Abstract
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus. As an emerging virus, CHIKV imposes a threat to public health. Currently, there are no vaccines or antivirals available for the prevention of CHIKV infection. Lycorine, an alkaloid from Amaryllidaceae plants, has antiviral activity against a number of viruses such as coronavirus, flavivirus and enterovirus. In this study, we found that lycorine could inhibit CHIKV in cell culture at a concentration of 10 μmol/L without apparent cytotoxicity. In addition, it exhibited broad-spectrum anti-alphavirus activity, including Sindbis virus (SINV), Semliki Forest virus (SFV), and Venezuelan equine encephalomyelitis virus (VEEV). The time of addition studies indicated that lycorine functions at an early post-entry stage of CHIKV life cycle. The results based on two different CHIKV replicons provided further evidence that lycorine exerts its antiviral activity mainly by inhibiting CHIKV translation. Overall, our study extends the antiviral spectrum of lycorine.
Keywords: Chikungunya virus (CHIKV), Alphavirus, Lycorine, Antiviral
Introduction
Chikungunya virus (CHIKV) is an important re-emerging mosquito-transmitted pathogen within the Alphavirus genus of the Togaviridae family. It generally causes high fever, headache, rashes, myalgia, arthralgia, and occasionally crippling arthritis that may persist for months or even years (Schwartz and Albert 2010). More severe symptoms, including encephalitis, hemorrhagic disease and mortality have also been reported during recent epidemics (Das et al. 2010). Additionally, perinatal CHIKV infection with severe outcomes has been reported recently (Gerardin et al. 2014; Bandeira et al. 2016; Contopoulos-Ioannidis et al. 2018; van Enter et al. 2018). To date, neither licensed vaccines nor specific antiviral drugs are available to prevent CHIKV infection.
CHIKV is a positive, single-stranded RNA virus, and the complete genome is around 12 kilobases (kb) with a 5′ cap and a 3′ poly (A) tail that contains two ORFs. The first ORF locating at the 5′ two-thirds of the CHIKV genome encodes four nonstructural proteins (nsP1–nsP4) responsible for viral replication and transcription. The second ORF, found at the 3′-end of the genome, encodes the structural proteins (capsid (C), E3, E2, 6 K/TF and E1) that function in the assembly of new virus particles and the attachment and entry to new cells. After entry into host cells, the viral genome is directly used as mRNA for the translation of nonstructural proteins (nsP1–nsP4) that form viral replication complex to initiate the synthesis of the negative-strand RNA using viral genome as the template (Kaur et al. 2013). The resulting negative-strand RNA then serves as a template for the synthesis of viral genomic and 26S subgenomic (sg) RNAs. Among which, the sgRNA is transcribed from the remaining 3′ one-third of the genome with a sg promoter that is presented on the full-length negative-stranded RNA. The sgRNA is subsequently translated into structural proteins. Efforts have been made to develop compounds against CHIKV infection that target different steps of viral life cycle, for example harringtonine, a cephalotaxine ester derived from the Japanese plum yew, inhibited CHIKV infection by interfering with the process of viral protein synthesis (Kaur et al. 2013); ribavirin and 6-azauridine that are nucleotide analogs target the steps of viral RNA synthesis (Briolant et al. 2004; Abdelnabi et al. 2015).
Lycorine is an alkaloid extracted from Amaryllidaceae plants (Zou et al. 2009). It has been reported to exhibit different biological activities such as anti-inflammatory, anti-malarial, anti-leukemia as well as antiviral activities. Numerous viruses have been reported to be sensitive to lycorine treatment including severe acute respiratory syndrome-associated coronavirus (SARS-CoV) (Shen et al. 2019), SARS-CoV-2 (Zhang et al. 2020), human immunodeficiency virus (HIV-1) (Yang et al. 2019), hepatitis C virus (HCV) (Chen et al. 2015), influenza virus (He et al. 2013), flavivirus (Zou et al. 2009) and enteroviruses (Liu et al. 2011; Wang et al. 2019).
In this paper, we report that lycorine inhibits multiple alphaviruses propagation in cell culture without detectable cytotoxicity. Mode-of-action analysis indicates that lycorine inhibits CHIKV infection at the post-entry step of CHIKV life cycle, mainly viral translation.
Materials and Methods
Cell Lines, Viruses and Compounds
BHK-21, Vero, Huh7 and A549 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 100 units/mL of penicillin, 100 µg/mL of streptomycin in 5% CO2 at 37 °C.
The wild type (WT) CHIKV (GenBank accession No. KC488650) and eGFP-CHIKV stocks were produced from the infectious cDNA clones (Deng et al. 2016) and stored in aliquots at −80 °C. The WT Sindbis virus (SINV, strain AR339) and Semliki Forest virus (SFV, strain SFV4) were kindly provided by Prof. Qing-Zhen Liu from Wuhan University and Prof. Xi Zhou from Wuhan Institute of Virology, respectively. The Venezuelan equine encephalitis virus (VEEV, strain TC-83) was generated by lipofection (DMRIE-C Reagent, Invitrogen) of Vero cells with the transcribed viral genomic RNA from the linearized infectious cDNA clone of the TC-83 vaccine strain.
The lycorine was purchased from Sichuan Victory Biological Technology Co., Ltd., China.
