Abstract
The microbiome is an integrated part of the human body that can modulate a variety of disease processes and affect prognosis, treatment response, complications and outcomes. The importance of allogeneic hematopoietic cell transplantation in cancer treatment has resulted in extensive investigations on the interaction between the microbiome and this treatment modality and is beginning to lead to clinical trials of microbiome-targeted interventions. Here we review some of these discoveries, as well as describe strategies being investigated to manipulate the microbiome for favorable outcomes.
Introduction
Increasingly, evidence has accumulated demonstrating that the community of commensal bacteria residing in our gastrointestinal (GI) tract is an important modulator of disease at biochemical and molecular levels1. The human microbiome project2 uncovered a surprising degree of microbiome variability between healthy individuals and has been a great tool, paving the way to help researchers investigate associations between disease parameters and clinical outcomes with microbiome heterogeneity, both at baseline and as disease advances.
The evidence has been particularly convincing for intestinal inflammatory diseases, including ulcerative colitis, Crohn’s disease, and graft-versus-host disease (GVHD), which is an immune-mediated condition mediated by cells derived from the graft infused into an allogeneic hematopoietic cell transplant (allo HCT) recipient3. Given that these are all intestinal inflammatory syndromes, the question arises of whether close proximity is an important contributor to microbiome-disease interactions.
Microbiota damage in allo HCT is common.
Allo HCT is a lengthy treatment modality that often results in multiple insults to the recipient’s microbiome structure. One way this structure can be characterized is by quantifying alpha diversity, an ecological measure which combines levels of richness of distinct bacteria and evenness in their relative abundances. Alpha diversity is commonly used as a convenient measure of microbiome health and is frequently reduced in patients with diseased states. Longitudinal monitoring of the microbiome of allo HCT recipients has demonstrated that changes in the microbiome, including loss of diversity, can be observed early during the treatment course when patients receive chemotherapeutic agents, radiation and the donor’s allogeneic hematopoietic cells4. Reduced diversity is often accompanied by prominent decreases in the abundances of commensal intestinal bacteria, many of which have been shown to have beneficial contributions to normal intestinal homeostasis including digestion, barrier function, and immune tolerance5.
Microbiota changes are associated with various poor outcomes
The intestinal microbiome during and after allo HCT has been examined by several groups with largely concordant results where many adverse outcomes are strongly associated with dysbiosis of the gut microbiota. Here we summarize some of these outcomes and how the microbiota is affected.
GVHD
Intestinal GVHD is common after allo HCT and inflammation is the hallmark of this disease. In mouse models, loss of bacterial diversity was noted to occur early after allo HCT in mice with GVHD6. Different mechanisms may explain microbiota contributions to GVHD; α-defensins are antimicrobial peptides produced by specialized granule-harboring epithelial cells known as Paneth cells that can target potentially pathogenic bacteria. In GVHD, Paneth cells are injured resulting in lower expression of antimicrobial peptides and possibly predisposing to dysbiosis by allowing harmful bacteria to expand7.
Febrile neutropenia in patients undergoing allo HCT is common and requires early treatment with empiric broad-spectrum antibiotics. Several of these antibiotics are considered clinically equivalent but interestingly can vary in their degree of collateral damage to the microbiome. This will be discussed in detail later in this review. Shono, et al found that GVHD severity, along with microbiome damage, can differ depending on the type of antibiotic used in both humans and in mice models. Specifically, antibiotics with stronger activity against obligate anaerobes, including piperacillin-tazobactam and carbapenems, resulted in increased disruption to the microbiome, higher intestinal barrier damage, and more severe GVHD, compared to a narrower-spectrum antibiotic such as aztreonam8.
