214–219. doi: 10.1097/MOP.0b013e3283223db7. [DOI] [PubMed] [Google Scholar]
- 13.Subcommittee of the Tenth Edition of the RDAs, Food and Nutrition Board, Commission on Life Sciences, National Research Council. FNB recommended dietary allowances, 10th ed. National Academy Press, Washington, 1989. [Google Scholar]
- 14.Sellmeyer DE, Schloetter M, Sebastian A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J Clin Endocrinol Metab. 2002;87:2008–2012. doi: 10.1210/jcem.87.5.8470. [DOI] [PubMed] [Google Scholar]
- 15.Pak CY, Peterson RD, Poindexter J. Prevention of spinal bone loss by potassium citrate in cases of calcium urolithiasis. J Urol. 2002;168:31–34. [PubMed] [Google Scholar]
- 16.Caudarella R, Vescini F, Buffa A, Stefoni S. Citrate and mineral metabolism: kidney stones and bone disease. Front Biosci. 2003;8:s1084–s1106. doi: 10.2741/1119. [DOI] [PubMed] [Google Scholar]
- 17.Marangella M, Di Stefano M, Casalis S, Berutti S, D'Amelio P, Isaia GC. Effects of potassium citrate supplementation on bone metabolism. Calcif Tissue Int. 2004;74:330–335. doi: 10.1007/s00223-003-0091-8. [DOI] [PubMed] [Google Scholar]
- 18.Vescini F, Buffa A, La Manna G, Ciavatti A, Rizzoli E, Bottura A, Stefoni S, Caudarella R. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest. 2005;28:218–222. doi: 10.1007/BF03345376. [DOI] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009;94:96–102. doi: 10.1210/jc.2008-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert H, Frassetto L, Moore JB, Torgerson D, Gannon R, Burckhardt P, Lanham-New S. The effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysis. Osteoporos Int. 2015;26:1311–1318. doi: 10.1007/s00198-014-3006-9. [DOI] [PubMed] [Google Scholar]
- 21.Granchi D, Baldini N, Ulivieri FM, Caudarella R. Role of Citrate in Pathophysiology and Medical Management of Bone Diseases. Nutrients. 2019;11 doi: 10.3390/nu11112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tekin A, Tekgul S, Atsu N, Bakkaloglu M, Kendi S. Oral potassium citrate treatment for idiopathic hypocitruria in children with calcium urolithiasis. J Urol. 2002;168:2572–2574. doi: 10.1016/S0022-5347(05)64218-8. [DOI] [PubMed] [Google Scholar]
- 23.Sarica K, Erturhan S, Yurtseven C, Yagci F. Effect of potassium citrate therapy on stone recurrence and regrowth after extracorporeal shockwave lithotripsy in children. J Endourol. 2006;20:875–879. doi: 10.1089/end.2006.20.875. [DOI] [PubMed] [Google Scholar]
- 24.Lalande A, Roux S, Denne MA, Stanley ER, Schiavi P, Guez D, De Vernejoul MC. Indapamide, a thiazide-like diuretic, decreases bone resorption in vitro. J Bone Miner Res. 2001;16:361–370. doi: 10.1359/jbmr.2001.16.2.361. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak MM, De Joussineau C, Carter DH, Pisitkun T, Knepper MA, Gamba G, Kemp PJ, Riccardi D. Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone. J Am Soc Nephrol. 2007;18:2509–2516. doi: 10.1681/ASN.2007030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int. 2007;18:479–486. doi: 10.1007/s00198-006-0259-y. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:516–526. doi: 10.7326/0003-4819-133-7-200010030-00010. [DOI] [PubMed] [Google Scholar]
- 28.Penido MG, Diniz JS, Guimarães MM, Cardoso RB, Souto MF, Penido MG. [Urinary excretion of calcium, uric acid and citrate in healthy children and adolescents] J Pediatr (Rio J) 2002;78:153–160. [PubMed] [Google Scholar]
- 29.Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993;123:393–397. doi: 10.1016/s0022-3476(05)81738-x. [DOI] [PubMed] [Google Scholar]
