Abstract
Background
Older patients hospitalized for acute decompensated heart failure (ADHF) have high rates of physical frailty, poor quality-of-life, delayed recovery, and frequent rehospitalizations. However, physical frailty is not addressed in ADHF care pathways.
Methods
This was a multi-center, randomized, attention-controlled trial of a novel, transitional, tailored, progressive, multi-domain (strength, balance, mobility, endurance) rehabilitation intervention beginning early after hospital admission for ADHF. The primary outcome was the Short Physical Performance Battery (SPPB) score, a standardized measure of physical function validated in frail older persons, assessed at 3 months by blinded observers. The secondary outcome was 6-month all-cause rehospitalization rate.
Results
We enrolled 349 patients aged 60–99 years: 52% women, 49% non-white, 53% preserved ejection fraction. At baseline, patients had markedly impaired physical function, averaged five co-morbidities, and 97% were frail or pre-frail. Intervention adherence (87%) and retention (91%) were excellent. At 3-month follow-up in the intervention group relative to attention control, SPPB increased 1.5±0.4 units (p<0.001), 6-minute walk distance increased 34±11 meters (p=0.003), Fried frailty criteria improved by −0.3±0.1 (p=0.033), quality-of-life (Kansas City Cardiomyopathy Questionnaire) increased 7±3 units (p=0.007), and depression (Geriatric Depression Survey-15) decreased 0.7±0.3 units (p=0.018). At 6-month follow-up, there were no significant differences in all-cause rehospitalizations, which were frequent (1.2/participant), or death. There were 3 serious adverse events possibly related to the intervention, all self-limited.
Conclusions
A novel, transitional, tailored, progressive, multi-domain physical rehabilitation intervention was feasible, safe, and resulted in improved physical function, frailty, quality-of-life, and depression in a diverse population of older patients hospitalized for ADHF.
Funded by National Institute on Aging; REHAB-HF ClinicalTrials.gov number: NCT02196038
Keywords: Heart Failure, Aging, Frailty, Physical Function, Rehabilitation, Clinical trial, Rehospitalization
INTRODUCTION
Acute decompensated heart failure (ADHF) is the leading cause of hospitalization in older persons.1 Hospitalized ADHF is associated with poor health-related quality-of-life, frequent rehospitalizations, high mortality and costs >$39 billion/year.1,2 Fifty-percent of older ADHF patients experience rehospitalization or death within 6 monhths.2 Most intervention trials aimed at improving ADHF outcomes have been neutral, suggesting that outcomes in older ADHF patients may be driven partly by mechanisms that have been overlooked.3–5
Among older ADHF patients, physical function is markedly impaired and frailty rates and comorbidity burden are high.5–8 Even when stable and well-compensated, older patients with chronic HF have severe impairments in physical function due to combined effects of aging, cardiovascular dysfunction, and skeletal muscle dysfunction.9,10 As patients with chronic HF transition to ADHF, physical function worsens further and is exacerbated by the hospital experience and bedrest.8 These deficits often persist. Many patients never recover baseline function, lose independence, and have high rates of re-hospitalization and death following discharge (“post-hospital syndrome”).4,5,11–14
However, management guidelines do not address physical dysfunction in patients hospitalized for ADHF,15 and prior studies of exercise training in HF generally excluded patients with recent hospitalization.10,16 To address these important gaps in ADHF management, we conducted a randomized, attention-controlled, single-blind trial of a novel, transitional, tailored, progressive, multi-domain physical rehabilitation intervention. We hypothesized that this intervention would improve physical function and reduce all-cause 6-month rehospitalizations.
METHODS
Trial design and oversight
Trial design and intervention methods have been previously described.17,18 The steering committee designed the trial and oversaw operations. The trial protocol was approved by institutional review boards at sites. An independent data and safety monitoring committee evaluated patient safety. The authors vouch for the accuracy and completeness of the data and all analyses and for the fidelity of this report to the trial protocol.
Population.
Participants were screened at hospital admission and enrolled prior to discharge. The trial included patients ≥60 years old who were admitted for ADHF with reduced or preserved ejection fraction (HFrEF, HFpEF), could walk ≥4 meters with assistive device if needed, were functionally independent prior to admission, and were expected to be discharged home. Key exclusion criteria were: end-stage HF or kidney disease, inability to participate due to dementia, stroke or other disorders, and already participating in regular exercise.17 Full inclusion/exclusion criteria are in Supplement pages 4-5.
Trial conduct
Randomization.
Following consent and completion of baseline testing, participants were randomized with equal probability to either Rehabilitation Intervention or Attention Control using block randomization and stratification by ejection fraction (<45% or ≥45%) and clinical site.
Usual care.
Both study arms received usual care as recommended by their medical providers, which could include inpatient or outpatient physical therapy and standard cardiac rehabilitation.
Attention Control.
The Attention Control group participants received bi-weekly phone contact and in-person clinic visits at 1-month and 3-month intervals following discharge from the index hospitalization.17 Symptoms, events, and non-study rehabilitation therapy were collected. They received no specific recommendations regarding exercise; but were encouraged to adhere to prescribed therapy and follow-up appointments.
The REHAB-HF Intervention.
As previously described,18,19 the intervention was an innovative, transitional, tailored, progressive, one-on-one, multi-domain physical rehabilitation program developed for frail, older patients with ADHF and designed to start as soon as possible following hospital admission. The intervention focused on 4 domains: strength, balance, mobility, and endurance which were stratified at every session from a level of 1–4 (Table S2). The progression of exercise intensity and modes was individualized based upon performance level within each domain.18 A key goal was to increase endurance (walking time), but doing this safely required first addressing deficits in balance, strength, and mobility.