Congruent with these antibiotic associations, multiple studies have found that preservation of obligately anaerobic bacteria is associated with protection from GVHD. Abundance of bacteria from the genus Blautia, an abundant commensal member of the Clostridia class of Firmicutes, has been associated with better survival, less GVHD-related or relapse-related mortality, and less need for GVHD treatment with systemic steroids9. An independent study of adult patients also found that bacteria from the family Lachnospiraceae (which includes Blautia and belongs to the class Clostridia) are associated with less GVHD10. Pediatric patients with Clostridia were also found to have reduced GVHD in independent cohorts from two transplant centers11. Mechanisms for this association remain unclear, but one study found that recovery of mucosal-associated invariant T cells, an immune regulatory population, is highly heterogeneous after allo HCT but is associated with increased abundance of intestinal Blautia species12. A metabolite commonly produced by Clostridia, butyrate, was found to directly alleviate GVHD in mouse models, via support of intestinal epithelial cells13. In addition to Clostridia, other clinical studies have found that Bacteroides species, another intestinal obligately anaerobic commensal, was associated with less GVHD14. One open question is whether, in addition to the host microbiome, the composition of the HCT donor’s microbiome can have any potential impact on GVHD severity.
Clostridium difficile infection
Clostridium difficile infection (CDI) in allo HCT patients requires close monitoring. Watery diarrhea is often the presenting symptom that prompts testing but sometimes treatment can be started while awaiting test results15. A single center retrospective study showed that the majority of CDI cases occur in the first 6 months following allo HCT. Total body irradiation (TBI) and GVHD are known risk factors as both result in gut dysbiosis and intestinal barrier dysfunction, predisposing to local infection and bacteremia respectively with less clear effects on overall mortality16.
The majority of cases of CDI occur in the peri-transplantation period. Healthier patients without comorbid diseases are less likely to develop early CDI in the pre-engraftement period but CDI in post-engraftement period is common and may potentially warrant outpatient surveillance17, though this is not yet recommended by guidelines. Presence at engraftment of usually abundant obligate anaerobes, including Bacteroidetes, Lachnospiraceae, and Ruminococcaceae, was associated with a 60% lower risk of CDI in the postengraftement period18. Since antibiotics are routinely administered during the course of allo HCT, CDI in this patient population frequently recurs and is associated with loss of the same obligately anaerobic intestinal bacteria19.
Bacteremia
Bacteremia is a potentially lethal complication in patients undergoing allo HCT. In patients who develop this complication, reduced microbiome diversity is frequently observed20. They also display loss of obligately anaerobic commensal bacteria, and instead often show gut domination by pathogenic bacteria, including Enterococcus and Proteobacteria (which includes E. coli, Klebsiella, and other gram-negative facultatively anaerobic bacteria) which match the bacteria isolated from the bloodstream, suggesting that the pathophysiology of bacteremia may require dysbiosis as well as impaired intestinal barrier function. Domination with Enterococcus, including strains expressing genes that encode vancomycin resistance, has been particularly associated with metronidazole use. Interestingly, Proteobacterial domination was reduced with by levofloxacin use, an example where antibiotics can have a positive clinical effect via the microbiome. In another study, a broadly-active combination of ciprofloxacin and metronidazole prophylaxis was associated with domination by Enterococcus which was more pronounced when GVHD is present21. This emphasizes the importance of antibiotic selection in the peri-transplantation period which will be further discussed below.
Recently, high-resolution metagenomic sequencing of paired blood and stool samples from HCT patients with bacteremia confirmed that in many but not all cases, commensal intestinal bacteria and blood pathogens shared significant genetic similarity indicating a highly probable gut origin22. Interestingly, this was also true for some pathogens that are less known to be enteric, such as Pseudomonas aeruginosa and Staphylococcus epidermidis, suggesting that the traditional teaching that these pathogens are normally rare in the intestinal tract is not necessarily true in all patient populations.