Plasmid Construction
We have constructed two kinds of replicons to compare the replication efficiency: the CHIKV-sg-Rluc and CHIKV-nsP3-Rluc-replicon. The former was constructed by replacing the structural protein genes of the WT CHIKV with a Renilla luciferase gene that was initiated by a sg promoter, the latter was constructed by inserting the Renilla luciferase gene after codon 490 of the nsP3 gene (Kummerer et al. 2012). An inactivating GDD to GAA mutation in the catalytic site of nsP4 was introduced to obtain the CHIKV-nsP3-Rluc-nsP4mut-replicon (Utt et al. 2016). For the purpose of comparison of translation efficiency, two constructs were designed. One is the T7 promoter-based construct expressing firefly luciferase that contains the 5′ and 3′ UTR of CHIKV, the 77 N-terminal amino acid residues of nsP1, a firefly luciferase reporter, and a polyadenylation region followed by a T7 transcription terminator (Utt et al. 2016). The other is a T7 promoter-based sgRNA expressing Renilla luciferase. The sgRNA includes the sg-5′UTR and 3′UTR of CHIKV, a Renilla luciferase reporter and a polyadenylation region.
Electroporation
Approximately 5 μg of RNA was electroporated into 8 × 106 BHK-21 cells in 0.8 mL of cold phosphate buffered saline (PBS, pH 7.5) in a 0.4-cm cuvette with the GenePulser apparatus (Bio-Rad) using settings of 0.85 kV and 25 μF, pulsing three times with 3 s intervals. After a 10 min recovery at room temperature, the transfected cells were diluted in 20 mL of DMEM containing 10% FBS with or without 10 μmol/L of lycorine and transferred into 12-well plates. The cell lysates collected at the indicated time points were subjected to the luciferase assay as described previously (Li et al. 2019).
Antiviral Assay of Lycorine
eGFP-CHIKV reporter virus was used for rapid detection of inhibitory effects of lycorine and its derivatives. Vero cells seeded in 12-well plates (2 × 105 cells per well) were infected with eGFP-CHIKV at an MOI of 0.01. At 36 hours post infection (hpi), the eGFP expression was detected under a fluorescent microscope (Nikon Eclipse TE2000) in the presence of different concentrations of lycorine or 10 μmol/L of derivatives. For dose-dependent inhibition of lycorine on CHIKV in different cell lines or other alphaviruses, Vero, BHK-21, Huh7, A549 cells infected with WT CHIKV or Vero cells infected with WT SINV, SFV and VEEV at an MOI of 0.01 were treated with increasing concentrations of lycorine. The culture media were collected at 12 hpi for SINV and at 24 hpi for SFV and VEEV. Viral titers were quantified by plaque assay in BHK-21 cells as described previously (Zhang et al. 2016, 2019). The antiviral activity of lycorine was expressed as 50% effective concentration (EC50) which indicated the compound concentration required to achieve 50% of viral titer reduction and was calculated by nonlinear regression using GraphPad Prism software 5.0.
Cytotoxicity Assays
The cytotoxicity of lycorine or its derivatives on cells was examined with CCK-8 assay kit. In brief, different cells were seeded in 96-well plates (1 × 104 cells per well) and allowed to grow for 24 h before treatment. Afterwards, the twofold serial dilutions of lycorine were added to the cells. At 48 h, the cells were incubated with 10 μL of CCK8 reagent (cell counting kit-8, Bimake) for 1 h at 37 °C. The absorbance at 450 nm was recorded by a multimode microplate reader (Varioskan Flash; Thermo Fisher, Finland). Cell viability was expressed as a percentage of the treated cells to the control (untreated) cells. For each lycorine concentration, six wells were performed in parallel and the mean values of the cell viability were calculated. The 50% cytotoxic concentration (CC50) was calculated by nonlinear regression using GraphPad Prism software 5.0 to determine the cytotoxic concentration at which 50% of the cells are viable.
Disinfection Test
To exclude the possibility of degermation of lycorine, a disinfectant test was designed. Briefly, equal volumes of eGFP-CHIKV (2 × 104 PFU in 100 μL) and 10 μmol/L of lycorine were mixed and incubated at 37 °C for 1 h. The MicroSpin S-400 HR columns (GE Healthcare) were used to trap the lycorine before inoculation into Vero cells. At 24 hpi, the eGFP expression was captured under a fluorescence microscope.
Time-of-Addition Assay
To determine the step inhibited by lycorine during viral life cycle, a time-of-addition assay was performed. Vero cells infected with WT CHIKV at an MOI of 10 for 1 h at 37 °C were treated with 10 μmol/L of lycorine at the following time points: pre-infection (−2–0 h), during infection (0–1 h), and postinfection (2, 4, 6, 8, 10 h). Meanwhile, 0.1% DMSO was added at the same time as a control. The supernatants collected at 12 hpi were subjected to viral titer quantification by plaque assay in BHK-21 cells as described above.
Statistical Analysis
The two-way ANOVA was used to determine whether there were significant differences (P < 0.05) in all experiments. The statistical analyses were performed using the nonparametric test in GraphPad Prism 5.0.
