Abstract
Aim:
To test the effectiveness of a motivational interviewing (MI) intervention using the mobile phone among adults with alcohol use problems.
Design:
A randomized clinical trial of mobile MI and standard in-person MI with 1- and 6-month follow-up, including a one-month waitlist control followed by mobile MI.
Setting:
A primary health center in rural Kenya.
Participants:
Three hundred adults screening positive for alcohol use problems were randomized and received immediate mobile MI (n=89), in-person MI (n=65), or delayed mobile MI (n=76) for waitlist controls one month after no treatment, with 70 unable to be reached for intervention.
Intervention and comparator:
One MI session was provided either immediately by mobile phone, in-person at the health center, or delayed by one month and then provided by mobile phone.
Measurements:
Alcohol use problems were repeatedly assessed using the Alcohol Use Disorder Identification Test (AUDIT) and the shorter AUDIT-C. The primary outcome was difference in alcohol score one month after no intervention for waitlist control vs. one month after MI for mobile MI. The secondary outcomes were difference in alcohol score for in-person MI vs. mobile MI one and six months after MI.
Findings:
For our primary outcome, average AUDIT-C scores were nearly three points higher (Difference=2.88, 95% CI: 2.11, 3.66) for waitlist controls after one month of no intervention vs. mobile MI one month after intervention. Results for secondary outcomes supported the null hypothesis of no difference between in-person and mobile MI at one month (Bayes Factor=.22) but were inconclusive at six months (Bayes Factor=.41).
Conclusion:
Mobile phone-based motivational interviewing may be an effective treatment for alcohol use problems among adults visiting primary care in Kenya. Providing mobile motivational interviewing may help clinicians in rural areas reach patients needing treatment for alcohol use problems.
Keywords: Mobile intervention, motivational interviewing, alcohol use disorders, HIV/AIDS, Kenya, mHealth
INTRODUCTION
Alcohol use disorders (AUDs) are among the most prevalent mental disorders worldwide and constitute a major public health burden. An estimated one in 20 deaths are attributable to AUDs and they contribute 5.1% of disability adjusted life years(1). Treatments for AUDs in countries in Sub-Saharan Africa, like Kenya, is lacking, and the association between AUDs and sexually transmitted infections like HIV/AIDS(2–4), further burdens the developing healthcare system. AUDs contribute to poor adherence to HIV/AIDS medications, riskier sexual behavior among alcohol discordant couples, and accelerates HIV progression(3, 5–9). Not treating comorbid AUDs as a part of the President’s Emergency Plan for AIDS Relief slows the probability of achieving the Sustainable Development Goal of ending the AIDS epidemic by 2030(10). More interventions that are cost-effective, sustainable, and culturally appropriate to address AUDs are needed in Kenya.
AUDs affect 18–40% of attendees in primary care facilities worldwide, including Kenya(11–13). Compared to specialist settings, early screening and intervention for AUDs in primary care has shown good results(14–16). Multiple factors make delivering effective treatment for AUDs challenging in low and middle income countries(17). In Kenya, apart from universal challenges such as large geographic spread of health facilities, provider incompetence to deliver the interventions, stigmatizing attitudes by providers towards AUDs and governance that accords low priority for AUDs, heavy workload contributed by staffing shortages in primary care settings further limits provider bandwidth to address AUDs. This limits access to AUD services only to specialist mental health clinicians clustered in urban areas. Brief, innovatively-delivered, cost-effective solutions that empower providers in primary care settings to effectively treat AUDs are urgently needed.
Evidence-based brief intervention models for AUDs have been tested in primary care settings in high-income countries but less so in low and middle-income countries(18–25). Motivational interviewing (MI) is a type of brief intervention that uses open-ended questions, affirmations, reflective listening, and summarizing as key tools(26). MI has been shown to treat a range of problem behaviors, including AUDs, by helping patients identify and address ambivalence towards changing behaviors(27, 28). MI is delivered in a communicative style promoting autonomy and self-efficacy(29, 30). Studies from high income countries show that MI results in improved outcomes for non-injection drug users(31) with AUDs(32–34) and improved treatment response(35). Interventions must be adapted to the local context(36) for similar outcomes to be replicable in low income populations(37). Few studies have been conducted in low-resource primary care settings. A study in South Africa reported positive results for using adapted MI but showed no difference between MI and controls in treating AUDs in emergency departments(38). Similar studies using MI have reported good results in Zambia(39), South Africa(40–42) and Nigeria(43). In Sub-Saharan Africa, studies using MI have shown mixed results due to poor attendance to sessions, poor fidelity of the intervention, and high attrition rates(44). Two studies in Kenya have utilized MI, focusing on obstetric care and heroin use(45, 46). No MI studies have been conducted on AUDs in Kenya, so more research on the efficacy of MI to treat AUDs is needed.
Innovative methods of delivering MI could help hard-to-reach populations who have limited access to healthcare in Kenya. This study tests an innovative approach to deliver MI over the mobile phone (mHealth), enabling MI-trained clinicians to deliver treatment remotely. MHealth has emerged as a dynamic innovation with diverse uses in Sub-Saharan Africa(47). It is estimated that nearly 500 projects using mHealth were implemented in Sub-Saharan Africa prior to 2017(48). The Kenyan eHealth Strategy acknowledges that mHealth-related interventions require mobile phones and infrastructure to be accessible to the population(49).There has been an increase in the use of mobile technology across Kenya, and in 2013 mobile penetration for those living on less than US $2.50 per day was already 60%(50).
MHealth can be used to enhance follow-up, treatment monitoring and communication about mental disorders within and across health facilities, as well as improve healthcare outcomes(51, 52). This approach can also increase awareness within communities in which mental illness is highly stigmatized(53). Mobile phone-based interventions targeting drug or alcohol abstinence have demonstrated significant improvements compared to controls(54, 55). MI enhanced with technology for people with HIV/AIDS and AUDs in primary care has been shown to have positive outcomes, is acceptable to patients in resource-limited settings and is feasible without extensive additional staff involvement(56–58). Studies conducted in Kenya involving mHealth show good results(59–62). To date, no studies in Kenya have evaluated the efficacy of mobile phone-based brief interventions among individuals with AUDs in primary care, although there are considerable opportunities(63). This study was grounded in a biopsychosocial model that linked AUDs with interrelated physical, mental health, and social problems. The objectives of this study were (1) test whether mobile MI reduced alcohol use one month after intervention compared to waitlist controls, and (2) test whether there was a difference in alcohol use between mobile MI and standard in-person MI at one month and six months after intervention.