Respiratory complications
The upper respiratory tract hosts the majority of the microbiome which can potentially relocate to the lungs, and associations between the intestinal microbiome and pulmonary outcomes after allo HCT have also been observed. Challenges in studying the microbiome of the respiratory tract include variability in sampling methods23, but significant progress is being made. Parallel RNA/DNA sequencing of lower respiratory specimens in immune compromised pediatric patients could identify pathogens in many patients where no clinical isolate could be identified using current microbiological methods, indicating that sequencing approaches can augment standard techniques24. Interestingly, evidence for a gut-lung axis also exists. A study by Harris, et al25 showed that low baseline GI microbiome diversity and at the time of engraftment was associated with more early pulmonary complications including infections, transfusion reactions, interstitial pneumonitis. Peri-transplant antibiotic use has been found to be a risk factor for development of respiratory viral infections in allo HCT recipients26, and GI colonization with butyrate-producing commensal bacteria were associated with protection from lower respiratory tract viral infection following allo HCT27, suggesting that intestinal bacteria-derived butyrate could potentially support systemic antiviral immunity. Similarly, gut domination of Proteobacteria in allo HCT recipients was associated with low survival and more respiratory complications28.
Overall survival
Studies have shown that intestinal microbiota diversity at the time of engraftment is a potential clinical predictor of mortality in allo HCT29. This association appears to be driven by treatment-related mortality (TRM), a composite endpoint which includes GVHD-related mortality and mortality due to infectious complications, which have individually been associated with microbiome damage as described above, and are often inextricably linked clinically. CMV, for example, reactivates frequently in patients treated with immune suppression for GVHD, but reactivation can also precede GVHD30. Notably, TRM excludes relapse, and relapse as an adverse outcome has not been associated with reduced microbiome diversity31. Potential relationships that exist between disease relapse following allo HCT and host microbiome are understudied. Peled, et al31 have reported an association between presence and higher abundance of Eubacterium limosum with decreased risk for relapse in these patients. Interestingly, not all disease types were equally associated; freedom from relapse of acute myelogenous leukemia and multiple myeloma were particularly associated with having Eubacterium limosum in the intestinal tract. Whether this observation can be replicated by other centers is still unknown.
The microbiome as a target of intervention
The observation that the intestinal microbiome composition is associated with a variety of important clinical outcomes after allo HCT, including that microbiome diversity often precedes TRM from allo HCT, leads to hypotheses that have yet to be formally tested clinically. One is that loss of diversity is a biomarker that can robustly and reproducibly predict survival after allo HCT. Multicenter efforts are currently underway to address this hypothesis, including a trial by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1703/1801) (NCT03959241). A second, potentially more intriguing hypothesis, is that damage to the intestinal microbiome can be prevented or treated, and this may lead to improved clinical outcomes.
Reducing the complexity of microbiome data to an alpha diversity metric is very useful, given the relative ease of intuitive understanding, as well as the strength of its association with outcomes such as overall survival. There are important limitations, however, to using alpha diversity as a biomarker32. Some are methodological – there are many ways to quantify and weigh the relative contributions of evenness and richness, and it is unclear which methods are more predictive of outcomes. There are also upstream computational decisions that would have to be standardized to allow characterization of a “normal range” of diversity, including when and how to rarefy sequences to deal with inherent differences in sequencing depth. It is also possible to have “false positives” for high diversity – for example, if many normal commensal bacteria are missing, but bacteria that are present happen to be equally abundant. Finally, current sequencing technologies require batching hundreds of samples in order for 16S sequencing to be cost-effective, and thus a quick turn-around time that would be necessary for microbiome diversity to guide clinical practice has yet to be made feasible and available.
An alternative to directly measuring microbiome diversity by sequencing is to detect its metabolic activity. Weber, et al33 developed a tool that can indirectly quantify microbiota composition by measuring levels of 3-indoxyl sulfate in the urine. This metabolite is produced from indole that is a by-product of metabolism of dietary tryptophan by intestinal bacteria. The authors found that higher levels of urinary 3-indoxyl sulfate early after transplantation predicted reduced transplant-related mortality and improved overall survival.