Results
Identification of Lycorine as an Inhibitor of CHIKV
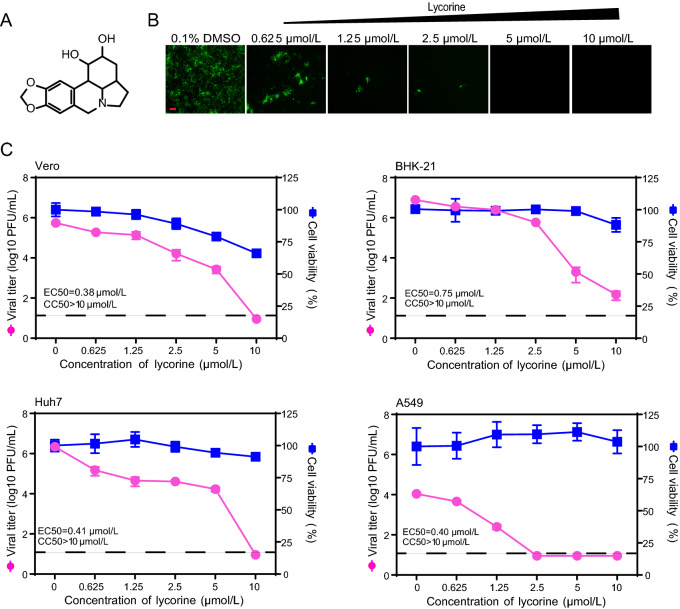
To rapidly evaluate the antiviral activity of lycorine (Fig. 1A) against CHIKV, we used a previously constructed CHIKV reporter virus (eGFP-CHIKV) harboring an additional subgenomic promoter to express the reporter gene, an enhanced green fluorescent protein (eGFP) (Deng et al. 2016). As shown in Fig. 1B, treatment with lycorine led to a dose-dependent inhibition of eGFP expression in eGFP-CHIKV infected Vero cells, indicating that lycorine could efficiently inhibit CHIKV replication. We then performed a viral titer reduction assay using WT CHIKV to further confirm the results from reporter virus. Consistently, the dose-dependent reductions in viral titers were observed in different lycorine-treated cells including Vero, BHK-21, Huh7 and A549 cells with the EC50 values (the compound concentration required to inhibit 50% of viral titer) of 0.38, 0.75, 0.41 and 0.4 μmol/L, respectively (Fig. 1C). To exclude the possibility that the inhibition of viral replication was due to cytotoxicity caused by the compound, we performed a cell proliferation-based cytotoxicity assay. Lycorine is less cytotoxic to these cells at least at a concentration of 10 μmol/L when the viral titers of CHIKV were decreased by 103–105-fold (Fig. 1C). Taken together, our data confirm that lycorine could efficiently inhibit CHIKV replication in cell culture, which are not cell type dependent.
Fig. 1.
Inhibitory effects of lycorine on CHIKV infection. A Chemical structure of lycorine. B Detection of eGFP expression in Vero cells infected with eGFP-CHIKV when treated with different concentrations of lycorine. The eGFP expression was detected under a fluorescent microscope at 36 hpi. The length of the scale bar (displayed in a red line segment) represents 100 μm. C Dose-dependent inhibition of lycorine on CHIKV infection in different cell lines. Vero, BHK-21, Huh7, A549 cells were infected with WT CHIKV at an MOI of 0.01 and treated with the increasing concentrations of lycorine. The viral titers in culture media at 24 hpi were quantified by plaque assay. The cytotoxicity of lycorine on cells was examined with CCK-8 assay kit. The concentration–response curves for antiviral and cytotoxic activities of lycorine are shown in pink and blue lines, respectively. The dashed lines in panel C represent the limit of detection. Two independent experiments were performed in triplicate. Data represent the mean ± standard deviation (SD) of the triplicate measurements in a representative experiment.
Broad Antiviral Spectrum of Lycorine Against Alphavirus
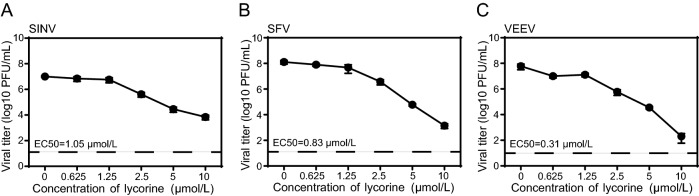
Several other alphaviruses, including SINV, SFV, and VEEV were then chosen to examine whether lycorine has broad antiviral spectrum against alphavirus. As shown in Fig. 2, lycorine also displayed dose-dependent inhibitory effects on the replications of SINV, SFV, and VEEV with EC50 values of 1.05, 0.83 and 0.31 μmol/L, respectively (Fig. 2A–2C). These results demonstrate that lycorine broadly inhibits the replication of alphavirus in cell culture.
Fig. 2.
The antiviral activities of lycorine against other alphaviruses. A–C Dose-dependent inhibition of SINV, SFV and VEEV infection by lycorine. Vero cells infected with different alphaviruses at an MOI of 0.01 were treated with increasing concentrations of lycorine. The supernatants were harvested at 12 hpi for SINV and at 24 hpi for SFV and VEEV. Viral titers were determined by plaque assay. The EC50 are displayed at the lower left for each virus. The dashed lines in panels A–C represent the limit of detection. Two independent experiments were performed in triplicate. Data represent the mean ± standard deviation (SD) of the triplicate measurements in a representative experiment.
Lycorine Acts at the Early Step of Viral Life Cycle
To further define the mode of action of lycorine, we first examined whether lycorine acts as a viricidal agent through direct inactivation of CHIKV virion particles. After pretreating eGFP-CHIKV with 10 μmol/L of lycorine for 1 h, the mixture went through MicroSpin S-400 HR columns (GE Healthcare) to get rid of lycorine and was used to infect Vero cells (Fig. 3A). No significant loss of eGFP expression was observed within the eGFP-CHIKV infected cells with or without lycorine treatment (Fig. 3B), which clearly demonstrate that preincubation of CHIKV with lycorine did not impair the infectivity of CHIKV in Vero cells. It indicates that lycorine is not a viricidal agent against CHIKV infection.
Fig. 3.
