Abstract
Background
High-risk coronary artery plaque (HRP) is associated with increased risk of acute coronary syndrome.
We aimed to investigate the prevalence of HRP in asymptomatic patients with type 2 diabetes (T2D), and its relation to patient characteristics including cardiovascular risk factors, diabetes profile, and coronary artery calcium score (CACS).
Methods
Asymptomatic patients with T2D and no previous coronary artery disease (CAD) were studied using coronary computed tomography angiography (CCTA) in this descriptive study. Plaques with two or more high-risk features (HRP) defined by low attenuation, positive remodeling, spotty calcification, and napkin-ring sign were considered HRP. In addition, total atheroma volume (TAV), proportions of dense calcium, fibrous, fibrous-fatty and necrotic core volumes were assessed. The CACS was obtained from non-enhanced images by the Agatston method. Cardiovascular and diabetic profiles were assessed in all patients.
Results
In 230 patients CCTA was diagnostic and 161 HRP were detected in 86 patients (37%). Male gender (OR 4.19, 95% CI 1.99–8.87; p < 0.01), tobacco exposure in pack years (OR 1.02, 95% CI 1.00–1.03; p = 0.03), and glycated hemoglobin (HbA1c) (OR 1.04, 95% CI 1.02–1.07; p < 0.01) were independent predictors of HRP. No relationship was found to other risk factors. HRP was not associated with increased CACS, and 13 (23%) patients with zero CACS had at least one HRP.
Conclusion
A high prevalence of HRP was detected in this population of asymptomatic T2D. The presence of HRP was associated with a particular patient profile, but was not ruled out by the absence of coronary artery calcium. CCTA provides important information on plaque morphology, which may be used to risk stratify this high-risk population.
Trial registration This trial was retrospectively registered at clinical trials.gov January 11, 2017 trial identifier NCT03016910.
Keywords: Coronary computed tomography angiography, Atherosclerosis, Asymptomatic coronary artery disease, High-risk plaque, Coronary artery calcium score, Type 2 diabetes
Introduction
Acute myocardial infarction (MI) is mainly caused by the rupture of a coronary artery plaque and subsequent coronary artery occlusion. Ruptured plaques are characterized as lipid-rich plaques with positive remodeling (PR) covered by a thin fibrous cap infiltrated by inflammatory cells [1, 2]. Beyond the assessment of coronary artery stenosis coronary computed tomography angiography (CCTA) enables evaluation of coronary artery plaque morphology and burden. Mounting evidence suggests, that plaque morphology assessed by CTTA [3], and by invasive coronary imaging [4, 5], is an important prognostic factor. In particular, plaques with both low attenuation and positive remodeling have a strong association with cardiac events, and may be considered high-risk plaques (HRP) [6–8]. However, the characteristic of patients with high-risk plaque and the relation to atherosclerotic burden is less clear.
Large epidemiological studies have demonstrated that patients with type 2 diabetes (T2D) and no prior MI have the same cardiovascular risk as non-diabetics with a previous MI [9, 10]. Furthermore, patients with T2D have a 30% greater mortality when suffering a MI compared to patients without T2D [11]. Despite effective risk-factor control, individuals with T2D still have twice the risk of MI as non-diabetics [11]. This residual risk of MI implies that intrinsic properties of diabetes are involved in the atherosclerotic process. However, the mechanisms underlying the increased frequency of MI in T2D are not yet well understood. The objectives of this study were the following: (1) to examine the prevalence of HRP in asymptomatic T2D; (2) to investigate the relationship between HRP and cardiovascular risk factors and diabetes profile; (3) to investigate the relationship between HRP and the coronary artery calcium score (CACS).