Optimizing antibiotic use
Empiric and targeted treatment for neutropenic fever
Antibiotics are used frequently in allo HCT patients either empirically for febrile neutropenia or culture-targeted for known infections. Current guidelines for the treatment of neutropenic fever do not take into consideration microbiome bystander effects, and many recommended antibiotics are in fact highly damaging to obligately-anaerobic intestinal commensals. Shono, et al8 compared common antibiotics used for febrile neutropenia and found that imipenem-cilastatin and piperacillin-tazobactam were associated with higher GVHD-related mortality, compared to aztreonam and cefepime. Mice experiments showed that imipenem-treated mice had aggravated GVHD and this was attributable to expansion of intestinal mucus-degrading bacteria Akkermansia muciniphila. Holler, et al21 found that gut dysbiosis characterized by Enterococcus predominance was commonly seen in patients receiving ciprofloxacin and metronidazole prophylaxis. In pediatric patients undergoing allo HCT, exposure to clindamycin was seen to associated with depletion of Clostridia as well as with more GVHD11. A recent retrospective review found that patients who received oral broad-spectrum antibiotics as bacterial prophylaxis had higher rates of GVHD34. Finally, the timing of antibiotic administration has also been retrospectively associated with GVHD; patients who began receiving antibiotics for neutropenic fever pre-transplant had worsened outcomes compared to those who received antibiotics post-transplant, with reductions in stool Clostridia abundance and urinary 3-indoxyl sulfate as well as increased TRM35.
A randomized clinical trial is currently underway investigating how the use of piperacillin-tazobactam and cefepime as empiric antibiotic therapy for febrile neutropenia in allo HCT can affect Clostridiales abundance in the intestine (NCT03078010).
Prophylactic strategies – oral decontamination
In contrast to recent studies indicating that antibiotic use leads to microbiome injury and aggravates GVHD, early studies in the 1970s in mice found that gut decontamination with oral antibiotics reduced GVHD and improved survival36, and initial clinical studies showed similar benefits37, as well as a more recent randomized study38. Results of subsequent studies, however, have been mixed39. A recent retrospective microbiological analysis of stool specimens collected from patients who received decontamination found that success of decontamination was only roughly 50%, and successful decontamination was associated with less acute GVHD40. A clinical trial is planned to re-evaluate oral decontamination followed by 3rd-party fecal microbiota transplantation (FMT) in selected allo HCT patients who require antibiotic treatment with meropenem or piperacillin-tazobactam, both of which have been seen to be associated with increased GVHD (NCT03862079).
Prophylactic strategies – selective decontamination
Per joint American Society of Clinical Oncology /Infectious Diseases Society of America guidelines, fluoroquinolones are the preferred prophylaxis in febrile neutropenia41. Levofloxacin was very effective in reducing infections and bacteremia in patients with high-risk neutropenia42. More recent studies have found that quinolone prophylaxis is associated with reduced intestinal domination by gram-negative bacteria20. Thus quinolones appear to mediate a benefit by selectively reducing potentially pro-inflammatory bacteria without broadly targeting obligately-anaerobic commensals. Polymixin B, which similarly targets gram-negative bacteria, showed a similar benefit in a mouse model of GVHD by reducing the abundance of intestinal E. coli7.
A possible alternative to fluoroquinolones for bacterial prophylaxis allo HCT is rifaximin. The transplant center at Regensburg changed its institutional practice from prophylaxis with ciprofloxacin and metronidazole to rifaximin, and compared outcomes in two cohorts of patients before and after the switch43. They found comparable episodes of fever, infections and bacteremia, suggesting similar efficacy for infectious prophylaxis. Intriguingly, they also noted that patients receiving rifaximin had reduced gut dysbiosis with higher urinary 3-indoxyl sulfate, as well as reduced transplant-related mortality and improved overall survival.