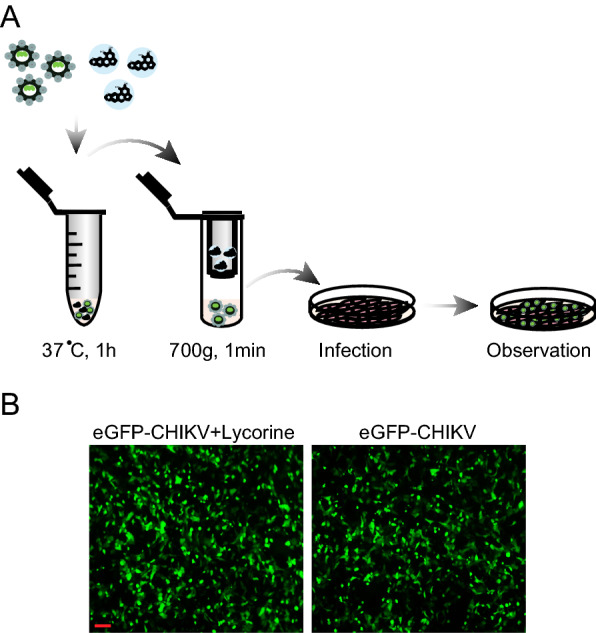
Disinfection test of lycorine. A Schematic representation of the disinfection test. After sequentially incubated at 37 °C for 1 h and passed through MicroSpin S-400 HR column, the mixture of eGFP-CHIKV and 10 μmol/L of lycorine was inoculated into Vero cells. The eGFP expression was detected under a fluorescent microscope at 24 hpi (B). The eGFP-CHIKV only was used as a control. The length of the scale bar (displayed in a red line segment) represents 100 μm. At least three independent experiments were performed, and one representative experiment was presented.
Then, a time-of-addition assay was performed to identify which step of viral life cycle is blocked by lycorine. Vero cells were infected with CHIKV at an MOI of 10, and the compound (10 μmol/L) was added before and after infection as indicated in Fig. 4A. The culture supernatants were reclaimed for plaque assays at 12 hpi. As shown in Fig. 4B, pretreatment with lycorine for 2 h prior to CHIKV infection (Fig. 4B, panel 2) or co-incubation with virus for 1 h (Fig. 4B, panel 3) showed little inhibitory effects on viral infection, indicating that lycorine did not inhibit CHIKV entry. A maximal reduction in viral titers was observed when lycorine was added at the 0- and 2-hpi time points, and thereafter, the inhibitory effects were gradually decreased (Fig. 4B). These results indicate that lycorine may exert its effect at early stages of post-entry, such as genome translation and replication.
Fig. 4.
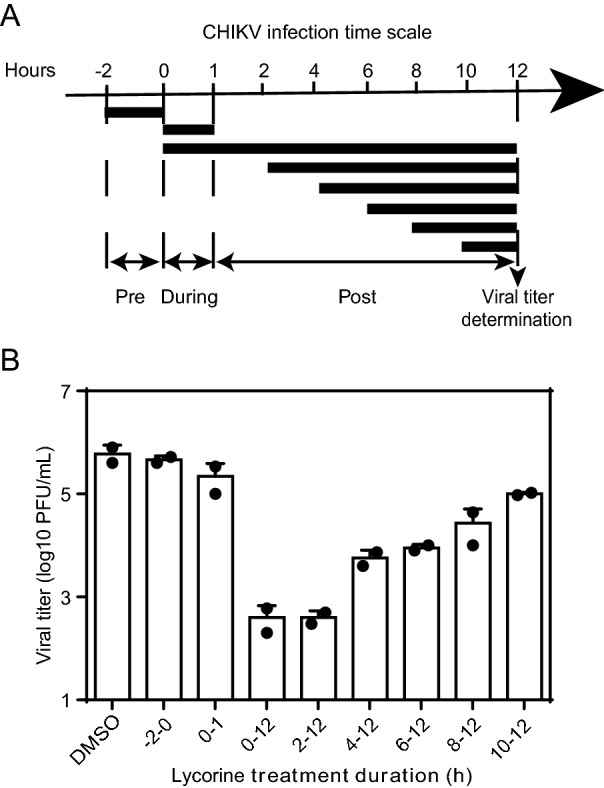
Time-of-addition experiment of lycorine. A Schematic illustration of the time-of-addition experiment. Vero cells were infected with WT CHIKV at an MOI of 10, and treated with 10 μmol/L of lycorine pre (−2–0 h), during (0–1 h) and post (2, 4, 6, 8, 10 h) infection. 0.1% DMSO was added at the same time as a control. B Viral titer determination. At 12 hpi, the supernatants were harvested and viral titers were determined by plaque assay. At least three independent experiments were performed, and one representative experiment was presented. The data were represented as mean ± standard deviation (SD) of the duplicate measurements.