METHODS
Trial Design
This was a randomized clinical trial with a parallel design including two treatment modalities (mobile MI and standard in-person MI) with 1- and 6-month follow-up, and a one-month waitlist followed by delayed mobile MI.
Study site
This study site was a Tier 2 facility (primary care health center) in a county in Eastern Province, 100 kilometers East of Nairobi, Kenya. At the time of data collection, this county was reported to have consistently high levels of alcohol consumption and was reported to be the third highest in the country(64). The health center offered primary care services including a comprehensive HIV/AIDS care clinic (CCC), voluntary HIV testing and counseling (HTC), and basic obstetric care. The health center had 810 patients enrolled in the CCC and provided 4138 instances of HTC services in 2015.
Participant recruitment
Between October 2014 and March 2015, community health workers, peer educators and clinicians (‘health center staff’) in the HTC and CCC clinics at the health center screened convenience samples of adults for alcohol use problems using a translated three question version of the Alcohol Use Disorder Identification Test (AUDIT)(65, 66) described below. The research team at the Africa Mental Health foundation (AMHF) trained health center staff in the HTC and CCC to conduct an informed consent process with all patients that scored positive for alcohol use problems. The AMHF clinicians were visiting researchers and the health center staff conducting the consent were high school graduates with no college training working as peer educators at the center.
Volunteers were consented confidentially in private rooms. Those who declined consent received services as usual. Twenty-eight adults (10 from the HTC; 18 from the CCC) screened positive for alcohol use problems, were eligible for the study, but declined consent and were excluded (Figure 1). Reasons for declining consent included wanting monetary compensation, promising to return later, and disinterest in participating. Persons younger than 18 years old, with severe psychiatric morbidity, or cognitive impairment were not eligible for the study. Assessment and consent documents were locked separately at AMHF Nairobi offices, and participants were assigned a unique study ID, maintained by AMHF research staff as a separate locked document linking the study ID and the participant’s name. AMHF clinicians did not know the HIV status of the participants.
Figure 1:
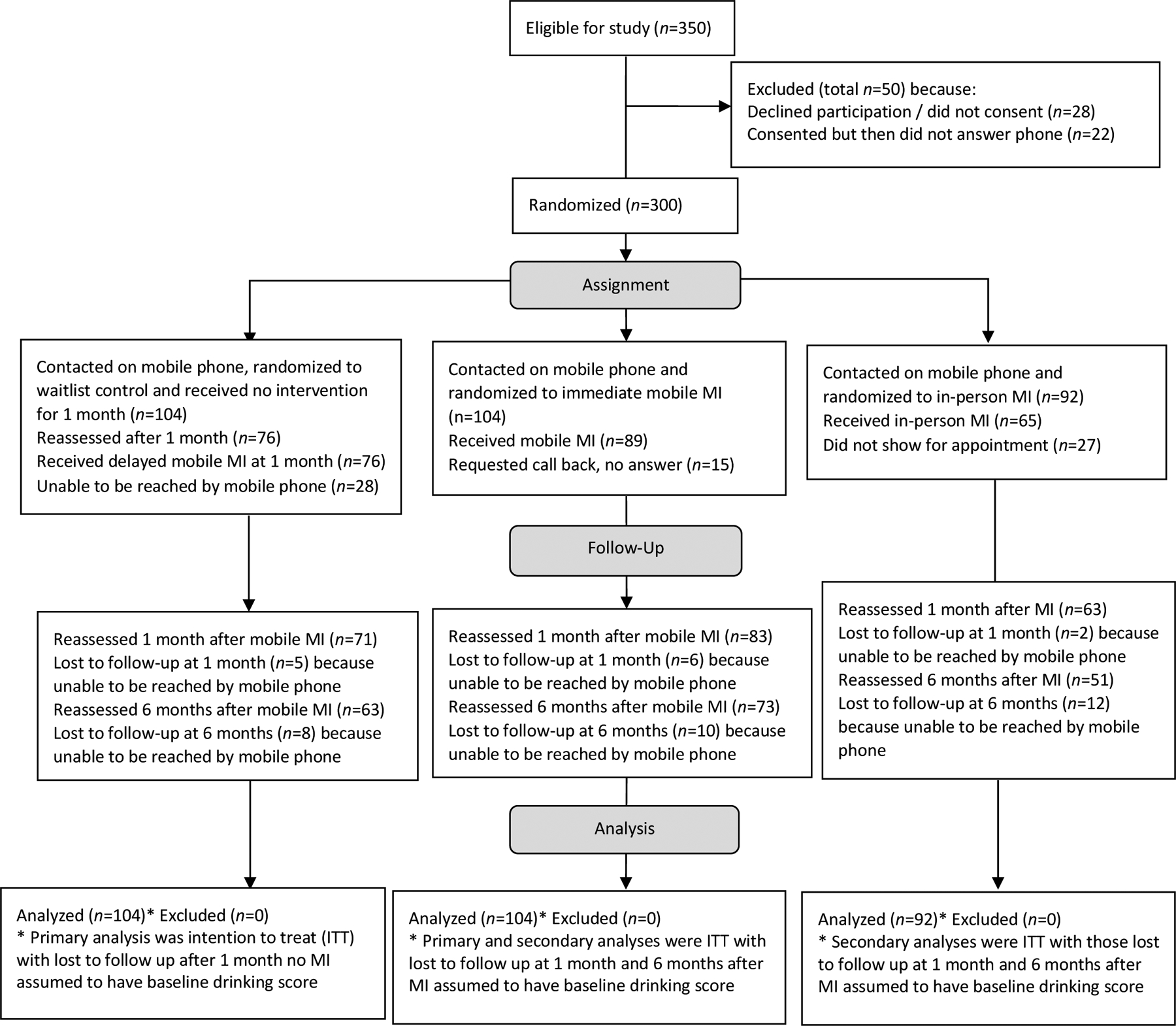
Flow of eligible participants through random assignment to three study modalities, follow-up reassessment of alcohol use at one and six months after motivational interviewing intervention, and analyses.
Study measures
Sociodemographic Questionnaire:
This questionnaire included self-report information about gender, age, marital status, education, employment, and contact information including primary and alternatemobile phone numbers.