The intervention began in the hospital whenever possible, and transitioned to the outpatient facility as soon as possible after discharge. If needed, home-based sessions were provided by therapists initially until the participant was physically able to attend the facility-based outpatient sessions. Outpatient sessions were 60 minutes, 3 days/week for 12 weeks or 36 sessions. Facility-based outpatient sessions were complemented by home exercise (low-intensity walking and strengthening exercises, gradually increasing toward a goal of 30 minutes daily) on non-program days initiated following a study staff visit to evaluate the participant’s home environment.18 A key goal which was addressed early and throughout the first 3 months of one-on-one supervised sessions was to prepare the patient to transition to the independent maintenance phase during months 4–6. At the 3-month visit, participants were transitioned to the maintenance phase, provided with individualized exercise prescriptions, and followed by phone calls. Additional intervention details are on Supplement pages 7-8.
Participant retention and intervention adherence and fidelity were reviewed and discussed every two weeks by the Sustaining Participant Engagement Committee using methods consistent with the NIH Behavior Change Consortium Treatment Fidelity Workgroup recommendations.18
Blinding.
Physical and cognitive function outcome measures were assessed by personnel who were blinded to randomization assignment.
Assessment schedule.
Physical function, quality-of-life, depression, and cognition function were assessed at baseline in the hospital and at 3-months.17 Clinical events were collected throughout follow-up by monthly interview and medical record review.
Trial outcomes
Primary outcome.
The primary outcome was total Short Physical Performance Battery (SPPB) score at 3 months. The SPPB is a standardized, reproducible measure of global physical function validated in frail older persons that predicts a wide range of clinical outcomes.20,21 It has 3 components: a standing balance test, gait speed (4-meter walk) test, and strength test (time to complete 5 chair rises). Each component is scored 0–4 for a total score ranging from 0–12 where lower scores indicate more severe physical dysfunction.
Secondary outcome.
The secondary outcome was all-cause rehospitalizations at 6 months, defined as a hospital stay >24 hours. Rehospitalizations were further categorized as non-cardiovascular, HF, or other cardiovascular by an independent, blinded adjudicator.
Other key outcomes.
Additional physical function outcomes included 6-minute walk distance (6MWD), Fried frailty criteria, hand-grip strength, and gait speed. Quality-of-life was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the EuroQol Visual Analog Scale. Other measures included the Geriatric Depression Scale-15 (GDS-15) and the Montreal Cognitive Assessment (MoCA).
Statistical Analyses.
Based on pilot trial results,19 258 evaluable patients were required for 80% power to detect a 10% (0.6 units) difference in mean SPPB score at 3 months, and 334 evaluable patients were required to provide 80% power to detect a 25% difference in 6-month rehospitalization rate. To allow for ~7% dropouts, recruitment targeted 360 participants.
Analyses were conducted in SAS Enterprise Guide version 7.11 and SAS 9.4 (Cary, NC). Baseline characteristics were summarized using means±standard deviations for continuous variables or counts and percentages for categorical variables. Analyses of covariance were used to assess differences between intervention groups for 3-month outcomes (including primary outcome) with adjustment for baseline measure, clinical site, EF category, age, and sex. Group differences in clinical events based on counts were assessed with Poisson regression with adjustment for clinical site, EF category, age, and gender. The secondary outcome, all-cause rehospitalization, was also adjusted for baseline SPPB score. Differences in proportion-based clinical measures were analyzed with logistic regression adjusted for clinical site, ejection fraction category, age, and sex. All-cause rehospitalization days were analyzed using negative binomial regression due to over-dispersion. A p-value of 0.05 was used for determining statistical significance. Testing was not hierarchical. Confidence intervals for secondary and exploratory outcomes were not adjusted for multiplicity to allow comparisons with other studies. The consistency of intervention effects among pre-specified subgroups was described using forest plots and tests for interactions. Analyses for missingness using inverse probability weighting did not materially alter results.
RESULTS
Study Enrollment and Follow-up.
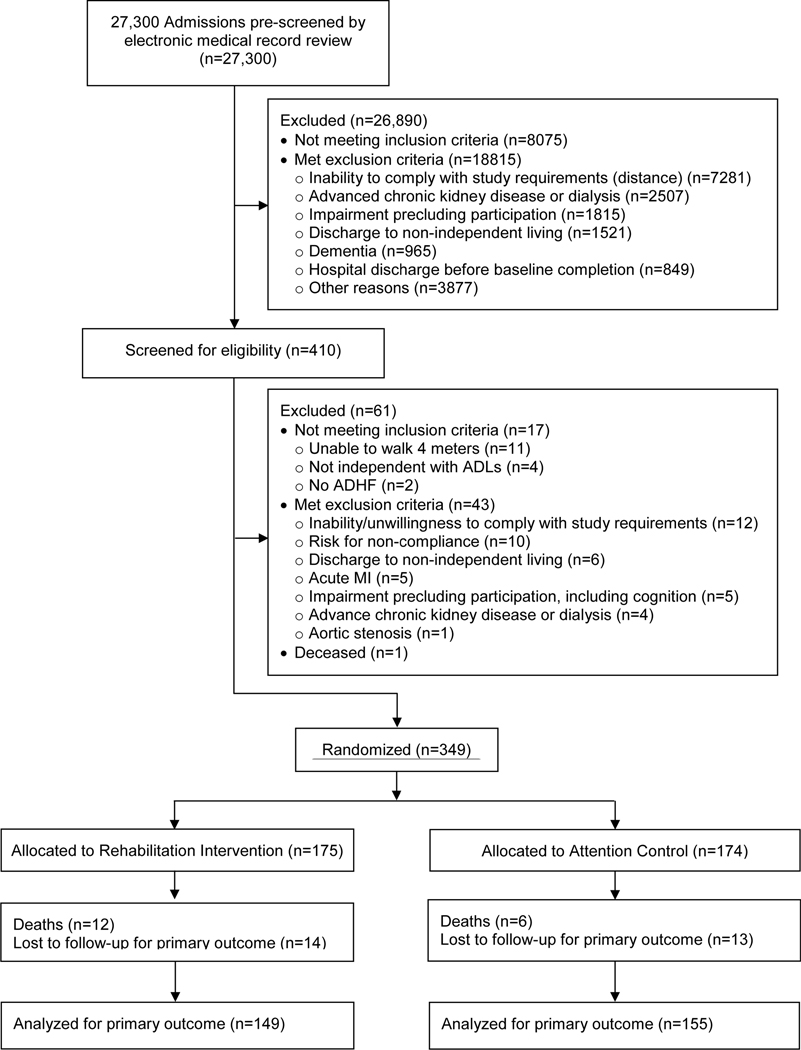
The DSMB approved stopping enrollment at 349 participants due to lower than expected dropout rate; 175 were randomly assigned to Rehabilitation Intervention and 174 to Attention Control. The primary outcome could not be collected in 18 participants due to death and in 27 for other reasons (87.1% collection rate; Figure 1). The secondary outcome was obtained in 99.4% (Figure S1).