Lycorine Inhibits Viral Replication Through Blocking Viral Translation
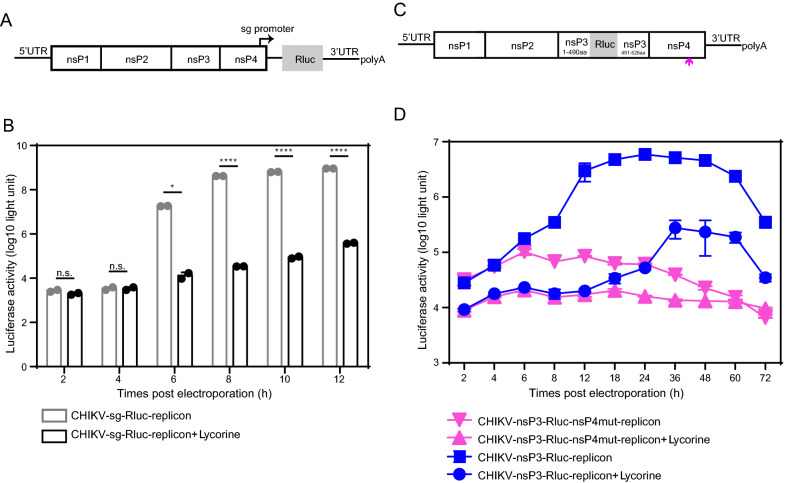
Furthermore, we analyzed the inhibitory effect of lycorine on viral RNA synthesis using a CHIKV-sg-Rluc replicon in which the structural proteins are replaced with Renilla luciferase (Rluc) reporter gene (Fig. 5A). As shown in Fig. 5B, CHIKV-sg-Rluc replicon RNA generated a sharp increase in luciferase signals at 6 hours post transfection (hpt) followed by steadily climbing till the end time-point of the experiment (12 hpt), while lycorine treatment dramatically inhibited luciferase activities from 6 to 12 hpt.
Fig. 5.
Inhibition of CHIKV RNA replication by lycorine. A Schematic representation of a sg promoter-initiating luciferase-reporter replicon of CHIKV. The CHIKV-sg-Rluc-replicon was constructed by replacing the structural protein genes with a Renilla luciferase reporter gene that was initiated by a sg promoter. B Comparison of the replication efficiencies of the CHIKV-sg-Rluc-replicon in the presence of lycorine or not. Equal amounts of the replicon RNAs (5 μg) were electroporated into BHK-21 cells with or without 10 μmol/L of lycorine and assayed for luciferase activities at the indicated time points post transfection. C Schematic representation of the CHIKV-nsP3-Rluc-replicon. The construct was obtained by inserting the Renilla luciferase reporter gene into the non-structural protein gene nsP3. The arrow below the diagram denotes the position of the GDD to GAA mutation in the catalytic site of nsP4. D Comparison of the replication efficiencies of the CHIKV-nsP3-Rluc replicon and CHIKV-nsP3-Rluc-nsP4mut replicon constructs in the presence of lycorine or not. Equal amounts of the construct RNAs (5 μg) were electroporated into BHK-21 cells with or without 10 μmol/L of lycorine and assayed for luciferase activities at the indicated time points post transfection. At least three independent experiments were performed, and one representative experiment was presented. The data were represented as mean ± standard deviation (SD) of the duplicate measurements. Statistical analysis was performed with two-way ANOVA, and the asterisks denote statistical differences between the indicated groups. *P < 0.05; ****, P < 0.0001; n.s., no statistical difference.
Because it was difficult to differentiate between replicon RNA translation and RNA synthesis using the CHIKV-sg-Rluc system, we then used a CHIKV-nsP3-Rluc replicon in which the structural proteins were deleted and a Rluc reporter gene was fused in frame within nsP3 (Fig. 5C) to examine the alterations of Rluc signals when treated with lycorine. Similarly, treatment with lycorine resulted in a significant reduction in luciferase signals in Vero cells transfected with CHIKV-nsP3-Rluc RNA, occurring earlier at 2 hpt. In order to determine the time point when viral translation switches to viral RNA replication, an RNA-dependent RNA polymerase (RdRP)-inactivated replicon in the context of CHIKV-nsP3-Rluc replicon, CHIKV-nsP3-Rluc-nsP4mut containing GDD-to-GAA amino acid mutations in the conserved catalytic motif of nsP4 RdRP domain was constructed. CHIKV-nsP3-Rluc and CHIKV-nsP3-Rluc-nsP4mut replicon RNAs generated similar extents of Rluc signal increase at 2 and 4 hpt, whereas Rluc activities in CHIKV-nsP3-Rluc-nsP4mut-transfected cells remained at a low level at 6 hpt (about 105) and decreased thereafter due to its disability to synthesize viral RNA in contrast to peaking to as high as about 107 at 24 hpt in CHIKV-nsP3-Rluc-transfected cells. Such discrepancies of time-dependent signal changes between these two replicons indicate that for the first four hours, translation of input replicon RNA takes place; and after 4 h Rluc signals reflect replicon RNA replication. It should be noted that, in the case of CHIKV-nsP3-Rluc-nsP4mut transfection, an early inhibitory effect of lycorine on Rluc activities was also observed at 2 hpt. It thus indicates that lycorine might exert its antiviral activity through direct inhibition of translation.
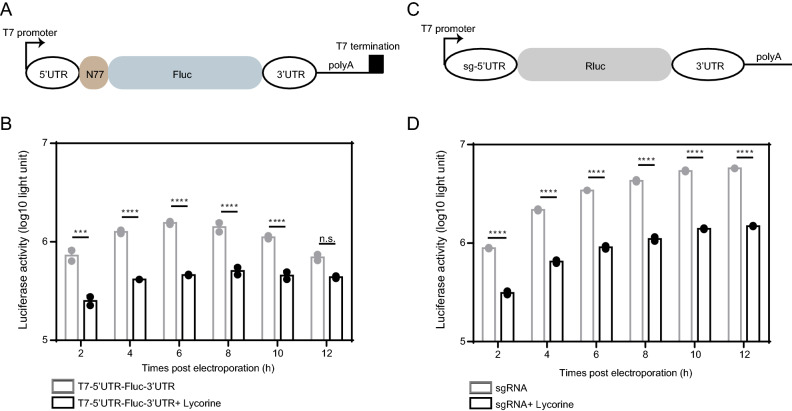
To further confirm the inhibition of viral RNA translation by lycorine, we prepared two sets of CHIKV mini-genome systems: the 5′UTR-Fluc-3′UTR CHIKV mini-genome in which firefly luciferase (Fluc) reporter gene was inserted in frame between 5′ and 3′ UTRs of CHIKV (Fig. 6A) and the 5′sub-UTR-Rluc-3′UTR CHIKV mini-genome in which 5′UTR of subgenomic RNA was used to replace 5′UTRs of viral genome (Fig. 6C). The activities of Fluc and Rluc could be used to surrogate viral RNA translation directed by 5′-UTR and the sg promoter of CHIKV, respectively. As shown in Fig. 6B and 6D, both luciferase activities were greatly inhibited by lycorine. Altogether, these data suggest that lycorine inhibits viral propagation by targeting the translation of both genomic and subgenomic RNAs.