Alcohol Use Disorders Identification Test (AUDIT):
The AUDIT(65) is a widely used instrument that identifies risky or harmful alcohol consumption and alcohol dependence and provides a framework for intervention to help hazardous drinkers reduce or stop alcohol consumption and avoid the harmful consequences of their drinking(67). It has good psychometric properties when used in both developed and developing countries(68, 69). The authors, three of the health center staff, and a group of community members met three times and took pictures of commercially produced and homebrewed alcohol (beer and spirits) that was frequently consumed in the area. These images were included on the AUDIT to make it relevant to the local context. It was not possible to convert the homebrewed alcohol into standard units as no information was available on alcohol content. The English AUDIT questions were translated into Kiswahili (the national language) and Kikamba (the local language) by a trained translator fluent in all three languages, making sure the questions were well understood in the Kenyan cultural context. The AUDIT was then back translated into English by another trained translator fluent in all three languages and reviewed by the research team and community members to ensure no meaning was lost. The final versions were pre-tested with community members.
Alcohol Use Disorders Identification Test-Consumption (AUDIT-C):
The first 3-items from the AUDIT identify individuals at risk of hazardous drinking or who have AUDs (66, 70, 71). The AUDIT-C is scored on a 0–12 point scale and scores of three or more in females (sensitivity: 0.66; specificity: 0.94) and four or more in males (sensitivity: 0.86; specificity: 0.72) (66) are considered positive. In this study, a score of four or more on the AUDIT-C was used for all participants as a positive screen, regardless of sex. The AUDIT-C assessed at one month was the primary outcome measure.
Reliability
The research team conducted a pilot study of test-retest reliability of the Kiswahili version of the AUDIT at a different primary care health center. Fifty-six Kenyan adults participated (average age 31 years old, 48% female, 50% married, 36% high school graduates, 21% unemployed, and 34% AUDIT score>8), and 30 returned for reassessment an average 6.4 days later (range 5–8 days). The test-retest reliability between baseline and reassessment AUDIT and AUDIT-C scores were high for Pearson’s correlations>.94 and Spearman’s rhos>.98 (Ps<.0005). There was substantial agreement using an AUDIT cutoff at eight (Kappa=.75, P<.0005) and almost perfect agreement using an AUDIT-C cutoff at four (Kappa=.83, P<.0005).
Ethical approval and consent
This study was approved by the Institutional Review Boards at the Kenya Medical Research Institute and the University of Vermont, USA. The protocol was registered after study completion and is publicly available at clinicaltrials.gov.
Study procedures
After obtaining consent, health center staff administered the sociodemographic questionnaire and AUDIT to 322 participants, and they communicated the participant’s mobile phone numbers to the AMHF clinicians the same day. AMHF clinicians on the research team (a medical doctor, clinical psychologist, and nurse) were able to contact 300 of these participants (93%) through the mobile phone number provided. The 22 participants that were never reached after at least three more attempts within two weeks were excluded (Figure 1). Once contacted, the AMHF clinicians randomized participants to receive one session of MI through three modalities (Figure 1). The experimental intervention, mobile MI, was prioritized in the randomization each day by the clinicians with the first eligible participant randomized to mobile MI (n=104), followed by waitlist (n=104), and third to in-person MI (n=92), although not all received the intervention. The in-person group was smaller because some days not enough participants were enrolled to be randomized to in-person. In-person MI was the standard intervention where participants met an AMHF clinician at the health center for a single face-to-face session. This session was scheduled within a maximum of two weeks from the enrollment date. In-person participants that did not show for their appointment were called and appointments rescheduled three more times before being designated as no show. Mobile MI was the experimental treatment where participants spoke with an AMHF clinician on their mobile phone for one MI session (referred to as immediate mobile MI throughout), preferably on this first phone call. If the time was inconvenient for the participant, the mobile MI intervention was re-scheduled the same day if possible, or within the week. AMHF clinicians attempted calling the participant back at least three more times after first contact before designating the participant as no answer. The one-month waitlist controls were contacted by the AMHF clinician on their mobile phone to introduce themselves, provide no intervention, and inform the waitlist participants they would call again in one month.
Follow up:
After one month, AMHF clinicians called the waitlist controls to first reassess alcohol using the AUDIT-C and then provide one session of mobile MI (referred to as delayed mobile MI throughout). AMHF clinicians attempted calling the waitlist controls at least three more times within two weeks before designating the waitlist participant as unable to be reached. All participants receiving MI were followed up for mobile re-assessments one month after the MI session (using the AUDIT-C) and six months later (using the AUDIT, which included the three question AUDIT-C), and again AMHF clinicians attempted calling participants at least three more times before designating them as lost to follow up (Figure 1). Mobile numbers being disconnected, phones not being charged, or participants purposefully avoiding calls were a few of the main barriers experienced using mobile phones in this study.
MI intervention
The intervention consisted of a single brief MI session by a trained clinician lasting approximately 30 minutes. AMHF clinicians used MI to encourage motivation for change on participants’ alcohol use patterns while rolling with resistance in an empathic style. The three clinicians who delivered MI had a Master’s degree in nursing, doctoral degree in clinical psychology, or a medical degree, and were fluent in all three languages. They were trained over a six-month period on how to deliver MI. The training included a four-week online course (8 hours over four weeks) by the Health Education and Training Institute titled ‘Motivational Interviewing: advancing the practice’ for which a certificate was awarded on completion of all the modules including a practical exam. Clinicians met weekly for an hour (24 hours over six months) of mentored discussions with the study principal investigator and for peer-to-peer learning on applying MI methods in practice. Each clinician spent additional time conducting at least three role-play MI sessions and three practical MI sessions with patients receiving services in a mental health clinic (each approximately 30 minutes). The practical sessions were recorded and discussed during subsequent peer-to-peer sessions for improvement. Adapted versions of the MI Interview Rating Worksheet and MI Competency and Adherence Feedback scale by Martino and colleagues(72) were used by clinicians to evaluate each other’s practical sessions during these peer-to-peer sessions. This process helped maintain the fidelity of the MI intervention and produced a reference document of translations of reflective statements and terms that could be used as a quick guide for clinicians.
Sample size
Pilot study data of In-person MI showed a large effect size of .93 for the decrease in alcohol use score one month post MI. Given mobile MI was an experimental treatment, we estimated a conservative medium effect size of .5, a two-tailed test, alpha error of .05 and beta error of .10, resulting in needing approximately 86 participants in each group for our primary pairwise comparison. We anticipated an approximate 25% lost to follow up based on previous work in Kenya(73, 74), and so health center staff screened and consented 322 participants for all three groups (Figure 1) to approach our estimated sample sizes for our primary outcome at one month.