Figure 1.
CONSORT Diagram for the Primary Outcome (SPPB at 3-month Follow-up).
Participant Characteristics.
Baseline characteristics are in Table 1 and Table S3; reasons for exclusion are in Table S2. Patients were age 72.7±8.1 years with 52% women and 49% non-white (94% Black/African-American). HFpEF was more frequent than HFrEF (53% vs. 47%). Patients had high comorbidity burdens and high rates of all-cause (~45%) and HF hospitalization (~25%) in the previous 6 months. Most (97%) participants met Fried criteria for frail or pre-frail. Geriatric conditions, including urinary incontinence, falls, and cognitive dysfunction were common. Over 50% of participants had diabetes mellitus, with higher prevalence in the intervention group (58% vs. 47%).
Table 1.
Baseline Characteristics of REHAB-HF Participants by Treatment Group
| Characteristics | Rehabilitation Intervention (N=175) | Attention Control (N=174) |
|---|---|---|
| Age | 73.1±8.5 | 72.2±7.7 |
| Female | 85 (49%) | 98 (56%) |
| Non-white | 81 (46%) | 91 (52%) |
| BMI (kg/m2) | 32.9±8.2 | 33.0±8.9 |
| Ejection fraction ≥45% | 93 (53%) | 92 (53%) |
| NYHA Class | ||
| II | 33 (19%) | 34 (20%) |
| III | 100 (57%) | 90 (52%) |
| IV | 41 (23%) | 51 (29%) |
| B-type natriuretic peptide, pg/mL (n=204), median (IQR) | 595 (259–1292) | 645 (381–1072) |
| N-terminal proBNP, pg/mL (n=117), median (IQR) | 2527 (1395–4858) | 3615 (1874–8637) |
| Days Hospitalized at Index Hospitalization, median (IQR) | 4 (3–7) | 5 (3–7) |
| Previous Hospitalizations in previous 6 months | 76 (43%) | 80 (46%) |
| Total Comorbidities | 5.4 ± 2.0 | 5.0 ± 1.9 |
| Hypertension | 159 (91%) | 162 (93%) |
| History of myocardial infarction | 31 (18%) | 32 (18%) |
| History of coronary revascularization (PCI, CABG) | 55 (31%) | 47 (27%) |
| Atrial fibrillation | 89 (51%) | 87 (50%) |
| Diabetes mellitus | 101 (58%) | 81 (47%) |
| Hyperlipidemia | 110 (63%) | 120 (69%) |
| Chronic obstructive pulmonary disease | 54 (31%) | 44 (25%) |
| Chronic kidney disease | 59 (34%) | 58 (33%) |
| Stroke | 26 (15%) | 26 (15%) |
| Peripheral vascular disease | 27 (15%) | 13 (7%) |
| Arthritis, muscle/joint pain, or connective tissue disease | 84 (48%) | 70 (40%) |
| History of Cancer | 42 (24%) | 33 (19%) |
| Sleep apnea or sleep disordered breathing | 68 (39%) | 57 (33%) |
| Depression (by EMR documentation) | 29 (17%) | 33 (19%) |
| Geriatric Conditions | ||
| Dementia or cognitive impairment (by EMR documentation) | 6 (3%) | 4 (2%) |
| Frailty (by Fried Criteria) | 92 (53%) | 100 (57%) |
| Urinary incontinence* | 19 (13%) | 21 (15%) |
| Patients with falls in previous 3 months† | 24 (16%) | 20 (14%) |
| Cardiac Medications at discharge | ||
| Loop diuretic | 162 (93%) | 164 (95%) |
| Beta-blocker | 138 (79%) | 138 (80%) |
| Angiotensin-converting enzyme inhibitors | 65 (37%) | 66 (38%) |
| Angiotensin II receptor blockers | 38 (22%) | 37 (21%) |
| Aldosterone antagonist | 29 (17%) | 34 (20%) |
| Sacubitril/valsartan | 1 (1%) | 1 (1%) |
| Digoxin | 8 (5%) | 11 (6%) |
Values shown as N (%) or mean±SD, unless otherwise specified. Abbreviations: BMI: body mass index; NYHA: New York Heart Association; BNP: B-type natriuretic peptide; HF: heart failure; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; EMR: electronic medical record.