Methods
Study population
This was a single-center cross-sectional study performed at Odense University Hospital, Svendborg, Denmark, from March 2016 to September 2017. A total of 261 consecutive patients with asymptomatic T2D, over the age of 18, gave written informed consent for participation in the study. There was no clinical indication for CCTA, and the examination was exclusively performed with the purpose of a descriptive study. Patients were recruited at the Endocrinology Outpatient Clinic and the hospital Retina Photography Clinic. The diagnostic criteria for type 2 diabetes included hbA1c ≥ 48 mmol/mol on two separate occasions and the absence of glutamatic acid decarboxylase antibodies [12]. Patients were not eligible if they had a history of coronary artery disease (CAD), documented heart failure, reduced glomerular filtration (< 45 ml/min), or allergy to iodine contrast. A detailed health status was recorded in all patients. Blood was drawn for creatinine, glycated hemoglobin (HbA1c), lipid profile, CRP and troponin T. All patients had a spot urine was collected to assess micro- and macroalbuminuria. Albuminuria was defined as albumin-creatinine ratio ≥ 30 mg/L in at least two independent samples and no symptoms of urinary tract infection. Blood pressure was measured in a seated position after at least 10 min of rest. Patients receiving one or more antihypertensive drugs were categorized as having hypertension. Patients treated with one or more lipid-lowering drugs were categorized as having hypercholesterolemia. Patients underwent annual screening for retinopathy and neuropathy as a part of their clinical controls. Retinopathy was graded from 0 to 4 [13], and peripheral neuropathy was graded as normal, reduced, or absent. Diabetic complications were defined as presence of any degree of albuminuria, retinopathy, and/or neuropathy. Smoking history was evaluated to assess the total tobacco exposure in terms of pack years (1 pack year = 20 cigarettes per day for one year) [14].
CTTA acquisition
The CCTA images were acquired using a standardized protocol for a 256-detector system (GE-revolution CT, GE healthcare, Waukesha, Wisconsin, USA). An unenhanced scan was initially performed to assess coronary artery calcium.
Patients were prepared for the scan with tablet ivabradine 7.5 mg once daily two days prior to the scan. If needed, intravenous beta blocker was administered on the day of the scan in patients with increased heart rate (> 65 beats per minute) [15]. Sublingual fast acting nitrates were administered just before the enhanced scan. Images were obtained by an ECG-gated prospective acquisition in the 75% of the R-R interval with an additional padding of 45 ms (ms) to allow additional reconstruction. In patients with a high heart rate an additional phase was acquired in the 40% phase of the R-R interval, and a repeated scan was acquired if the heart rhythm was irregular. A total of 60 ml of iodine contrast (Visipaque 320 mg iodine/ml) was injected at 5 ml/s, and the scan was timed visually, when maximal attenuation was detected in the ascending aorta. Tube voltage and current were modulated to the patient’s body size with a tube voltage between 80 to 140 kV and a tube current between 150 and 700 milliampere according to body mass index (BMI). Gantry rotation time was 280 ms with 16 cm axial coverage. The median radiation dose per scan was 2.01 millisievert.
The slice thickness and interval for reconstruction were both 0.625 mm, and 40% adaptive statistical iterative reconstruction (ASiR) was adopted. All available phases were reconstructed, and images with superior image quality were selected for analysis.
Quantitative CCTA analysis
Reconstructed images were analyzed on an offline workstation with validated semiautomatic software (Qangio CT Research Edition ver. 3.1.3.18, Medis NL) [16]. Experienced observers (LJH and TRA) analyzed all images while blinded to patient characteristics. Plaque location was designated according to the American Heart Association modified 17 segment model [17]. Centerlines of each coronary artery were automatically extracted by the software. On the basis of longitudinal images, cross sectional lumen and vessel contours were created and manually corrected by the observer if necessary. Segments with insufficient image quality were excluded, and the software automatically excluded segments with a lumen diameter less than 1.5 mm. Diagnostic CCTA was considered as an overall image quality allowing qualitative and quantitative plaque analysis. Patients with severe motion artifacts or otherwise compromised image quality were excluded from the analysis.
All available segments were screened for the presence of plaque, and segments without visible plaque were excluded from the analysis [18]. For each patient the following parameters were calculated: total atheroma volume (TAV) (total vessel volume—total lumen volume), percent atheroma volume (PAV) (total vessel volume—total lumen volume/total vessel volume × 100%), and normalized atheroma volume (NAV) (total vessel volume—total lumen volume/mean segment length).