Microbiome interventions
Diet
When medically not contraindicated, oral intake is usually encouraged in allo HCT patients, and clinicians monitor oral nutrition as a measure of a healthy prognosis, including a reduced incidence of severe GVHD44. One study showed that in allo HCT patients, supplemental enteral nutrition, compared to parenteral nutrition, was associated with better overall survival and protection from severe acute GVHD45. Similar results were found with home-based care for allo HCT as these patients had better oral intake than those transplanted in the inpatient hospital setting46. Another study showed that parenteral nutrition in allo HCT patients, compared to enteral feeding, was associated with more infections and increased escalation of medical care with central access and ICU transfer with higher overall mortality from infections47,48. One limitation to drawing conclusions from non-randomized studies associating improved oral nutrition with better outcomes is that patients who are able to tolerate oral intake are generally healthier and more welling to initiate oral feed in contrary to the other group. Importantly, a phase 3 clinical trial by a group in France is currently underway comparing enteral to parenteral nutrition (NCT01955772).
The type of oral nutrition in the peri-HCT period may also be an important factor. Traditionally, allo HCT patients are recommended to limit their diet to well-cooked foods and peeled fruits, known as a neutropenic diet. In recent years, this practice has been examined and been found to have a limited benefit, or even potentially some harm49. One center recently made an institutional practice switch from a neutropenic diet to a more liberalized diet, and found no increase in infections or other adverse outcomes50. A randomized study addressing the same question also found no benefit to a neutropenic diet51. The short term52 and long-term53 effects of dietary differences on the microbiome have been reported in healthy volunteers, and the effects in the allo HCT population warrant further study.
Prebiotics
Prebiotics are dietary supplements designed to promote the growth or modify the metabolism of beneficial intestinal commensal bacteria, and are commonly used to target the microbiome. In allo HCT patients, a small number of studies have been performed. One study in Japan showed that allo HCT patients who were given supplemental products enriched with glutamine, fiber and oligosaccharides (polydextrose, lactosucrose and dextrin) had less diarrhea, mucositis, enterococcus bacteremia and better survival54. Potato starch (NCT02763033), gluten-free diet (NCT03102060), and fructose oligosaccharides (NCT02805075) are all currently being studied for potential benefit in HCT patients.
Probiotics
Probiotics are ingestible formulations of live bacteria that can modulate intestinal homeostasis. Because they are only regulated for safety and not for medical efficacy, they are widely available, and many questions regarding their effects on the microbiome and health persist55. Some probiotic studies have been performed in the allo HCT population. Lactobacillus plantarum was recently found to be safe to administer during neutropenia in the pediatric allo HCT population56 and a multi-center study evaluating for potential benefit for GVHD is underway (NCT03057054).
Results with Lactobacillus rhamnosus GG (LGG) have been mixed. In a murine model, administration of LGG resulted in lower GVHD scores and less pathologic sequelae and improved survival57. A recent randomized clinical trial, however, did not show any appreciable protection from GVHD in humans receiving LGG58. Patients receiving LGG did not show measurable increases in the abundance of Lactobacillus, which is in accordance with prior studies in healthy volunteers indicating lack of persistent colonization, though colonoscopic biopsies appear to be more sensitive for detecting LGG than stool samples59.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) has been reliably shown to be effective in treating recurrent Clostridium difficile infection60,61, including in allo HCT patients with recurrent Clostridium difficile infection62–64. In the allo HCT population, FMT has also been performed as therapy for GVHD, reported now in several small case series65–68. In all of these, patients with steroid-dependent or steroid-refractory GVHD responded to FMT and were noted to have improvements in microbiome composition. The treatment was generally well-tolerated without serious adverse effects.
The prophylactic use of FMT in allo HCT recipients without symptoms of GVHD has also begun to be investigated by two groups, including a 13-patient single-arm study of oral frozen 3rd-party FMT capsules69, and a randomized study of 25 patients, 14 of whom received an autologous FMT collected prior to HCT conditioning70. Both studies administered FMT following neutrophil recovery, and both show evidence of improved microbiome composition in patients following FMT. There has yet, however, to be any evidence of improvements in clinical outcomes with prophylactic FMT, and follow-up as well as larger studies are being planned.