Data collection in Attention Control=142, Rehabilitation Intervention=144;
Data collection in Attention Control=143, Rehabilitation Intervention=146
Baseline Measures.
At baseline, physical function was severely impaired with mean SPPB score of 6.0±2.7 units (Table 2; Figures S3, S4), 6MWD of 194±105 meters, and gait speed of 0.60±0.23 m/sec. Disease-specific (KCCQ) and general quality-of-life (Visual Analog Scale) were severely impaired. Cognitive impairment by MoCA and depression by GDS-15 scores were common.
Table 2.
Outcome Measures by Treatment Group
| Rehabilitation Intervention (n=149) | Attention Control (n=155) | Effect Size (95%CI) | p-value | |||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||
| 3-month Outcomes | ||||||
| SPPB Score (Primary Outcome) | 6.0±2.8 | 8.3±0.2 | 6.1±2.5 | 6.9±0.2 | 1.5 (0.9, 2.0) | <0.0001 |
| Balance Score | 2.6±1.3 | 3.2±0.1 | 2.7±1.3 | 2.9±0.1 | 0.4 (0.1, 0.6) | 0.007 |
| 4-meter Walk Score | 2.3±1.0 | 3.0±0.1 | 2.3±1.0 | 2.5±0.1 | 0.5 (0.2, 0.7) | <0.0001 |
| Chair Rise Score | 1.1±1.2 | 2.1±0.1 | 1.2±1.2 | 1.5±0.1 | 0.6 (0.4, 0.9) | <0.0001 |
| 6 Minute Walk Distance (m) | 194±104 | 293±8 | 193±107 | 260±8 | 34 (11, 56) | 0.003 |
| Gait Speed (m/s) | 0.60±0.23 | 0.80±0.02 | 0.61±0.22 | 0.68±0.02 | 0.12 (0.07, 0.16) | <0.0001 |
| Handgrip Strength (kg) | ||||||
| Men | 30.3±9.5 | 30.1±0.7 | 30.5±10.7 | 30.6±0.7 | -0.5 (−2.6, 1.7) | 0.67 |
| Women | 20.7±7.3 | 21.2±0.6 | 19.6±6.6 | 21.4±0.6 | -0.1 (−1.7, 1.5) | 0.88 |
| Modified Fried Frailty Criteria*, # | 2.3±1.1 | 1.4±0.1 | 2.4±1.1 | 1.6±0.1 | -0.3 (−0.5, −0.0) | 0.033 |
| KCCQ Overall Score | 40±21 | 69±2 | 42±21 | 62±2 | 7 (2, 12) | 0.007 |
| EuroQol Visual Analogue Scale | 58±22 | 71±2 | 58±21 | 64±2 | 7 (3, 12) | 0.003 |
| Cognition (MoCA Score) | 21.9±4.2 | 22.2±0.3 | 21.8±4.6 | 22.5±0.3 | -0.2 (−1.1, 0.6) | 0.56 |
| Depression (GDS-15 Score) | 4.6±3.3 | 3.3±0.2 | 4.7±3.3 | 4.1±0.2 | -0.7 (−1.3, −0.1) | 0.018 |
| 6-month Clinical Events | Rehabilitation Intervention (n=174) | Attention Control (n=173) | Effect Size (95%CI) | p-value | ||
| All-cause rehospitalizations at 6 months follow-up, rate (Secondary Outcome) | 1.22 (1.04, 1.43) | 1.35 (1.15, 1.57) | 0.90 (0.74, 1.10) | 0.32 | ||
| Deaths, rate | 0.11 (0.07, 0.19) | 0.09 (0.05, 0.16) | 1.17 (0.61, 2.27) | 0.63 | ||
| Combined all-cause rehospitalizations and death at 6 months, rate | 1.36 (1.17, 1.59) | 1.47 (1.27, 1.70) | 0.93 (0.77, 1.12) | 0.44 | ||
| Heart failure rehospitalizations, rate | 0.59 (0.46, 0.75) | 0.69 (0.55, 0.87) | 0.85 (0.65, 1.13) | 0.26 | ||
| Patients with ≥2 all-cause rehospitalizations, proportion | 0.29 (0.22, 0.38) | 0.37 (0.29, 0.46) | 0.71 (0.44, 1.13) | 0.14 | ||
| Patients with ≥2 heart failure rehospitalizations, proportion | 0.12 (0.07, 0.20) | 0.15 (0.09, 0.24) | 0.78 (0.41, 1.46) | 0.43 | ||
| All-cause rehospitalization days | 8.9 (6.2, 12.7) | 9.1 (6.6, 12.6) | 0.98 (0.64, 1.49) | 0.92 | ||
| Patients with ≥1 fall, proportion | 0.25 (0.18, 0.34) | 0.34 (0.26, 0.43) | 0.67 (0.42, 1.06) | 0.09 | ||
| Patients with ≥1 injurious fall, proportion | 0.06 (0.03, 0.12) | 0.09 (0.05, 0.15) | 0.66 (0.30, 1.47) | 0.31 | ||
Baseline data presented as means±SD. Follow-up data presented as LSmeans±SE adjusted for baseline value, clinical site, age, sex, and EF category. Clinical event data presented as LSmeans (95% CI) of rate (counts per patient) or proportion of patients adjusted for clinical site, age, sex, and EF category. All-cause rehospitalization at 6 months also adjusted for baseline SPPB score. Clinical event effect sizes of rates are shown as rate ratios and proportions are shown as odds ratios. KCCQ and Visual Analogue Scale scores range 0–100, with higher score meaning better health status. MoCA score ranges 0–30 with higher score meaning better cognitive function. GDS-15 score ranges 0–15 with higher score meaning worse depressive symptoms. Abbreviations: SPPB: short physical performance battery; KCCQ: Kansas City Cardiomyopathy Questionnaire; MoCA: Montreal Cognitive Assessment; GDS-15: Geriatric Depression Scale.