Fig. 6.
Inhibition of CHIKV translation by lycorine. A Schematic representation of the T7 promoter-based construct expressing firefly luciferase. The T7-5′UTR-Fluc-3′UTR construct contains the 5′ and 3′UTR of CHIKV, the 77 N-terminal amino acid residues of nsP1, a firefly luciferase reporter gene, and a polyadenylation region followed by a T7 transcription terminator. B Comparison of the translation efficiencies of the T7-5′UTR-Fluc-3′UTR construct in the presence of lycorine or not. Equal amounts of the construct RNAs (5 μg) were electroporated into BHK-21 cells with or without 10 μmol/L of lycorine and assayed for luciferase activities at the indicated time points posttransfection. C Schematic representation of the T7 promoter-based sgRNA expressing Renilla luciferase. The sgRNA contains the sg-5′UTR and 3′UTR of CHIKV, a Renilla luciferase reporter gene and a polyadenylation region. D Comparison of the translation efficiencies of the sgRNA in the presence of lycorine or not. Equal amounts of the sgRNAs (5 μg) were electroporated into BHK-21 cells with or without 10 μmol/L of lycorine and assayed for luciferase activities at the indicated time points post transfection. At least three independent experiments were performed, and one representative experiment was presented. The data were represented as mean ± standard deviation (SD) of the duplicate measurements. Statistical analysis was performed with two-way ANOVA, and the asterisks denote statistical differences between the indicated groups. ***, P < 0.001; ****, P < 0.0001; n.s., no statistical difference.
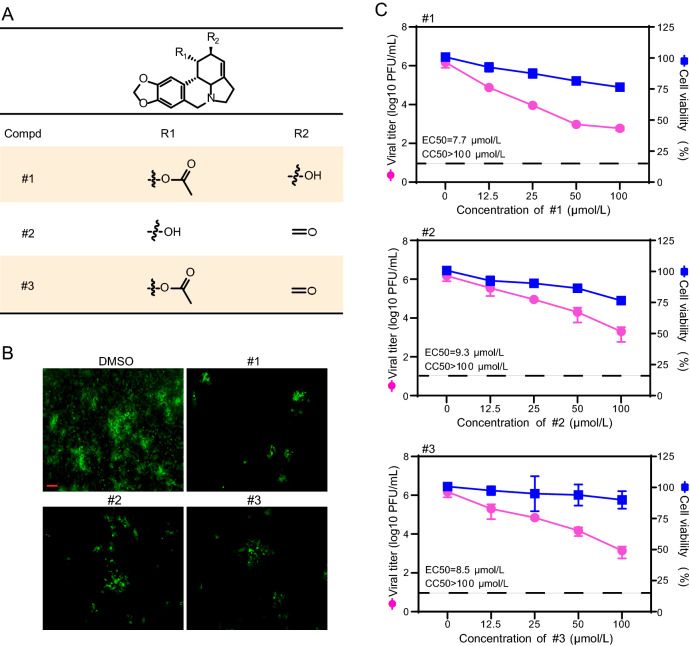
Antiviral Activity of Lycorine Derivatives Against CHIKV Infection
It has been reported previously that the antiviral activity of lycorine could be improved by modifying its two hydroxyl groups at C1 and/or C2 positions (Zou et al. 2009). We then tested the antiviral profiles of three lycorine derivatives with these two hydroxyl group modifications using a eGFP-CHIKV reporter virus (Deng et al. 2016) (Fig. 7A). Those derivatives of lycorine were identified to inhibit viral replication efficiently, as listed in Fig. 7B and 7C. Their antiviral activities highlight modified spots for improving antiviral efficiency although the tested derivatives displayed less efficacy to inhibit CHIKV replication than lycorine. Our results clearly indicate that the modification of lycorine is one of possible ways to improve antiviral profile of lycorine which is worth to explore in our future study.
Fig. 7.
Inhibitory effects of lycorine derivatives on CHIKV. A Chemical structures of lycorine derivatives. B Detection of eGFP expression by fluorescent microscope in the presence of different lycorine derivatives. Vero cells infected with eGFP-CHIKV at an MOI of 0.01 were treated with 10 μmol/L of lycorine derivatives. The eGFP expression was detected under a fluorescent microscope at 36 hpi. The length of the scale bar (displayed in a red line segment) represents 100 μm. C Dose-dependent inhibition of lycorine derivatives on CHIKV infection. Vero cells were infected with WT CHIKV at an MOI of 0.01 and treated with the increasing concentrations of lycorine derivatives. The viral titers in culture media at 24 hpi were quantified by plaque assay. The cytotoxicity of lycorine derivatives on cells was examined with CCK-8 assay kit. The concentration–response curves for antiviral and cytotoxic activities of lycorine derivatives are shown in pink and blue lines, respectively. The dashed lines in panel C represent the limit of detection. Two independent experiments were performed in triplicate. Data represent the mean ± standard deviation (SD) of the triplicate measurements in a representative experiment.