Statistical analyses
For our primary analysis, we used multiple linear regression to compare the average AUDIT-C for waitlist control and immediate mobile MI after one month, controlling for baseline AUDIT-C score as the main confounder. For our secondary analyses, we used multiple linear regressions to compare average AUDIT-C for in person MI and immediate mobile MI at one month, controlling for baseline AUDIT-C score, and we repeated this with six month data controlling for baseline AUDIT-C score. Other potential confounding effects of age, biological sex, marital status (married vs. other), education (at least high school graduate vs. less than high school graduate), employment (worked in the home, unemployed, self-employed, employed), and clinic where participant was recruited (CCC vs. HTC) were tested by adding covariates to all models. These other confounders were not kept in final models because there were no significant associations (Ps>.01). An intention-to-treat (ITT) approach was used, and missing AUDIT-C data at one month and six months due to lost to follow-up were filled in with baseline AUDIT-C scores. When no statistically significant differences were found, we calculated the Bayes Factor(75). The analysis plan was not pre-registered on a publically available website, and this should be taken into account when interpreting findings. All analyses were conducted using STATA SE 15.1, and results were considered significant at P<.05.
RESULTS
Sample characteristics
Table 1 shows demographics (age, sex, marital status, education, employment, and clinic where they were recruited) for 300 participants contacted by AMHF clinicians and randomized to three study modalities. Overall, the study sample was 38 years old on average, 78% male, 60% married, 36% high school graduates, 38% employed, and 47% recruited from the CCC. The 22 participants that were not able to be reached by AMHF clinicians on their mobile phone for randomization were not different on demographic variables from those reached (Ps>.05). Figure 1 shows the flow of participants after randomization that were reassessed one month after no intervention (n=76, 27% unable to be reached by mobile phone) and received delayed mobile MI, received immediate mobile MI (n=89, 14% requested a call back but did not answer), and received in-person MI (n=65, 29% did not show for appointment). At one month after intervention, there were losses to follow-up among waitlist controls (n=5, 7%), immediate mobile MI (n=6, 7%), and in-person MI (n=2, 3%) participants. At six months, there were eight additional participants from waitlist controls (11%), 10 from immediate mobile MI (12%), and 12 from in-person (19%) that were lost to follow-up.
Table 1:
Demographics of the contacted and randomized to either waitlist control, immediate mobile motivational interviewing (MI), or In-Person MI.
| Contacted on Mobile Phone by AMHF Clinician and Randomized | Randomized to Waitlist Control | Randomized to Immediate Mobile MI | Randomized to In-Person MI | |||||
|---|---|---|---|---|---|---|---|---|
| Demographics | N | Percent or Mean (SD) | N | Percent or Mean (SD) | N | Percent or Mean (SD) | N | Percent or Mean (SD) |
| Age | 300 | 38 (11.7) | 104 | 39 (12.0) | 104 | 39 (12.2) | 92 | 36 (10.6) |
| Gender | ||||||||
| Female | 65 | 22% | 24 | 23% | 18 | 17% | 23 | 25% |
| Male | 235 | 78% | 80 | 77% | 86 | 83% | 69 | 75% |
| Marital Status | ||||||||
| Single (never married) | 74 | 25% | 25 | 24% | 28 | 27% | 21 | 23% |
| Married | 181 | 60% | 63 | 61% | 58 | 56% | 60 | 65% |
| Separated | 28 | 9% | 10 | 10% | 11 | 11% | 7 | 8% |
| Widowed | 16 | 5% | 6 | 6% | 6 | 6% | 4 | 4% |
| missing | 1 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| Education | ||||||||
| No Schooling | 2 | 1% | 0 | 0% | 1 | 1% | 1 | 1% |
| Some Primary School | 61 | 21% | 31 | 30% | 20 | 19% | 11 | 12% |
| Completed Eight Grade (Standard 8) | 91 | 31% | 27 | 26% | 33 | 32% | 31 | 35% |
| Some High School | 36 | 12% | 12 | 12% | 12 | 12% | 12 | 13% |
| Completed High School (Form 4) | 108 | 36% | 34 | 33% | 38 | 37% | 36 | 39% |
| Employment | ||||||||
| Working in the Home | 21 | 7% | 9 | 9% | 4 | 4% | 8 | 9% |
| Unemployed | 29 | 10% | 7 | 7% | 11 | 11% | 11 | 12% |
| Self employed | 134 | 45% | 47 | 45% | 53 | 51% | 34 | 37% |
| Employed | 115 | 38% | 41 | 39% | 36 | 35% | 38 | 41% |
| missing | 1 | 0% | 0 | 0% | 0 | 0% | 1 | 1% |
| Clinic | ||||||||
| Testing for HIV | 158 | 53% | 46 | 44% | 59 | 57% | 53 | 58% |
| Comprehensive Care (HIV positive) | 142 | 47% | 58 | 56% | 45 | 43% | 39 | 42% |
Notes: AMHF=Africal Mental Health Foundation; SD=standard deviation;
Impact of the Intervention
For our primary outcome comparing average drinking scores for waitlist vs. mobile MI (using an ITT approach) we found that waitlist controls receiving no intervention for one month had an average AUDIT-C score that was nearly three points higher than participants receiving immediate mobile MI and being reassessed one month later (P<0005), controlling for baseline AUDIT-C scores (Table 2). Similar inferences were drawn among the sample of participants excluding those lost to follow-up at one month (Table 2). For our secondary outcomes (using an ITT approach) there was no difference between alcohol scores at one month after treatment comparing in-person to mobile MI (P=.63). Using the effect from our primary outcome for waitlist control vs. mobile MI after one month as the comparison, the Bayes Factor (assuming a half normal distribution) was 0.22, concluding there was evidence for the null hypothesis because Bayes was smaller than the generally accepted cutoff of one third(75). Results for our comparison of in-person to mobile MI at six months were inconclusive (P=.34) with a Bayes Factor of .41 not meeting the cutoff of less than one third. Inferences from all analyses were similar when participants lost to follow-up were dropped and analyses included participants with follow-up data only at one month and six months after MI (Table 2). Although there was no control group for comparison, the marked decrease in alcohol score seen at one month after MI for both mobile and in-person appeared to be sustained out to six months post intervention (Figure 2).