Furthermore, the volumes of plaque types dense calcium, fibrous, fibrous–fatty, and necrotic core were calculated on the basis of Hounsfield Units (HU). The software applied a dynamic algorithm that adapted the HU thresholds according to luminal contrast densities [19]. To address the differences in length of vessels analyzed in HRP and non-HRP patients TAV and volumes of fibrous, fibrous-fatty, necrotic core and necrotic core plaque were normalized according to vessel length by the following formula: (plaque volume/segment length) × mean segment length population.
Qualitative CCTA analysis
All non-calcified and mixed plaques were screened for the following plaque features:
Low attenuated plaque (LAP): non-calcified or mixed plaque with a volume > 1 mm3 containing voxels with attenuation less than 30 HU.
Positive remodeling (PR): Remodeling indices was calculated as lesion diameter/reference area, where the reference diameter is from a normal/minimal diseased proximal segment. PR was defined as more than 5% area remodeling [20, 21].
Spotty calcification (SC): Calcified elements less than 3 mm in length occupying less than 90 degrees of the coronary arc.
Napkin ring sign (NRS): A non-calcified ring like structure surrounding a central area with a lower attenuation. HRP was defined as a plaque with the presence of two or more high-risk features.
Furthermore, for all plaque the percent diameter stenosis was calculated by the following: 1 − (minimal lumen diameter plaque/lumen diameter reference segment) × 100%. The degree of stenosis were reported as mild (25–49%), moderate (50–69%) and severe stenosis (> 70%).
Coronary artery calcium score
The coronary artery calcium score (CACS) was calculated by the Agatston score using the unenhanced CT images. Patients were categorized in groups according to the CACS:
No CAD (CACS = 0), minimal CAD (CACS = 1–10), mild CAD (CACS = 11–100), moderate CAD (CACS = 101–400), and severe CAD (CACS > 400).
Statistical analysis
Statistical analyses were performed by STATA IC 14.2. Baseline parameters were presented as means with standard deviation. Risk factors with a non-normal distribution were presented as medians with interquartile range. A t-test was performed to assess the relationship between baseline parameters and HRP on a per-patient level. The association of HRP and variables with a p < 0.05 were tested in univariate and multivariate logistic analysis adjusted for confounders including age, sex, dyslipidemia, and systolic blood pressure. Inter- and intra-reader agreement were assessed by Pearson’s correlation coefficient (Pearson’s r) and Bland–Altman analysis using 95% limits of agreement (LOA) [22] for twenty random vessels.
Results
Study population
Of the 261 patients recruited for the study, 230 had a diagnostic CCTA and were included in the final analysis. Mean age was 62 ± 10 years and 73% of patients were men. The mean diabetes duration was 11 ± 8 years, and 54% of the patients had diabetic complications. Retinopathy was present in 52 patients (stage 1; n = 23, stage 2; n = 21, stage 3: n = 3, stage 4: n = 5). Albuminuria was present in 65 patients (microalbuminuria n = 61 and macroalbuminuria n = 4.) and neuropathy was present in 57 patient and 38 patients (17%) had two or more complications. In 144 (63%) of patients, diabetes care was managed by a specialist at the Endocrinology Outpatient Clinic, and the remaining patients were followed by their general practitioner (GP). We identified 161 high-risk plaques in 86 (37%) patients. Furthermore, we identified CAD without HRP in 117 (51%) patients, and 27 (12%) patients had no evidence of CAD.