- 30.Stapleton FB. Hematuria associated with hypercalciuria and hyperuricosuria: a practical approach. Pediatr Nephrol. 1994;8:756–761. doi: 10.1007/BF00869114. [DOI] [PubMed] [Google Scholar]
- 31.Chen YH, Lee AJ, Chen CH, Chesney RW, Stapleton FB, Roy S 3rd. Urinary mineral excretion among normal Taiwanese children. Pediatr Nephrol. 1994;8:36–39. doi: 10.1007/BF00868256. [DOI] [PubMed] [Google Scholar]
- 32.Kröger HPJ. Measurement of bone mass and density in children. In: Schönau E. Paediatric osteology: new developments in diagnostics and therapy. Elsevier Science, Amsterdam, 1996: 103-108. [Google Scholar]
- 33.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 34.del Rio L, Carrascosa A, Pons F, Gusinyé M, Yeste D, Domenech FM. Bone mineral density of the lumbar spine in white Mediterranean Spanish children and adolescents: changes related to age, sex, and puberty. Pediatr Res. 1994;35:362–366. doi: 10.1203/00006450-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Thomas KA, Cook SD, Bennett JT, Whitecloud TS 3rd, Rice JC. Femoral neck and lumbar spine bone mineral densities in a normal population 3-20 years of age. J Pediatr Orthop. 1991;11:48–58. doi: 10.1097/01241398-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Anthroplus software. [cited 15 June 2020]. In: World Health Organization [Internet]. Available from: http://www.who.int/growthref/tools/en/
- 37.Tiselius HG. Practical aspects of the handling of samples and evaluation of laboratory data. Scand J Urol Nephrol Suppl. 1980;53:105–108. [PubMed] [Google Scholar]
- 38.Hellerstein S, Simon SD, Berenbom M, Erwin P, Nickell E. Creatinine excretion rates for renal clearance studies. Pediatr Nephrol. 2001;16:637–643. doi: 10.1007/s004670100622. [DOI] [PubMed] [Google Scholar]
- 39.Spivacow FR, Negri AL, del Valle EE, Calviño I, Fradinger E, Zanchetta JR. Metabolic risk factors in children with kidney stone disease. Pediatr Nephrol. 2008;23:1129–1133. doi: 10.1007/s00467-008-0769-2. [DOI] [PubMed] [Google Scholar]
- 40.García-Nieto V, Monge-Zamorano M, González-García M, Luis-Yanes MI. Effect of thiazides on bone mineral density in children with idiopathic hypercalciuria. Pediatr Nephrol. 2012;27:261–268. doi: 10.1007/s00467-011-1987-6. [DOI] [PubMed] [Google Scholar]
- 41.El Hage R, Jacob C, Moussa E, Groussard C, Pineau JC, Benhamou CL, Jaffré C. Influence of the weight status on bone mineral content and bone mineral density in a group of Lebanese adolescent girls. Joint Bone Spine. 2009;76:680–684. doi: 10.1016/j.jbspin.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Eliakim A, Raisz LG, Brasel JA, Cooper DM. Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males. J Bone Miner Res. 1997;12:1708–1713. doi: 10.1359/jbmr.1997.12.10.1708. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca RM, de França NM, Van Praagh E. Relationship between indicators of fitness and bone density in adolescent Brazilian children. Pediatr Exerc Sci. 2008;20:40–49. doi: 10.1123/pes.20.1.40. [DOI] [PubMed] [Google Scholar]
- 44.Inomoto T. [Physical activity/sports and bone mineral density] Clin Calcium. 2008;18:1339–1348. [PubMed] [Google Scholar]
- 45.Sardinha LB, Baptista F, Ekelund U. Objectively measured physical activity and bone strength in 9-year-old boys and girls. Pediatrics. 2008;122:e728–e736. doi: 10.1542/peds.2007-2573. [DOI] [PubMed] [Google Scholar]
- 46.Akhtar F, Khalid F, Wang H, Zhang D, Gong X. The Effect of Thiazide Diuretics on Blood Lipid Profile in Hypertensive Adults: A Meta-analysis of Randomized Controlled Trials. Cureus. 2018;10:e2651. doi: 10.7759/cureus.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollifield JW. Thiazide treatment of hypertension. Effects of thiazide diuretics on serum potassium, magnesium, and ventricular ectopy. Am J Med. 1986;80:8–12. doi: 10.1016/0002-9343(86)90335-9. [DOI] [PubMed] [Google Scholar]