Modified Fried Frailty Criteria excludes weight loss criteria due to difficulty in ascertaining weight changes due to fluid status.
Intervention Retention, Adherence, and Progression.
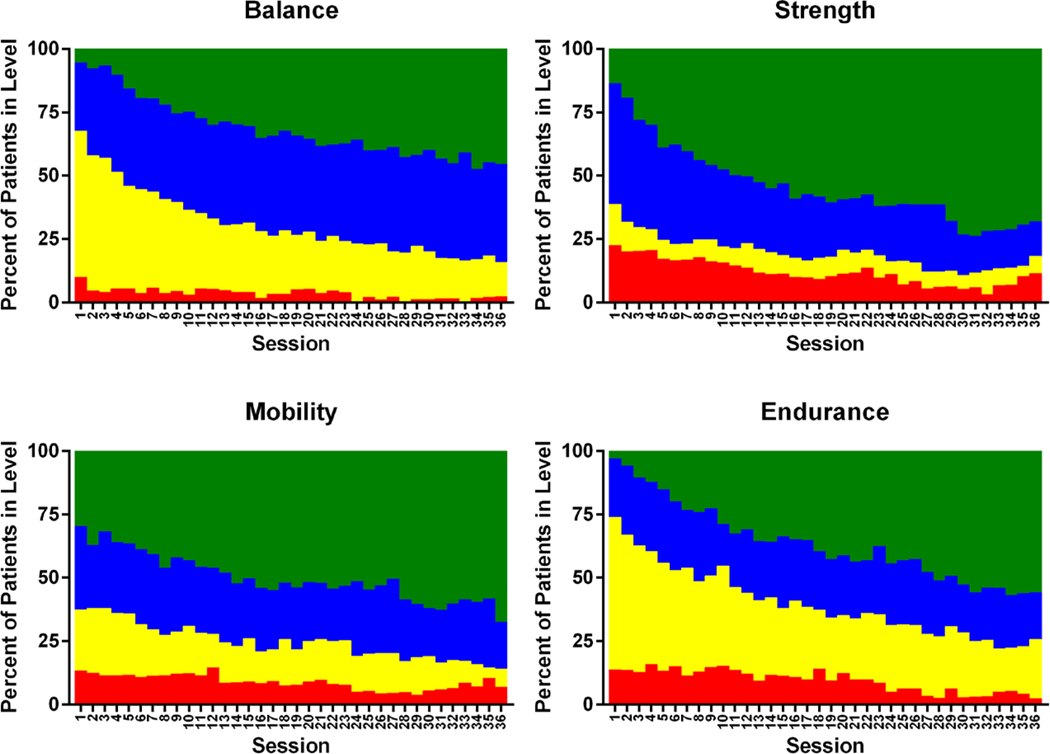
Of the 175 participants randomized to Rehabilitation Intervention, 12 died prior to completing the intervention, 14 dropped from the study, and 16 discontinued intervention participation but provided the primary and secondary outcomes. Intervention retention was 82%, excluding participants who died (Figure 1). Excluding participants who died or dropped but including participants who discontinued, participants completed 26.1±0.9 outpatient intervention sessions for an intervention adherence rate of 73±3%. After adjusting for sessions that had to be missed due to medical appointments and illness, intervention adherence rate for the outpatient facility-based sessions was 80±2% (Table S7). Participants progressed to higher stratification levels in each domain throughout the intervention (Figure 2). A key goal was increasing endurance exercise time, which progressed more than two-fold from 10.7±5.9 to 22.0±11.1 minutes from the first to last session. Additional data regarding exercise during inpatient, outpatient, and maintenance phases are in Tables S8-10; non-study therapy is in Table S11.
Figure 2.
Progression of physical function across intervention sessions by exercise domain. As previously described (Reeves, American Heart Journal, 2017; Pastva, Contemporary Clinical Trials, 2018), each exercise session was comprised of 4 domains (balance, strength, mobility, endurance) and four stratification levels (Level 1: red, Level 2: yellow; Level 3: blue; Level 4: green) corresponding to increasing thresholds of functional ability. For all domains, as number of sessions increased throughout the duration of the rehabilitation intervention, proportion of participants at higher levels (3 and 4) generally increased, while lower levels (1 and 2) generally decreased.
Safety.
Over the 4052 intervention sessions, 3 serious adverse events were judged by site investigators as possibly related to the intervention, all self-limiting (Table S4). There were 17 non-serious adverse events judged possibly related to the intervention, all self-limiting (Table S5).
Primary outcome.