Cardiovascular risk factors
High-risk plaques were more frequent in men (90 vs. 63%, p < 0.001) and were associated with a higher tobacco exposure in pack years (20.2 vs. 13.1, p = 0.001), and patients with HRP had a lower BMI (29.4 vs. 31.2 kg/m2, p = 0.001) (Table 1). In the univariate logistic regression male gender (OR 4.92, 95% CI: 2.28–10.6, p < 0.001) and smoking in pack years (OR 1.02, 95% CI: 1.00–1.03, p = 0.01) were significant predictors of HRP and remained significant in the multivariate analysis. BMI was negatively associated with HRP (OR 0.92, 95% CI: 0.86–0.97, p = 0.01) but was not significant in the multivariate analysis (Table 2). Baseline characteristics were compared between men and women and men had a greater tobacco exposure (17.5 ± 20.1 vs. 11.6 ± 15.9 pack years, p = 0.04). In addition, men had higher HbA1c (61.5 ± 14.4 vs. 55.9 ± 12.7 mmol/mol, p = 0.01), and a greater proportion of diabetic complications (64 vs. 47%, p = 0.01). The median CACS was higher in men (137.0 IQR: 13–699 vs. 4.0 IQR 0–121, p < 0.001). Baseline characteristics stratified by sex are shown in Table 5 in the appendix section.
Table 1.
Baseline characteristics of patients with and without high-risk plaque
| Variable | Overall n = 230 | HRP present n = 86 | HRP not present = 144 | p-value |
|---|---|---|---|---|
| Age | 62 ± 10 | 62 ± 9 | 61 ± 10 | 0.40 |
| Male, n (%) | 144 (63) | 77 (90) | 91 (63) | < 0.001 |
| BMI, kg/m2 | 30.6 ± 3.78 | 29.4 ± 3.78 | 31.23 ± 5.17 | 0.01 |
| Waist circumference, cm | 107 ± 15 | 106 ± 9 | 107 ± 15 | 0.47 |
| Waist / hip –ratio | 1.00 ± 0.1 | 1.02 ± 0.1 | 0.99 ± 0.1 | 0.02 |
| Outpatient diabetes care, n (%) | 144 (63) | 52 (60) | 92 (64) | 0.56 |
| Hypertension, n (%) | 160 (70) | 57 (66) | 102 (71) | 0.52 |
| Hypercholesterolemia, n (%) | 179 (78) | 65 (76) | 114 (79) | 0.53 |
| Current smoker, n (%) | 53 (23) | 25 (29) | 27 (19) | 0.07 |
| Pack years | 15.9 ± 19.2 | 20.2 ± 22.7 | 13.1 ± 16.2 | 0.001 |
| Systolic blood pressure, mmHg | 141.3 ± 15.7 | 141.5 ± 15.8 | 141.2 ± 15.3 | 0.87 |
| Diastolic blood pressure, mmHg | 85.8 ± 10.2 | 86.6 ± 9.74 | 85.4 ± 10.53 | 0.38 |
| Laboratory findings | ||||
| LDL cholesterol, mmol/L | 2.0 ± 0.8 | 2.1 ± 0.8 | 1.9 ± 0.8 | 0.15 |
| HDL cholesterol, mmol/L | 1.3 ± 0.7 | 1.2 ± 0.5 | 1.3 ± 0.7 | 0.97 |
| Triglycerides, mmol/L | 2.0 ± 1.1 | 2.2 ± 1.2 | 2.0 ± 1.1 | 0.21 |
| CRP, mg /L | 2.9 ± 3.7 | 2.6 ± 3.7 | 3.0 ± 3.7 | 0.35 |