Table 2:
Mean Difference in Alcohol Use Disorders Identification Test Consumption (AUDIT-C) Scores for Primary and Secondary Outcomes in an Intention to Treat Sample and a Sample of Subjects with Complete Follow Up.
| Multiple Linear Regression Results | ||||||
|---|---|---|---|---|---|---|
| Sample Size | Unadjusted Mean Difference | Unadjusted Standard Deviation | Adjusted* Mean Difference (Beta Coefficient) | 95% Confidence Interval | ||
| Low | High | |||||
| Intention To Treat Sample a | ||||||
| Primary Outcome: Waitlist vs. Mobile MI at 1 month |
208 | 2.75 | 0.39 | 2.88 | 2.11 | 3.66 |
| Secondary Outcome: In-Person MI vs. Mobile MI at 1 month |
196 | 0.33 | 0.45 | 0.20 | −0.60 | 1.00 |
| Secondary Outcome: In-Person MI vs. Mobile MI at 6 months |
196 | 0.50 | 0.46 | 0.44 | −0.47 | 1.36 |
| Participants With Follow Up b | ||||||
| Primary Outcome: Waitlist vs. Mobile MI at 1 month |
159 | 3.52 | 0.40 | 3.60 | 2.82 | 4.38 |
| Secondary Outcome: In-Person MI vs. Mobile MI at 1 month |
146 | −0.10 | 0.43 | −0.33 | −1.16 | 0.49 |
| Secondary Outcome: In-Person MI vs. Mobile MI at 6 months |
124 | −0.46 | 0.35 | −0.46 | −1.16 | 0.24 |
Notes:
Multiple linear regressions adjusted for baseline AUDIT-C score;
Intention to Treat Sample: Missing AUDIT-C data at one and six months were filled in with baseline AUDIT-C scores.
Participants With Follow Up: Participants with missing AUDIT-C data at one and six months were excluded from analyses.
Figure 2:
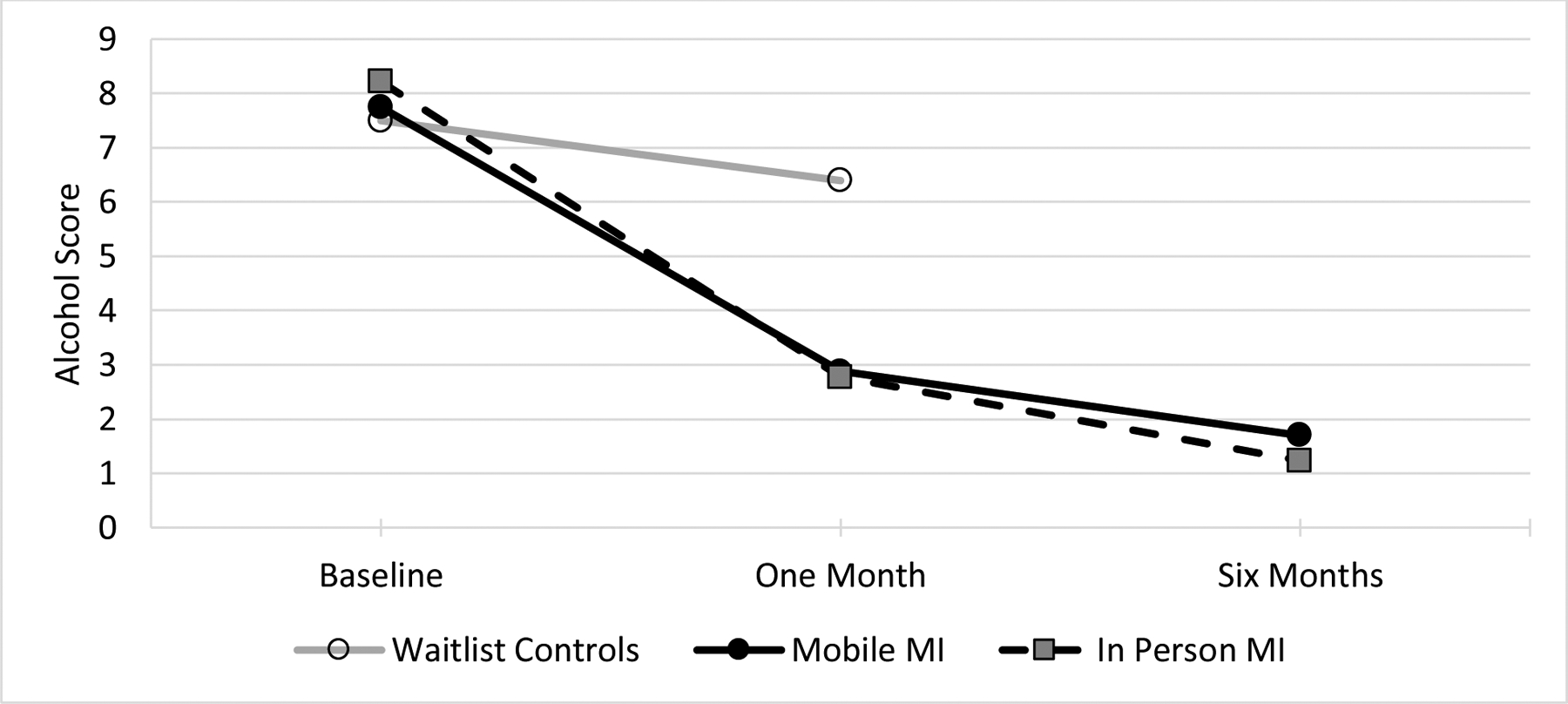
Average alcohol score at one month was significantly higher for waitlist controls compared to mobile MI. There were no differences in average alcohol scores between in-person and mobile MI at either one month or six months.
DISCUSSION
In line with our primary objective, results indicate that mobile MI is an effective treatment modality for reducing alcohol use among patients visiting primary care in Kenya, as compared to no intervention. Our study suggests that patients receiving one session of mobile MI have significantly lower alcohol use at one month compared to controls receiving no intervention. This marked reduction in alcohol use among our mobile MI group is sustained at six months, suggesting that motivation to change behavior continues after the immediate post-intervention phase. Our findings contribute to the existing evidence that brief interventions help to reduce alcohol use in high income(18–21) and low income countries(21, 24, 25, 76), including studies in Kenya at the intersection of alcohol abuse and HIV/AIDS(77, 78), and in some cases result in alcohol cessation(79).