Discussion
Being an extracted alkaloid, lycorine has various biological functions for cancer and infectious diseases treatment (Liu et al. 2011). In the present study, we have demonstrated that lycorine could inhibit multiple alphaviruses replication in cell culture without cytotoxicity, which further broadened its antiviral spectrum combined with previous antiviral studies of different viral species (Zou et al. 2009; Liu et al. 2011; He et al. 2013; Shen et al. 2019; Wang et al. 2019; Zhang et al. 2020).
Different antiviral mechanisms of lycorine have been proposed by different groups. For influenza virus, the results of intracellular localization of nucleoprotein (NP) indicated that lycorine could inhibit nuclear-to-cytoplasm export of the RNP complex of virus replication at an early stage of a single-round and multi-round of replication (He et al. 2013). For enteroviruses, it has been reported that lycorine inhibits EV71 and CVA16 infection through downregulating autophagy that is important for virus replication (Wang et al. 2019). Alternately, blocking the elongation of the viral polyprotein during translation is another possible mechanism of lycorine against EV71 infection in cell culture (Liu et al. 2011). However, drug resistant selection indicated that viral protease may be the target of lycorine to inhibit viral replication of both EV71 and HCV (Guo et al. 2016). F76L mutation in EV71 2A protease and R118L mutation in HCV NS3 protease confer the resistance to lycorine in both viruses, respectively (Guo et al. 2016). Additionally, a single-amino acid substitution in WNV 2 K peptide was identified to confer resistance to lycorine, partially through enhancement of viral RNA synthesis or through modulation of host immune response.
In the present study, the mode-of-action analyses indicated that lycorine inhibits CHIKV replication through the suppression of viral translation. The translation of alphavirus includes both nonstructural and structural proteins synthesis which are directed by genomic RNA (gRNA) and sgRNA, respectively. The early translation of nonstructural proteins (nsP1–4) is essential for the formation of replication complex which is responsible for viral RNA replication and transcription. The translation of structural proteins derived from sgRNA happens in the late phase of the virus life cycle and plays a key role in viral assembly. Lycorine may act on the translation of both gRNA and sgRNA, and thus inhibit both viral RNA synthesis and viral assembly which result in a cumulative and strong inhibitory effect on the production of infectious viruses in cell culture.
Similar to cellular mRNAs, the alphavirus genomes contain a cap structure at their 5′ end and a poly(A) tail, and are translated through the canonical mechanism with cap structure recognition. Such RNA viruses as alphaviruses can manipulate the cellular translation machinery for their own benefit (Jaafar and Kieft 2019). Thus, it is very possible, plus consideration of its broad-spectrum antiviral activities, that lycorine modulates alphavirus replication through targeting host factors instead of directly targeting viral factors. It is worth to explore it in our future studies regarding the underlying mechanism by which lycorine interferes with translation machinery as well as about whether it has an inhibitory effect on host protein translation. Furthermore, it is also important to assess the possible side effect of lycorine during preclinical studies although a drug containing lycorine as an effective component has been clinically used in Russia as an expectorant to remedy chronic and acute inflammatory processes in lungs and bronchial diseases (Nair et al. 2013; Shen et al. 2018).
Acknowledgements
We are grateful to the staff of BSL-3 laboratory (Hao Tang) at Wuhan Institute of Virology. This work was supported by the National Key Research and Development Program of China (2018YFA0507201). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions
BZ, HQY and LQS designed the experiments. NL, ZW, RW, ZRZ, YNZ and CLD carried out the experiments. NL and BZ wrote the paper. All authors read and approved the final manuscript.
Compliance With Ethical Standards
Conflict of interests
The authors declare no conflict of interests.
Animal and Human Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Na Li and Zhen Wang have contributed equally to this work.
Contributor Information
Bo Zhang, Email: zhangbo@wh.iov.cn.
Lu-Qing Shang, Email: shanglq@nankai.edu.cn.
Han-Qing Ye, Email: yehq@wh.iov.cn.