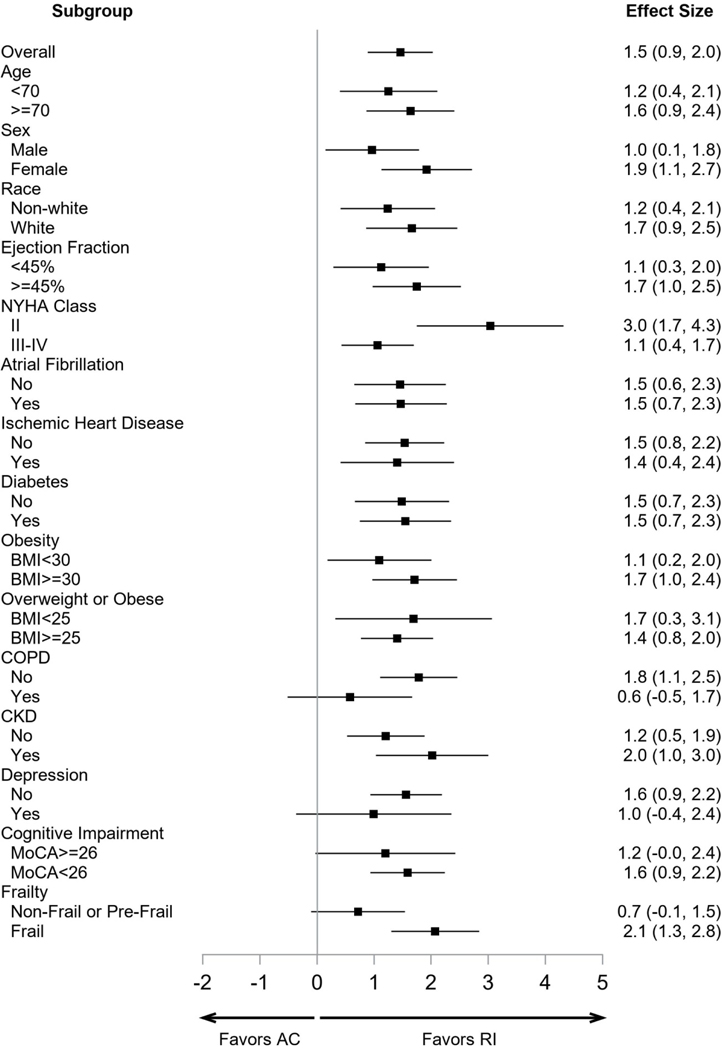
SPPB score at 3-months showed a large (1.5 units; CI:0.9–2.0) improvement in Rehabilitation Intervention relative to Attention Control (p<0.0001) (Table 2; Figure S3) which was relatively uniform across a wide variety of pre-specified subgroups (Figure 3). There were large improvements in all SPPB components (Table 2).
Figure 3.
Forest plot of the effect sizes (units and 95% Confidence Intervals) of the intervention of the pre-specified subgroups for the primary outcome, total SPPB Score. The effect size was relatively large and uniform across a broad range of key subgroups. Abbreviations: RI: Rehabilitation Intervention; AC: Attention Control.
Secondary outcome.
There was no significant difference in the secondary outcome, all-cause rehospitalization rate at 6-month follow-up (Table 2).
Other key outcomes.
There were large improvements in the Intervention versus Control group in 6MWD, gait speed, number of Modified Fried Frailty Criteria, quality-of-life, and depression (Table 2; Figure S4). The rate of overall falls was nominally lower (−33%; p=0.09) in the Rehabilitation Intervention group. Cognition was unchanged. There were 37 on-trial deaths, none related to intervention or study procedures. Causes of death are in Table S6. There were no significant differences in deaths or other clinical event outcomes (Table 2).
DISCUSSION
REHAB-HF was designed to address several critical evidence gaps regarding physical rehabilitation in HF patients. Prior trials excluded patients who were acutely hospitalized within the previous 6 weeks, which is when the severity of physical dysfunction and risk of clinical events are highest, and had relatively few older frail patients with multiple comorbidities who may require different approaches.3,10 REHAB-HF enrolled a diverse older cohort with high comorbidity burden, severe physical dysfunction, poor health-related quality-of-life, and high rates of frailty and depression, characteristics typical of older patients hospitalized for ADHF. Physical dysfunction, frailty, and depression are often unrecognized clinically in ADHF patients,8,22 are generally not addressed in clinical care pathways,10,23 and likely contribute to delayed, incomplete recovery and high rates of rehospitalization, death, and long-term loss of independence following hospital discharge.2,11,12,14 Indeed, the Attention Control group continued to demonstrate severe physical dysfunction three months following discharge. The novel, transitional, tailored, progressive, multi-domain REHAB-HF intervention was feasible, safe, had high adherence and retention, and was associated with large, significant, clinically meaningful improvements in SPPB. The SPPB is a well-established, easily administered, valid, reliable measure of physical function in frail older adults, and is predictive of important clinical outcomes, including disability, hospitalizations, nursing home admission, and death,20,21,24 including specifically in older patients hospitalized for ADHF.5 Improvements in 6MWD, Fried Frailty score, quality-of-life, and depression were also substantial. Over 6-months follow-up, there were high rates of all-cause rehospitalizations (average 1.2 per participant), HF rehospitalizations, and deaths (10%), but no significant intervention-related reduction.
Physical function impairments in these older, frail, hospitalized ADHF patients were much broader and more severe than observed in chronic stable HF.22 For example, in this cohort of patients who’d been stabilized and were being prepared for discharge to home, average baseline 6MWD was half that observed in the HF-ACTION trial,25 and severe lower extremity weakness prevented nearly one-third from standing even once from a seated position without the use of arms. The patients also had severe deficits in balance and mobility, and frequent history of falls and other geriatric conditions, which are not typically seen in chronic, stable HF and which are not addressed by conventional cardiac rehabilitation. Initiating standard endurance exercise training in such frail, older patients without first addressing deficits in balance and mobility can limit efficacy26 and increase the risk of injuries and falls.27,28
The novel REHAB-HF intervention was specifically designed to safely address this pattern of physical function deficits. The intervention was transitional (hospital-outpatient-home), tailored to individual patients’ deficits in each domain, and focused on improving deficits in balance, mobility, and strength before emphasizing endurance training. The result was steady progression in each of the four domains culminating in large improvements in overall functional performance and endurance (Figure 4) and low rate of falls. These salutary results were achieved in a diverse group of older participants with high rates of frailty, severe physical dysfunction, and high comorbidity burden. The novelty and efficacy of the REHAB-HF intervention are further supported by the fact that the large improvements in function relative to Attention Control were seen despite receipt of routine physical/occupational therapy and/or traditional cardiac/pulmonary rehabilitation as part of usual care by 43% of Attention Control participants (Table S11).