| Troponin, T ng/L | 10.0 ± 8.6 | 10.8 ± 9.8 | 9.5 ± 7.8 | 0.28 |
| HbA1c, mmol/mol | 60.1 ± 14.1 | 64.1 ± 15.8 | 57.7 ± 12.6 | < 0.001 |
| CACS, median [IQR] | 72.5 [1;460] | 113.0 [25;408] | 28.5 ± [0;612] | 0.11 |
| Duration of diabetes, years | 10.9 ± 8.0 | 10.1 ± 9.0 | 11.4 ± 7.3 | 0.21 |
| Lipid-lowering duration, years | 5.3 ± 5.7 | 5.1 ± 5.7 | 5.5 ± 5.7 | 0.58 |
| Diabetic complications, n (%) | 123 (54) | 55 (64) | 68 (47) | 0.01 |
| Retinopathy, n (%) | 52 (23) | 25 (29) | 27 (19) | 0.07 |
| Albuminuria, n (%) | 65 (28) | 30 (35) | 35 (24) | 0.09 |
| Neuropathy, n (%) | 57 (25) | 23 (27) | 34 (24) | 0.28 |
| Medication, n (%) | ||||
| Lipid-lowering, n (%) | 178 (77) | 65 (76) | 113 (78) | 0.61 |
| Aspirin, n (%) | 30 (13) | 10 (12) | 21 (15) | 0.30 |
| Beta blocker, n (%) | 23 (10) | 5 (6) | 18 (13) | 0.10 |
| ACE/ARB inhibitor, n (%) | 152 (66) | 56 (65) | 96 (67) | 0.81 |
| Insulin, n (%) | 90 (39) | 28 (33) | 62 (43) | 0.11 |
| GLP-1 inhibitor, n (%) | 54 (23) | 15 (17) | 39 (27) | 0.10 |
| DPP4 inhibitor, n (%) | 35 (15) | 17 (20) | 18 (13) | 0.14 |
| Biguanide, n (%) | 192 (83) | 77 (90) | 115 (80) | 0.06 |
| Sulfonylurea, n (%) | 40 (17) | 21 (24) | 19 (13) | 0.03 |
| SGLT2, n (%) | 21 (9) | 9 (10) | 12 (8) | 0.59 |
Baseline characteristics were compared between patients with and without HRP. A p < 0.05 was considered a significant
BMI body mass index, ACE/ARB ace converting enzyme inhibitor/angiotensin receptor blocker, LDL low-density lipoprotein, HDL high density lipoprotein, CRP c-reactive protein, CACS coronary artery calcium score, GLP-1 glucagon like peptid-1, DPP4 inhibitor dipeptidyl peptidase-4 inhibitors, SGLT-2 sodium glucose cotransport-2 inhibitor
Table 2.
Multivariate analysis of predictors for high-risk plaque. N = 230
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male | 4.92 | 2.28–10.6 | < 0.001 | 4.19 | 1.99—8.87 | < 0.001 |
| BMI | 0.92 | 0.86–0.97 | 0.01 | 0.93 | 0.86–1.00 | 0.06 |
| Pack Years | 1.02 | 1.00–1.03 | 0.01 | 1.02 | 1.00–1.03 | 0.03 |
| Any diabetic complication | 1.95 | 1.13–3.38 | 0.02 | 1.74 | 0.97–3.10 | 0.06 |
| HbA1c | 1.03 | 1.01–1.05 | 0.001 | 1.04 | 1.02–1.07 | 0.01 |
| Sulfonylureas | 2.14 | 1.08–4.27 | 0.03 | 1.98 | 0.96–4–07 | 0.06 |
In the multivariate analysis predictors were adjusted for age, sex, hypercholesterolemia, and systolic blood pressure
HbA1c Hemoglobin A1c
Table 5.