Conclusion
Early insights tend to resurge as development of new technologies allow a better understanding of patterns long-before observed in the past. The recent discoveries of changes in the microbiome during allo HCT is a perfect example of this phenomenon. The importance of clinical decision-making that incorporates cognizance of the microbiome is increasingly apparent, and additional strategies to protect, restore, and perhaps even augment the microbiome may yet provide approaches to further optimize outcomes in this vulnerable patient population.
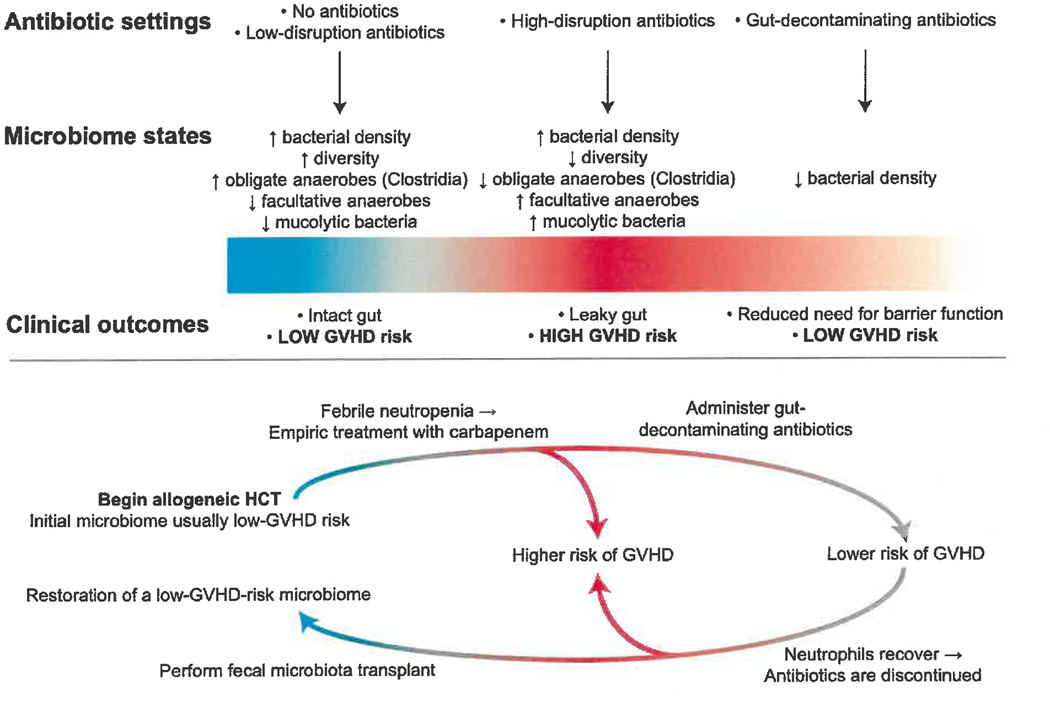
Figure 1.
Empiric antibiotics used for febrile neutropenia in hematopoietic cell transplant (HCT) patients have been linked to a dysbiotic microbiome and worsened outcomes, including increased graft-versus-host disease (GVHD). Fecal microbiota transplantation can potentially correct this disruption and potentially reduce GVHD.
Footnotes
Chapter Name
This template is formatted for submission of your manuscript to a book publisher. Styles have been created to keep the font and spacing formatting properly applied to your text. Pages have one-inch margins on all sides, and the text is double-spaced an in the Times New Roman font, which is a fixed-space font. Paragraphs are indented five spaces, or one-half inch, from the left and are left-justified.
You should submit your manuscript with an approximate word count in the upper right-hand corner of the title page (rounded to the nearest one hundred words). You can use Microsoft Word’s convenient word count feature to get this number by choosing Word Count from the Proofing section of the Review menu.