- 48.Krieger NS, Asplin JR, Frick KK, Granja I, Culbertson CD, Ng A, Grynpas MD, Bushinsky DA. Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J Am Soc Nephrol. 2015;26:3001–3008. doi: 10.1681/ASN.2014121223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones G, Riley MD, Whiting S. Association between urinary potassium, urinary sodium, current diet, and bone density in prepubertal children. Am J Clin Nutr. 2001;73:839–844. doi: 10.1093/ajcn/73.4.839. [DOI] [PubMed] [Google Scholar]
- 50.Duff TL, Whiting SJ. Calciuric effects of short-term dietary loading of protein, sodium chloride and potassium citrate in prepubescent girls. J Am Coll Nutr. 1998;17:148–154. doi: 10.1080/07315724.1998.10718740. [DOI] [PubMed] [Google Scholar]
- 51.Greger JL, Kaup SM, Behling AR. Calcium, magnesium and phosphorus utilization by rats fed sodium and potassium salts of various inorganic anions. J Nutr. 1991;121:1382–1388. doi: 10.1093/jn/121.9.1382. [DOI] [PubMed] [Google Scholar]
- 52.García Nieto VM, Luis Yanes MI, Tejera Carreño P, Perez Suarez G, Moraleda Mesa T. The idiopathic hypercalciuria reviewed. Metabolic abnormality or disease? Nefrologia (Engl Ed) 2019;39:592–602. doi: 10.1016/j.nefro.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Alon US, Zimmerman H, Alon M. Evaluation and treatment of pediatric idiopathic urolithiasis-revisited. Pediatr Nephrol. 2004;19:516–520. doi: 10.1007/s00467-004-1422-3. [DOI] [PubMed] [Google Scholar]
- 54.Spivacow FR, Pailler M, Martínez P. [Idiopathic hypercalciuria: can the diuretics be avoided? Medicina (B Aires) 2019;79:477–482. [PubMed] [Google Scholar]
- 55.Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int. 2011;79:393–403. doi: 10.1038/ki.2010.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall TJ, Schaueblin M. Hydrochlorothiazide inhibits osteoclastic bone resorption in vitro. Calcif Tissue Int. 1994;55:266–268. doi: 10.1007/BF00310404. [DOI] [PubMed] [Google Scholar]
- 57.Caudarella R, Vescini R, Rizzol E, Ulivieri FM. The effect of Thiazides on Bone Markers, Bone Mineral Density and Fractures. Clinic Rev Bone Miner Metab. 2015;13:173–184. [Google Scholar]
- 58.Spivacow FR, Negri AL, del Valle EE. Long Term Effect of Thiazides on Bone Mass in Women with Hypercalciuric Nephrolithiasis. Nefrología Diálisis y Trasplante. 2013;33:180–187. [Google Scholar]
- 59.Cheng L, Zhang K, Zhang Z. Effectiveness of thiazides on serum and urinary calcium levels and bone mineral density in patients with osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:3929–3935. doi: 10.2147/DDDT.S179568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Burgh AC, Oliai Araghi S, Zillikens MC, Koromani F, Rivadeneira F, van der Velde N, Hoorn EJ, Uitterlinden AG, Ikram MA, Stricker BH. The impact of thiazide diuretics on bone mineral density and the trabecular bone score: the Rotterdam Study. Bone. 2020;138:115475. doi: 10.1016/j.bone.2020.115475. [DOI] [PubMed] [Google Scholar]
- 61.Reusz GS, Dobos M, Vásárhelyi B, Sallay P, Szabó A, Horváth C, Byrd DJ, Thole HH, Tulassay T. Sodium transport and bone mineral density in hypercalciuria with thiazide treatment. Pediatr Nephrol. 1998;12:30–34. doi: 10.1007/s004670050398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at: mariagorettipenido@yahoo.com.br. Participants gave informed consent for data sharing. Data are anonymized and there were no risk of identification.