While novel, the REHAB-HF intervention has high potential for dissemination and incorporation into clinical care pathways. The intervention is adaptable to a wide range of function and settings, is based on principles utilized in physical therapy, cardiac rehabilitation, and management of frailty,18,29 utilized readily available equipment, and was able to be conducted with high fidelity across multiple sites, including community hospitals.
The intervention-related improvements in physical function and quality-of-life were broad, large in magnitude, and clinically meaningful. The average increase in SPPB score (1.5 units) was 3-fold larger than the accepted threshold for clinically meaningful change (0.5 units), and 50% larger than the threshold for a large change (1.0 units).21,30 All components of the SPPB, corresponding to balance, strength, and mobility, improved significantly. The average increase in 6MWD (34 meters) exceeded the accepted threshold (30 meters) for meaningful change, despite very low baseline values. The average increase the KCCQ score (7 units) also exceeded the accepted threshold for clinically meaningful change (5 units).31,32 Compared to the HF-ACTION trial, the intervention-related improvement in 6MWD was >2-fold larger and in KCCQ score was 35% larger.16,31 The improvement in depression is of interest since depression is common in HF patients and is associated with frequent rehospitalizations,33 and the few intervention trials specifically targeting depression in HF patients have been neutral. Of note, HF-ACTION also found a modest improvement in depressive symptoms.34
The relatively large, uniform improvements in these clinically meaningful outcomes may be due to both the severity of baseline deficits and the robust, broad systemic effects of physical exercise which favorably alters energy metabolism, oxidative stress, inflammation, tissue repair, growth factor response, and regulatory pathways.35 Older HF patients have many features of severe skeletal muscle myopathy that contribute to physical dysfunction and improve with exercise.9
Despite large, clinically meaningful improvements in physical function and quality-of-life, no significant reductions in rehospitalizations or mortality were observed. However, the trial was insufficiently powered for events since the effect size we observed on all-cause rehospitalizations (−10%) was smaller than that assumed in the power calculation (−25%). Nevertheless, patient preference studies in HF patients indicate that improving physical function and maintaining independence is highly valued aside from clinical events.36
To our knowledge, this is the first multi-center, randomized, controlled, single-blinded trial of physical rehabilitation that enrolled older HF patients during hospitalization when physical dysfunction and risk are greatest. The few previous ‘early’ rehabilitation HF studies enrolled patients and began the intervention on average seven weeks after hospital discharge, used mostly traditional exercise training,37,38 enrolled patients who were much younger and much less frail and diverse,38 lacked control groups,37,39 were unblinded38,40 and were small single, center.40 The largest previous trial, EJECTION-HF, found no intervention-related improvement in physical function (6MWD), hospitalizations, and death, but had low rates of intervention adherence (43%).38 Otherwise, findings from previous studies generally support REHAB-HF findings.
The present study has several strengths, including a diverse group of older frail ADHF patients with high comorbidity burdens, high rates of frailty, severe baseline physical dysfunction, and poor quality-of-life, and both community and tertiary care sites, enhancing generalizability of results, and a carefully designed and conducted innovative physical rehabilitation intervention.
The present study also has some potential limitations, in addition to insufficient power for events. Although staff assessing the primary outcome were blinded to group assignment, it was not possible to blind participants. Despite randomization, there were imbalances in some baseline characteristics, such as diabetes and peripheral vascular disease, which potentially disfavored the intervention group; however, overall results were not changed significantly in models adjusting for these variables.
CONCLUSION
A novel, transitional, tailored, progressive, multi-domain, physical function rehabilitation intervention that began early and extends for 12 weeks following discharge for ADHF was feasible, safe, and markedly improved physical function, frailty, quality-of-life, and depression. Future studies are needed to determine the impact of the intervention on clinical events.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge Alain Bertoni, MD, Peter Brubaker, PhD, and Jack Rejeski, PhD for critical review and editing of the manuscript.
Funding Support: This study was supported in part by the following research grants from the National Institutes of Health: R01AG045551; R01AG18915; P30AG021332; - P30AG028716; U24AG059624. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and by the Oristano Family Fund at Wake Forest School of Medicine.
Footnotes
Disclosures:
DWK reported receiving honoraria outside the present study from Consultant for AbbVie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, and Astra Zeneca, and stock ownership in Gilead Sciences.
DJW received research support and consulting fees from Amgen, CVRx, Cytokinetics, FibroGen, Novartis, NovoNordisk.
PD reported grant finding from NIH/NINDS; PCORI; AHRQ; and ownership in Care Directions.
RJM reported receiving research support and honoraria outside the present study from: Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor.
SDR reported receiving research support outside the presents study from: Abbott Vascular, AstraZeneca, Janssen Research & Development, Monteris, PhRMA Foundation, and TESARO and consulting income from Sanofi/Regeneron, NovoNordisk, SVC Systems and Minomic International, Inc.
MAE reported funding for serving on a Steering Committee for Boehringer Ingelheim and a DSMB for Ironwood Pharmaceuticals.
CMO reported receiving research support and honoraria outside the present study from: Bayer, Merck, BMS, and Abiomed.
Other authors report no disclosures.