Each chapter should begin on a new page. Keep in mind while writing your manuscript that you should follow each sentence with two spaces. Also, spell out numbers as words, do not hyphenate words that do not ordinarily contain a hyphen, and use underlining instead of italics. These are editors’ preferences which make reading your manuscript easier.
Starting on page two, your last name, the title, and the page number appear in the header section. Your last name and the title update automatically in the header when you type them in on the title page.
References
- 1.Slingerland AE, Schwabkey Z, Wiesnoski DH, Jenq RR. Clinical Evidence for the Microbiome in Inflammatory Diseases. Front Immunol. 2017;8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taur Y. Intestinal microbiome changes and stem cell transplantation: Lessons learned. Virulence. 2016;7(8):930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montassier E, Batard E, Massart S, et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol. 2014;67(3):690–699. [DOI] [PubMed] [Google Scholar]
- 6.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120(1):223–231. [DOI] [PubMed] [Google Scholar]
- 8.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(8):1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golob JL, Pergam SA, Srinivasan S, et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin Infect Dis. 2017;65(12):1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simms-Waldrip TR, Sunkersett G, Coughlin LA, et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol Blood Marrow Transplant. 2017;23(5):820829. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya A, Hanafi LA, Sheih A, et al. Graft-Derived Reconstitution of Mucosal-Associated Invariant T Cells after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(2):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biagi E, Zama D, Nastasi C, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015;50(7):992–998. [DOI] [PubMed] [Google Scholar]
- 15.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willems L, Porcher R, Lafaurie M, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2012;18(8):1295–1301. [DOI] [PubMed] [Google Scholar]
- 17.Dubberke ER, Reske KA, Olsen MA, et al. Risk for Clostridium difficile Infection After Allogeneic Hematopoietic Cell Transplant Remains Elevated in the Postengraftment Period. Transplant Direct. 2017;3(4):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Arguello ES, Jenq RR, et al. Protective Factors in the Intestinal Microbiome Against Clostridium difficile Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. J Infect Dis. 2017;215(7):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–438. [DOI] [PubMed] [Google Scholar]
- 20.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med. 2018;24(12):1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15(5):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinter MS, Dvorak CC, Mayday MY, et al. Pulmonary Metagenomic Sequencing Suggests Missed Infections in Immunocompromised Children. Clin Infect Dis. 2019;68(11):1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris B, Morjaria SM, Littmann ER, et al. Gut Microbiota Predict Pulmonary Infiltrates after Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med. 2016;194(4):450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogimi C, Krantz EM, Golob JL, et al. Antibiotic Exposure Prior to Respiratory Viral Infection Is Associated with Progression to Lower Respiratory Tract Disease in Allogeneic Hematopoietic Cell Transplant Recipients. Biol Blood Marrow Transplant. 2018;24(11):2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haak BW, Littmann ER, Chaubard JL, et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131(26):2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunker JJ, Flynn TM, Koval JC, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015;43(3):541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(9):1309–1314. [DOI] [PubMed] [Google Scholar]
- 31.Peled JU, Devlin SM, Staffas A, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol. 2017;35(15):1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner BD, Grunwald GK, Zerbe GO, et al. On the Use of Diversity Measures in Longitudinal Sequencing Studies of Microbial Communities. Front Microbiol. 2018;9:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber D, Oefner PJ, Hiergeist A, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015;126(14):1723–1728. [DOI] [PubMed] [Google Scholar]
- 34.Routy B, Letendre C, Enot D, et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology. 2017;6(1):e1258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber D, Jenq RR, Peled JU, et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(5):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45(3):577–588. [PubMed] [Google Scholar]