Another strength of this study is the application to people with HIV/AIDS receiving treatment within a primary health center. Studies applying similar behavioral interventions through different technologies have shown positive results, for example, for weight loss and injection drug use(56, 80). Studies have also shown that mobile phone-based interventions may help improve disease management and improve treatment outcomes(81), specifically for adherence to HIV medications and retention in treatment using text messaging(61) and self-regulation counseling(55). Our mobile intervention was delivered to all participants regardless of their HIV status, yet future studies may consider combining MI with these other successful mobile interventions. Thinking about other modification to the standard MI model for treatment, researchers in Zimbabwe are working to create a culturally contextualized treatment protocol that adapts the standard in-person MI to include cognitive behavioral therapy components for treating alcohol use problems among people living with HIV/AIDS(82). Looking to the future, combining mobile MI with existing prevention strategies may improve compliance with HIV prevention strategies including pre-exposure prophylaxis and treatment prevention or as a complementary element of these strategies whose adherence is directly affected by use of addictive substances(83). Primary care practitioners at the HTC and CCC may also benefit from training in MI or having staff that are MI trained to support their clinical practice, especially in low resource settings that have limited to no access to substance abuse care.
For our secondary outcome, no difference between average alcohol score one month after MI comparing mobile to in-person MI suggests that both treatments may be equally effective, at least in the short term. This evidence for the null hypothesis at one month is important in relation to the major advantages of mobile MI over in-person MI, such as lower cost because in-person MI requires travel for either the patient, provider, or both, and with the current mobile structure in Kenya, a patient receiving a call on a mobile phone is free. Mobile MI may also improve convenience and access to care because sessions can be conducted when the patient and the clinician are available in any location. These benefits combined may also reduce the burden on the primary healthcare system and could potentially reduce costs associated with access to services. Further studies can inform decisions about who benefits more from which delivery approach of MI based on individual participant sociodemographic and psychological parameters.
Our study has some limitations. Not having waitlist control data out to six months limited our ability to test the sustainability of interventions. We did not re-test the psychometric properties of our translated AUDIT in this study, however, the original AUDIT was developed in a Kenyan sample(65), and in an unpublished pilot study by this research team, we found the AUDIT and AUDIT-C Kiswahili translation to have high reliability. Clinicians were trained medical professionals, and they completed MI training through coursework, clinical training, and peer-evaluation, but were not certified MI counselors(84). There are few studies evaluating the level of MI skill associated with better health outcomes. A recent study by Palfai and colleagues shows that the quality of the brief MI skills within primary care is not associated with better drug use outcomes(85), suggesting that the highest level of skills may not be a critical predictor of improved outcomes, but more studies are needed. Another limitation is the fact that our controls waited one month, not six months, before receiving the intervention. A longer follow up period without intervention could provide better evidence of the effectiveness of mobile MI compared to a control group. Finally, dissemination of study results was delayed due to theft and damage at our Nairobi office, setting back our timeline, but fortunately, the original paper data and locked cabinets were not disturbed.
Conclusions
Mobile phone based MI may be an effective treatment for alcohol use problems among adults visiting primary care in Kenya. Providing mobile phone based MI may be a public health strategy to help clinicians in rural areas reach patients needing alcohol abuse treatment and potentially helping patients overcome access issues related to seeking mental health care.
Acknowledgements
We thank the health center staff and community members participating in this study and the Machakos County Health Management Team for permission to conduct the study.
Funding:
This research project was supported by the National Institutes of Health Fogarty International Center grant number R21TW009786.
Footnotes
Conflict of interest declaration: None
Clinical Trials Registration: NCT03573167
References
- 1.World Health Organization. Global Status Report on Alcohol and Health. 2018.
- 2.Parry C, Rehm J, Poznyak V, Room R. Alcohol and infectious diseases: an overlooked causal linkage? Addiction. 2009;104(3):331–2. [DOI] [PubMed] [Google Scholar]
- 3.Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167(4):789–97. [DOI] [PubMed] [Google Scholar]
- 4.Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, et al. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173(3):550–8. [DOI] [PubMed] [Google Scholar]
- 5.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine. 2001;16(2):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(4):572–7. [DOI] [PubMed] [Google Scholar]
- 8.Muturi N Alcohol consumption and reproductive health risks in rural Central Kenya. Sexual & reproductive healthcare : official journal of the Swedish Association of Midwives. 2014;5(2):41–6. [DOI] [PubMed] [Google Scholar]
- 9.Muturi N Community Perspectives on Communication Strategies for Alcohol Abuse Prevention in Rural Central Kenya. Journal of health communication. 2016;21(3):309–17. [DOI] [PubMed] [Google Scholar]
- 10.United Nations Division for Sustainable Development. Sustainable Development Goals 2015. [Available from: https://sustainabledevelopment.un.org/sdgs.