References
- 1.Heidenreich P, Albert N, Allen L, et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng R, Cox M, Neely M, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. American Heart Journal 2014;168:731–30. [DOI] [PubMed] [Google Scholar]
- 3.Fleg J Preventing Readmission After Hospitalization for Acute Heart Failure: A Quest Incompletely Fulfilled. JACC: Heart Failure 2018;6:153–5. [DOI] [PubMed] [Google Scholar]
- 4.Alahdab M, Mansour I, Napan S, Stamos T. Six Minute Walk Test Predicts Long-Term All-Cause Mortality and Heart Failure Rehospitalization in African-American Patients Hospitalized With Acute Decompensated Heart Failure. J Card Fail 2009;15:130–5. [DOI] [PubMed] [Google Scholar]
- 5.Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail 2010;16:390–5. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Kitzman D, Whellan D, et al. Frailty Among Older Decompensated Heart Failure Patients: Prevalence; Association with Patient-Centered Outcomes; Efficient Detection Methods. J Am Coll Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Lupón J, Vidán MT, et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. Journal of the American Heart Association 2018;7:e008251-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warraich H, Kitzman D, Whellan D, et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults >/=60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circulation: Heart Failure: American Heart Association; 2018:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman D, Haykowsky M, Tomczak C. Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleg J, Cooper L, Borlaug B, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail 2015;8:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumholz H Post-Hospital Syndrome: An Acquired, Transient Condition of Generalized Risk. N Engl J Med 2013;368:100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo M, de la Espriella R, Miñana G, et al. Clinical profile and 1-year clinical outcomes of super elderly patients admitted with acute heart failure. European Journal of Internal Medicine 2020;Epub: doi. 10.1016/j.ejim.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Huusko J, Tuominen S, Studer R, et al. Recurrent hospitalizations are associated with increased mortality across the ejection fraction range in heart failure. ESC Heart Fail 2020;Epub: 10.1002/ehf2.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill T, Allore H, Gahbauer E, Murphy T. Change in disability after hospitalization or restricted activity in older persons. JAMA 2010;304:1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman D, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal endpoint for therapies oriented to older adults with cardiovascular disease: AHA Scientific Statement. Circulation 2016;135:e894–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor C, Whellan D, Lee K, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves G, Whellan D, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) Trial: Design and Rationale. Am Heart J 2016;185:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastva A, Duncan P, Reeves G, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials 2018;64:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves G, Whellan D, O’Connor C, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: The REHAB-HF Pilot Study. JACC Heart Fail 2016;5:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavasini R, Guralnik J, Brown J, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soubra R, Aly C, Novella J. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Biomed Res Int 2019;Epub: 10.1155/2019/1354362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves G, Whellan D, Patel M, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients ³60 Years Hospitalized with Acute Decompensated Heart Failure vs Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol 2016;117:1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy C, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 24.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66A:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman D, Fleg J, Kitzman D, et al. 6-minute walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol 2012;60:2653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witham M, Fulton R, Greig C, et al. Efficacy and Cost of an Exercise Program for Functionally Impaired Older Patients With Heart Failure / Clinical Perspective. Circulation: Heart Failure 2012;5:209–16. [DOI] [PubMed] [Google Scholar]
- 27.Faber M RJ B, Chin P, Van Wieringen P. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil 2006;87:885–96. [DOI] [PubMed] [Google Scholar]
- 28.Giallauria F, Vigorito C, Tramarin R, et al. Cardiac Rehabilitation in Very Old Patients: Data From the Italian Survey on Cardiac Rehabilitation-2008 (ISYDE-2008)GÇöOfficial Report of the Italian Association for Cardiovascular Prevention, Rehabilitation, and Epidemiology. J Gerontol A Biol Sci Med Sci 2010;65A:1353–61. [DOI] [PubMed] [Google Scholar]
- 29.Dent E, Morley JE, Cruz-Jentoft A, et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. Journal of Nutrition, Health & Aging 2019;23:771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera S, Mody S, Woodman R, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–9. [DOI] [PubMed] [Google Scholar]
- 31.Flynn K, Pina L, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: Findings from the HF-ACTION Randomized controlled Study. JAMA 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spertus J, Jones P, Kim J, Globe D. Validity, reliability, and responsiveness of the Kansas City Cardiomyopathy Questionnaire in anemic heart failure patients. Qual Life Res 2008;17:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson TJ, Basu S, Pisani BA, et al. Depression Predicts Repeated Heart Failure Hospitalizations. J Card Fail 2012;18:246–52. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal J, Babyak M, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 2012;308:465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contrepois K, Wu S, Moneghetti KJ, et al. Molecular Choreography of Acute Exercise. Cell 2020;181:1112–30.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed S, Angelyn O, Johnson F, et al. Patients’ Willingness to Accept Mitral Valve Procedure-Associated Risks Varies Across Severity of Heart Failure Symptoms. Circulation 2019;12:e008051. [DOI] [PubMed] [Google Scholar]
- 37.Acanfora D, Scicchitano P, Casucci G, et al. Exercise training effects on elderly andmiddle-age patients with chronic heart failure after acute decompensation: A randomized, controlled trial Int J Cardiol 2016;225:313–23. [DOI] [PubMed] [Google Scholar]
- 38.Mudge A, Denaro C, Scott A, et al. Addition of supervised exercise training to a post-hospital disease management program for patients recently hospitalized with acute heart failure. JACC Heart Failure 2018;6:143–52. [DOI] [PubMed] [Google Scholar]
- 39.Scrutinio D, Passantino A, Catanzaro R, et al. Inpatient Cardiac Rehabilitation Soon After Hospitalization for Acute Decompensated Heart Failure: A Propensity Score Study. J Cardiopulm Rehabil Prev 2012;32:71–7. [DOI] [PubMed] [Google Scholar]
- 40.Babu AS, Maiya AG, George MM, Padmakumar R, Guddattu V. Effects of combined early in-patient cardiac rehabilitation and structured home-based program on function among patients with congestive heart failure: a randomized controlled trial. Heart Views 2011;12:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.