- 37.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–307. [DOI] [PubMed] [Google Scholar]
- 38.Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93(10):3267–3275. [PubMed] [Google Scholar]
- 39.Petersen FB, Buckner CD, Clift RA, et al. Laminar air flow isolation and decontamination: a prospective randomized study of the effects of prophylactic systemic antibiotics in bone marrow transplant patients. Infection. 1986;14(3):115–121. [DOI] [PubMed] [Google Scholar]
- 40.Vossen JM, Guiot HF, Lankester AC, et al. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS One. 2014;9(9):e105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018:JCO1800374. [DOI] [PubMed] [Google Scholar]
- 42.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977–987. [DOI] [PubMed] [Google Scholar]
- 43.Weber D, Oefner PJ, Dettmer K, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(8):1087–1092. [DOI] [PubMed] [Google Scholar]
- 44.Bechard LJ, Guinan EC, Feldman HA, Tang V, Duggan C. Prognostic factors in the resumption of oral dietary intake after allogeneic hematopoietic stem cell transplantation (HSCT) in children. JPEN J Parenter Enteral Nutr. 2007;31(4):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seguy D, Duhamel A, Rejeb MB, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94(3):287–294. [DOI] [PubMed] [Google Scholar]
- 46.Svahn BM, Remberger M, Heijbel M, et al. Case-control comparison of at-home and hospital care for allogeneic hematopoietic stem-cell transplantation: the role of oral nutrition. Transplantation. 2008;85(7):1000–1007. [DOI] [PubMed] [Google Scholar]
- 47.Seguy D, Berthon C, Micol JB, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006;82(6):835–839. [DOI] [PubMed] [Google Scholar]
- 48.Guieze R, Lemal R, Cabrespine A, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33(3):533–538. [DOI] [PubMed] [Google Scholar]
- 49.Trifilio S, Helenowski I, Giel M, et al. Questioning the role of a neutropenic diet following hematopoetic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(9):1385–1390. [DOI] [PubMed] [Google Scholar]
- 50.Taggart C, Neumann N, Alonso PB, et al. Comparing a Neutropenic Diet to a Food Safety-Based Diet in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 51.Moody KM, Baker RA, Santizo RO, et al. A randomized trial of the effectiveness of the neutropenic diet versus food safety guidelines on infection rate in pediatric oncology patients. Pediatr Blood Cancer. 2018;65(1). [DOI] [PubMed] [Google Scholar]
- 52.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyama S, Sato T, Tatsumi H, et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep Oncol. 2014;7(3):692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. [DOI] [PubMed] [Google Scholar]
- 56.Ladas EJ, Bhatia M, Chen L, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51(2):262–266. [DOI] [PubMed] [Google Scholar]
- 57.Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103(11):4365–4367. [DOI] [PubMed] [Google Scholar]
- 58.Gorshein E, Wei C, Ambrosy S, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2017;31(5). [DOI] [PubMed] [Google Scholar]
- 59.Alander M, Satokari R, Korpela R, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol. 1999;65(1):351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–508. [DOI] [PubMed] [Google Scholar]
- 61.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. [DOI] [PubMed] [Google Scholar]
- 62.Moss EL, Falconer SB, Tkachenko E, et al. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS One. 2017;12(8):e0182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bluestone H, Kronman MP, Suskind DL. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infections in Pediatric Hematopoietic Stem Cell Transplant Recipients. J Pediatric Infect Dis Soc. 2018;7(1):e6–e8. [DOI] [PubMed] [Google Scholar]
- 64.Webb BJ, Brunner A, Ford CD, Gazdik MA, Petersen FB, Hoda D. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2016;18(4):628–633. [DOI] [PubMed] [Google Scholar]
- 65.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spindelboeck W, Schulz E, Uhl B, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017;102(5):e210–e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaito S, Toya T, Yoshifuji K, et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018;2(22):3097–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi X, Li X, Zhao Y, et al. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease With Fecal Microbiota Transplantation: A Pilot Study. Front Immunol. 2018;9:2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2(7):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taur Y, Coyte K, Schluter J, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10(460). [DOI] [PMC free article] [PubMed] [Google Scholar]