- 11.Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption--I. Addiction. 1993;88(3):349–62. [DOI] [PubMed] [Google Scholar]
- 12.Abiodun OA. Alcohol-related problems in primary care patients in Nigeria. Acta Psychiatr Scand. 1996;93(4):235–9. [DOI] [PubMed] [Google Scholar]
- 13.Ndetei DM, Khasakhala LI, Ongecha-Owuor FA, Kuria MW, Mutiso V, Kokonya DA. Prevalence of substance abuse among patients in general medical facilities in Kenya. Substance Abuse. 2009;30(2):182–90. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell A, Wallace P, Kaner E. From Efficacy to Effectiveness and Beyond: What Next for Brief Interventions in Primary Care? Frontiers in Psychiatry. 2014;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oslin DW, Lynch KG, Maisto SA, Lantinga LJ, McKay JR, Possemato K, et al. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J Gen Intern Med. 2014;29(1):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaner EF, Wutzke S, Saunders JB, Powell A, Morawski J, Bouix JC, et al. Impact of alcohol education and training on general practitioners’ diagnostic and management skills: findings from a World Health Organization collaborative study. J Stud Alcohol. 2001;62(5):621–7. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins R, Baingana F, Ahmad R, McDaid D, Atun R. International and national policy challenges in mental health. Ment Health Fam Med. 2011;8(2):101–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Satre DD, Leibowitz A, Sterling SA, Lu Y, Travis A, Weisner C. A randomized clinical trial of Motivational Interviewing to reduce alcohol and drug use among patients with depression. 2016(1939–2117 (Electronic)). [DOI] [PMC free article] [PubMed]
- 19.D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojnar M, Jakubczyk A. Brief interventions for hazardous and harmful alcohol consumption in accident and emergency departments. Front Psychiatry. 2014;5:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaner EF, Dickinson HO, Beyer F, Pienaar E, Schlesinger C, Campbell F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev. 2009;28(3):301–23. [DOI] [PubMed] [Google Scholar]
- 22.Pengpid S, Peltzer K, Skaal L, Van der Heever H. Screening and brief interventions for hazardous and harmful alcohol use among hospital outpatients in South Africa: results from a randomized controlled trial. Bmc Public Health. 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltzer K, Matseke G, Azwihangwisi M. Evaluation of alcohol screening and brief intervention in routine practice of primary care nurses in Vhembe district, South Africa. Croat Med J. 2008;49(3):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsberg WA, Luseno WK, Karg RS, Young S, Rodman N, Myers B, et al. Alcohol, cannabis, and methamphetamine use and other risk behaviours among Black and Coloured South African women: A small randomized trial in the Western Cape. International Journal of Drug Policy. 2008;19(2):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic Cultural Adaptation of Cognitive-Behavioral Therapy to Reduce Alcohol Use Among HIV-Infected Outpatients in Western Kenya. AIDS Behav. 2010;14(3):669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett G Motivational Interviewing - Preparing People to Change Addictive Behavior - Miller,Wr, Rollnick,S. J Community Appl Soc. 1992;2(4):299–300. [Google Scholar]
- 27.D’Amico EJ, Osilla KC, Hunter SB. Developing a Group Motivational Interviewing Intervention for Adolescents At-Risk for Developing an Alcohol or Drug use Disorder. Alcohol Treat Q. 2010;28(4):417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollnick S, Miller WR, Butler C. Motivational interviewing in health care : helping patients change behavior. New York: Guilford Press; 2008. xiv, 210 p. p. [Google Scholar]
- 29.Jones SA, Latchford G, Tober G. Client experiences of motivational interviewing: An interpersonal process recall study. Psychol Psychother-T. 2016;89(1):97–114. [DOI] [PubMed] [Google Scholar]
- 30.Markland D, Ryan RM, Tobin VJ, Rollnick S. Motivational interviewing and selfdetermination theory. J Soc Clin Psychol. 2005;24(6):811–31. [Google Scholar]
- 31.Aharonovich E, Sarvet A, Stohl M, DesJarlais D, Tross S, Hurst T, et al. Reducing non-injection drug use in HIV primary care: A randomized trial of brief motivational interviewing, with and without HealthCall, a technology-based enhancement. J Subst Abuse Treat. 2017;74:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol Alcohol. 2006;41(3):32835. [DOI] [PubMed] [Google Scholar]
- 33.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psycho. 2005;1:91–111. [DOI] [PubMed] [Google Scholar]
- 34.Smedslund G, Berg RC, Hammerstrom KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Db Syst Rev. 2011(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug Alcohol Depend. 2006;81(3):301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peltzer K, Mosala T, Dana P, Fomundam H. Follow-up Survey of Women Who Have Undergone a Prevention of Mother-to-Child Transmission Program in a Resource-Poor Setting in South Africa. J Assoc Nurse Aids C. 2008;19(6):450–60. [DOI] [PubMed] [Google Scholar]
- 37.Field CA, Cochran G, Caetano R. Ethnic differences in the effect of drug use and drug dependence on brief motivational interventions targeting alcohol use. Drug Alcohol Depend. 2012;126(1–2):21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorsdahl K, Stein DJ, Corrigall J, Cuijpers P, Smits N, Naledi T, et al. The efficacy of a blended motivational interviewing and problem solving therapy intervention to reduce substance use among patients presenting for emergency services in South Africa: A randomized controlled trial. Subst Abuse Treat Pr. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thevos AK, Quick RE, Yanduli V. Motivational Interviewing enhances the adoption of water disinfection practices in Zambia. Health Promot Int. 2000;15(3):207–14. [Google Scholar]
- 40.Myers B, van der Westhuizen C, Naledi T, Stein DJ, Sorsdahl K. Readiness to change is a predictor of reduced substance use involvement: findings from a randomized controlled trial of patients attending South African emergency departments. BMC Psychiatry. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rendall-Mkosi K, Morojele N, London L, Moodley S, Singh C, Girdler-Brown B. A randomized controlled trial of motivational interviewing to prevent risk for an alcohol-exposed pregnancy in the Western Cape, South Africa. Addiction. 2013;108(4):725–32. [DOI] [PubMed] [Google Scholar]
- 42.Louwagie GMC, Okuyemi KS, Ayo-Yusuf OA. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction. 2014;109(11):1942–52. [DOI] [PubMed] [Google Scholar]
- 43.Holstad MM, Essien JE, Ekong E, Higgins M, Teplinskiy I, Adewuyi MF. Motivational Groups Support Adherence to Antiretroviral Therapy and use of Risk Reduction Behaviors in HIV Positive Nigerian Women: A Pilot Study. African journal of reproductive health. 2012;16(3):14–27. [PMC free article] [PubMed] [Google Scholar]
- 44.Mash RJ, Rhode H, Zwarenstein M, Rollnick S, Lombard C, Steyn K, et al. Effectiveness of a group diabetes education programme in under-served communities in South Africa: a pragmatic cluster randomized controlled trial. Diabetic Med. 2014;31(8):987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gisore P, Kaseje D, Were F, Ayuku D. Motivational Interviewing Intervention on Health-Seeking Behaviors of Pregnant Women in Western Kenya. J Appl Biobehav Res. 2014;19(2):144–56. [Google Scholar]
- 46.Beckerleg S Counselling Kenyan heroin users: cross‐cultural motivation? Health Education. 2001;101(2):69–73. [Google Scholar]
- 47.Betjeman TJ, Soghoian SE, Foran MP. mHealth in Sub-Saharan Africa. International journal of telemedicine and applications. 2013;2013:482324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, Cho YM, Kim SY. Mapping mHealth (mobile health) and mobile penetrations in sub-Saharan Africa for strategic regional collaboration in mHealth scale-up: an application of exploratory spatial data analysis. Globalization and health. 2017;13(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ministry of Health. Kenya National eHealth Policy 2016–2030. 2016.