References
- Abdelnabi R, Neyts J, Delang L. Towards antivirals against chikungunya virus. Antivir Res. 2015;121:59–68. doi: 10.1016/j.antiviral.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira AC, Campos GS, Sardi SI, Rocha VF, Rocha GC. Neonatal encephalitis due to chikungunya vertical transmission: first report in Brazil. IdCases. 2016;5:57–59. doi: 10.1016/j.idcr.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. In vitro inhibition of chikungunya and semliki forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antivir Res. 2004;61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Chen D, Cai J, Cheng J, Jing C, Yin J, Jiang J, Peng Z, Hao X. Design, synthesis and structure-activity relationship optimization of lycorine derivatives for HCV inhibition. Sci Rep. 2015;5:14972. doi: 10.1038/srep14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contopoulos-Ioannidis D, Newman-Lindsay S, Chow C, LaBeaud AD. Mother-to-child transmission of chikungunya virus: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006510. doi: 10.1371/journal.pntd.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Jaffar-Bandjee MC, Hoarau JJ, Krejbich Trotot P, Denizot M, Lee-Pat-Yuen G, Sahoo R, Guiraud P, Ramful D, Robin S, Alessandri JL, Gauzere BA, Gasque P. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog Neurobiol. 2010;91:121–129. doi: 10.1016/j.pneurobio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Deng CL, Liu SQ, Zhou DG, Xu LL, Li XD, Zhang PT, Li PH, Ye HQ, Wei HP, Yuan ZM, Qin CF, Zhang B. Development of neutralization assay using an eGFP chikungunya virus. Viruses. 2016;8(7):181. doi: 10.3390/v8070181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin P, Samperiz S, Ramful D, Boumahni B, Bintner M, Alessandri JL, Carbonnier M, Tiran-Rajaoefera I, Beullier G, Boya I, Noormahomed T, Okoi J, Rollot O, Cotte L, Jaffar-Bandjee MC, Michault A, Favier F, Kaminski M, Fourmaintraux A, Fritel X. Neurocognitive outcome of children exposed to perinatal mother-to-child chikungunya virus infection: the CHIMERE cohort study on reunion island. PLoS Negl Trop Dis. 2014;8:e2996. doi: 10.1371/journal.pntd.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Cao L, Wang P, Qing J, Zheng Q, Shang L, Yin Z, Sun Y. A conserved inhibitory mechanism of a lycorine derivative against enterovirus and hepatitis C virus. Antimicrob Agents Chemother. 2016;60:913–924. doi: 10.1128/AAC.02274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Qi WB, Wang L, Tian J, Jiao PR, Liu GQ, Ye WC, Liao M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir Viruses. 2013;7:922–931. doi: 10.1111/irv.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafar ZA, Kieft JS. Viral RNA structure-based strategies to manipulate translation. Nat Rev Microbiol. 2019;17:110–123. doi: 10.1038/s41579-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Thiruchelvan M, Lee RC, Chen H, Chen KC, Ng ML, Chu JJ. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57:155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerer BM, Grywna K, Glasker S, Wieseler J, Drosten C. Construction of an infectious chikungunya virus cDNA clone and stable insertion of mCherry reporter genes at two different sites. J Gen Virol. 2012;93:1991–1995. doi: 10.1099/vir.0.043752-0. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang YN, Deng CL, Shi PY, Yuan ZM, Zhang B. Replication-defective west nile virus with NS1 deletion as a new vaccine platform for flavivirus. J Virol. 2019;93:e00720–e00719. doi: 10.1128/JVI.00720-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang Y, Xu Y, Ma C, Qin C, Zhang L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol J. 2011;8:483. doi: 10.1186/1743-422X-8-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair JJ, Bastida J, Codina C, Viladomat F, Jv S. Alkaloids of the South African amaryllidaceae: a review. Nat Prod Commun. 2013;8:1335–1350. [PubMed] [Google Scholar]
- Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- Shen J, Zhang T, Cheng Z, Zhu N, Wang H, Lin L, Wang Z, Yi H, Hu M. Lycorine inhibits glioblastoma multiforme growth through EGFR suppression. J Exp Clin Cancer Res. 2018;37:157. doi: 10.1186/s13046-018-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Niu J, Wang C, Huang B, Wang W, Zhu N, Deng Y, Wang H, Ye F, Cen S, Tan W. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol. 2019 doi: 10.1128/JVI.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utt A, Quirin T, Saul S, Hellstrom K, Ahola T, Merits A. Versatile trans-replication systems for chikungunya virus allow functional analysis and tagging of every replicase protein. PLoS One. 2016;11:e0151616. doi: 10.1371/journal.pone.0151616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enter BJD, Huibers MHW, van Rooij L, Steingrover R, van Hensbroek MB, Voigt RR, Hol J. Perinatal outcomes in vertically infected neonates during a chikungunya outbreak on the island of curacao. Am J Trop Med Hyg. 2018;99:1415–1418. doi: 10.4269/ajtmh.17-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo T, Yang Y, Yu L, Pan X, Li Y. Lycorine derivative LY-55 inhibits EV71 and CVA16 replication through downregulating autophagy. Front Cell Infect Microbiol. 2019;9:277. doi: 10.3389/fcimb.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang JH, Zhang XL, Lao GJ, Su GM, Wang L, Li YL, Ye WC, He J. Tandem mass tag-based quantitative proteomic analysis of lycorine treatment in highly pathogenic avian influenza H5N1 virus infection. PeerJ. 2019;7:e7697. doi: 10.7717/peerj.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PT, Shan C, Li XD, Liu SQ, Deng CL, Ye HQ, Shang BD, Shi PY, Lv M, Shen BF, Qin CF, Zhang B. Generation of a recombinant west nile virus stably expressing the gaussia luciferase for neutralization assay. Virus Res. 2016;211:17–24. doi: 10.1016/j.virusres.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Zhang YN, Deng CL, Li JQ, Li N, Zhang QY, Ye HQ, Yuan ZM, Zhang B. Infectious chikungunya virus (CHIKV) with a complete capsid deletion: a new approach for a CHIKV vaccine. J Virol. 2019;93:e00504–00519. doi: 10.1128/JVI.00504-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YN, Zhang QY, Li XD, Xiong J, Xiao SQ, Wang Z, Zhang ZR, Deng CL, Yang XL, Wei HP, Yuan ZM, Ye HQ, Zhang B. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg Microbes Infect. 2020;9:1170–1173. doi: 10.1080/22221751.2020.1772676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G, Puig-Basagoiti F, Zhang B, Qing M, Chen L, Pankiewicz KW, Felczak K, Yuan Z, Shi PY. A single-amino acid substitution in west nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology. 2009;384:242–252. doi: 10.1016/j.virol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]