- 50.Donovan K Mobile usage at the base of the pyramid: research findings from Kenya and South Africa. Washington DC: World Bank Group; 2013. Report No.:84012. [Google Scholar]
- 51.Marangu E, Sands N, Rolley J, Ndetei D, Mansouri F. Mental healthcare in Kenya: exploring optimal conditions for capacity building. Afr J Prim Health Care Fam Med. 2014;6(1):E1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahu M, Grover A, Joshi A. Role of mobile phone technology in health education in Asian and African countries: a systematic review. Int J Electron Healthc. 2014;7(4):269–86. [DOI] [PubMed] [Google Scholar]
- 53.Kwan A mhealth solutions for improving mental health and illnesses in the aging process. 2013.
- 54.Dallery J, Jarvis B, Marsch L, Xie HY. Mechanisms of change associated with technology-based interventions for substance use. Drug Alcohol Depend. 2015;150:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalichman SC, Kalichman MO, Cherry C, Swetzes C, Amaral CM, White D, et al. Brief Behavioral Self-Regulation Counseling for HIV Treatment Adherence Delivered by Cell Phone: An Initial Test of Concept Trial. Aids Patient Care St. 2011;25(5):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aharonovich E, Greenstein E, O’Leary A, Johnston B, Seol SG, Hasin DS. HealthCall: Technology-based extension of motivational interviewing to reduce non-injection drug use in HIV primary care patients - a pilot study. Aids Care-Psychological and SocioMedical Aspects of Aids/Hiv. 2012;24(12):1461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, et al. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasin DS, Aharonovich E, Greenstein E. HealthCall for the smartphone: technology enhancement of brief intervention in HIV alcohol dependent patients. Addict Sci Clin Pract. 2014;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smillie K, Van Borek N, van der Kop ML, Lukhwaro A, Li N, Karanja S, et al. Mobile health for early retention in HIV care: a qualitative study in Kenya (WelTel Retain). Afr J AIDS Res. 2014;13(4):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet. 2011;378(9793):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. Aids. 2011;25(6):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odeny TA, Bailey RC, Bukusi EA, Simoni JM, Tapia KA, Yuhas K, et al. Effect of text messaging to deter early resumption of sexual activity after male circumcision for HIV prevention: a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;65(2):e50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsch LA, Dallery J. Advances in the psychosocial treatment of addiction: the role of technology in the delivery of evidence-based psychosocial treatment. Psychiatr Clin North Am. 2012;35(2):481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NACADA. Rapid situation assessment of drugs and substance abuse in Kenya. Nairobi, Kenya. 2012. [Google Scholar]
- 65.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 66.Substance Abuse and Mental Health Services Administration. AUDIT-C Overview [Available from: https://www.integration.samhsa.gov/images/res/tool_auditc.pdf.
- 67.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT : the Alcohol Use Disorders Identification Test : guidelines for use in primary health care2001:[1–41 pp.]. Available from: http://www.who.int/iris/handle/10665/67205.
- 68.Moretti-Pires RO, Corradi-Webster CM. Adaptação e validação do Alcohol Use Disorder Identification Test (AUDIT) para população ribeirinha do interior da Amazônia, Brasil. Cadernos de Saúde Pública. 2011;27:497–509. [DOI] [PubMed] [Google Scholar]
- 69.Nayak MB, Bond JC, Cherpitel C, Patel V, Greenfield TK. Detecting alcohol-related problems in developing countries: a comparison of 2 screening measures in India. Alcohol Clin Exp Res. 2009;33(12):2057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Arch Intern Med. 1998;158(16):17–95. [DOI] [PubMed] [Google Scholar]
- 71.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a Female Veterans Affairs Patient Population. Arch Intern Med. 2003;163(7):821–9. [DOI] [PubMed] [Google Scholar]
- 72.Martino SBS, Gallon SL, Hall D, Garcia M, Ceperich S, et al. Motivational interviewing assessment: Supervisory tools for enhancing proficiency. Salem, OR: Northwest Frontier Addiction Technology Transfer Center at Oregon Health and Science University; 2006. [Google Scholar]
- 73.Harder VS, Mutiso VN, Khasakhala LI, Burke HM, Ndetei DM. Multiple traumas, postelection violence, and posttraumatic stress among impoverished Kenyan youth. J Trauma Stress. 2012;25(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harder VS, Mutiso VN, Khasakhala LI, Burke HM, Rettew DC, Ivanova MY, et al. Emotional and Behavioral Problems among Impoverished Kenyan Youth: Factor Structure and Sex-Differences. J Psychopathol Behav Assess. 2014;36(4):580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dienes Z, Coulton S, Heather N. Using Bayes factors to evaluate evidence for no effect: examples from the SIPS project. Addiction. 2018;113(2):240–6. [DOI] [PubMed] [Google Scholar]
- 76.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. 2016(1879–0046 (Electronic)). [DOI] [PMC free article] [PubMed]
- 77.L’Engle KL, Mwarogo P, Kingola N, Sinkele W, Weiner DH. A randomized controlled trial of a brief intervention to reduce alcohol use among female sex workers in Mombasa, Kenya. J Acquir Immune Defic Syndr. 2014;67(4):446–53. [DOI] [PubMed] [Google Scholar]
- 78.Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction. 2011;106(12):2156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nyamathi A, Shoptaw S, Cohen A, Greengold B, Nyamathi K, Marfisee M, et al. Effect of Motivational Interviewing on Reduction of Alcohol Use. Drug Alcohol Depend. 2010;107(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity (Silver Spring, Md). 2012;20(2):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS medicine. 2013;10(1):e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madhombiro M, Dube-Marimbe B, Dube M, Chibanda D, Zunza M, Rusakaniko S, et al. A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART. 2017(1471–244X (Electronic)). [DOI] [PMC free article] [PubMed]
- 83.Madhombiro M, Dube-Marimbe B, Dube M, Chibanda D, Zunza M, Rusakaniko S, et al. A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART. BMC Psychiatry. 2017;17(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Widder R Learning to Use Motivational Interviewing Effectively: Modules. J Contin Educ Nurs. 2017;48(7):312–9. [DOI] [PubMed] [Google Scholar]
- 85.Palfai TP, Cheng DM, Bernstein JA, Palmisano J, Lloyd-Travaglini CA, Goodness T, et al. Is the quality of brief motivational interventions for drug use in primary care associated with subsequent drug use? Addict Behav. 2016;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