Baseline characteristics stratified by sex
| Variable | Overall n = 230 | Men n = 168 | Women n = 62 | p-value |
|---|---|---|---|---|
| Age | 62 ± 10 | 62 ± 10 | 61 ± 9 | 0.37 |
| BMI, kg/m2 | 30.6 ± 4.8 | 31.4 ± 5.8 | 30.3 ± 4.3 | 0.09 |
| Hypertension, n (%) | 160 (70) | 43 (66) | 117 (71) | 0.97 |
| Hypercholesterolemia, n (%) | 179 (78) | 128 (76) | 51 (82) | 0.34 |
| Current smoker, n (%) | 53 (23) | 41 (24) | 12 (19) | 0.42 |
| Pack years | 15.9 ± 19.2 | 17.5 ± 20.1 | 11.6 ± 15.9 | 0.04 |
| Laboratory findings | ||||
| LDL cholesterol, mmol/L | 1.99 ± 0.81 | 1.94 ± 0.82 | 2.12 ± 0.78 | 0.13 |
| HDL cholesterol, mmol/L | 1.3 ± 0.7 | 1.2 ± 0.7 | 1.3 ± 0.4 | 0.37 |
| HbA1c, mmol/mol | 60.0 ± 14.4 | 61.5 ± 14.4 | 55.9 ± 12.7 | 0.01 |
| CACS, median [IQR] | 72.5 [1;460] | 137.0 [13;699] | 4 0.0 [0;121] | < 0.001 |
| Duration of diabetes, years | 10.9 ± 8.0 | 11.1 ± 8.2 | 10.4 ± 7.4 | 0.55 |
| Lipid-lowering duration, years | 5.3 ± 5.7 | 5.3 ± 5.8 | 5.4 ± 5.4 | 0.86 |
| Diabetic complications, n (%) | 123 (54) | 55 (64) | 68 (47) | 0.01 |
| Medication | ||||
| Lipid-lowering, n (%) | 178 (77) | 127 (76) | 51 (82) | 0.28 |
| Insulin, n (%) | 90 (39) | 69 (41) | 21 (52) | 0.32 |
Diabetes-specific factors
Patients with HRP had 11% higher HbA1c levels compared to patients without HRP (64.1 vs. 57.7 mmol/mol, p = 0.01). The presence of any diabetic complication (retinopathy, albuminuria, and neuropathy), was more frequent in patients with HRP (64% vs. 47%, p = 0.01) and patients with HRP were more frequent sulfonylurea users (24 vs. 13%, p = 0.03) (Table 1).
In univariate analysis, Hb1Ac (OR 1.03, 95% CI: 1.01–1.05, p = 0.001), any diabetic complication (OR 1.95, 95% CI: 1.13- 3.38, p = 0.02), and use of sulfonylureas (OR 2.14, 95% CI: 1.08–4.27, p = 0.03) were significant predictors of HRP, but only HbA1c remained statistically significant in the multivariate analysis (Table 2).
Coronary artery calcium score
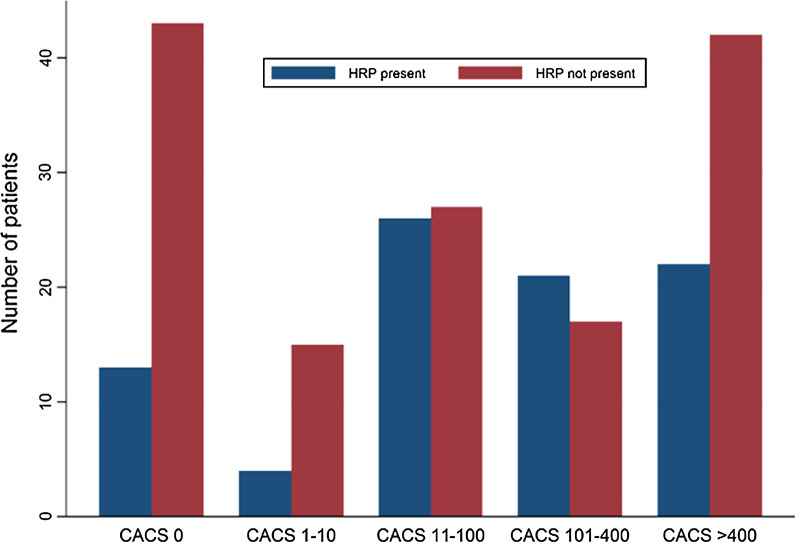
Median CACS was 75.5 (IQR: 1.00–460), and HRP was detected in all CACS groups. We identified 13 patients (23%) with HRP and CACS = 0 (Fig. 1). The highest frequency of patients with HRP was found in patients with moderate calcium score (CACS = 11–100) (Fig. 2). In the group of patients with HRP and no or minimal CACS fewer used lipid-lowering medication (59 vs. 80%, p = 0.07) and the duration of lipid-lowering treatment was significantly shorter compared to patients with HRP and mild to severe CACS (2.3 ± 3.2 vs. 5.7 ± 6.0 years, p = 0.03).
Fig. 1.
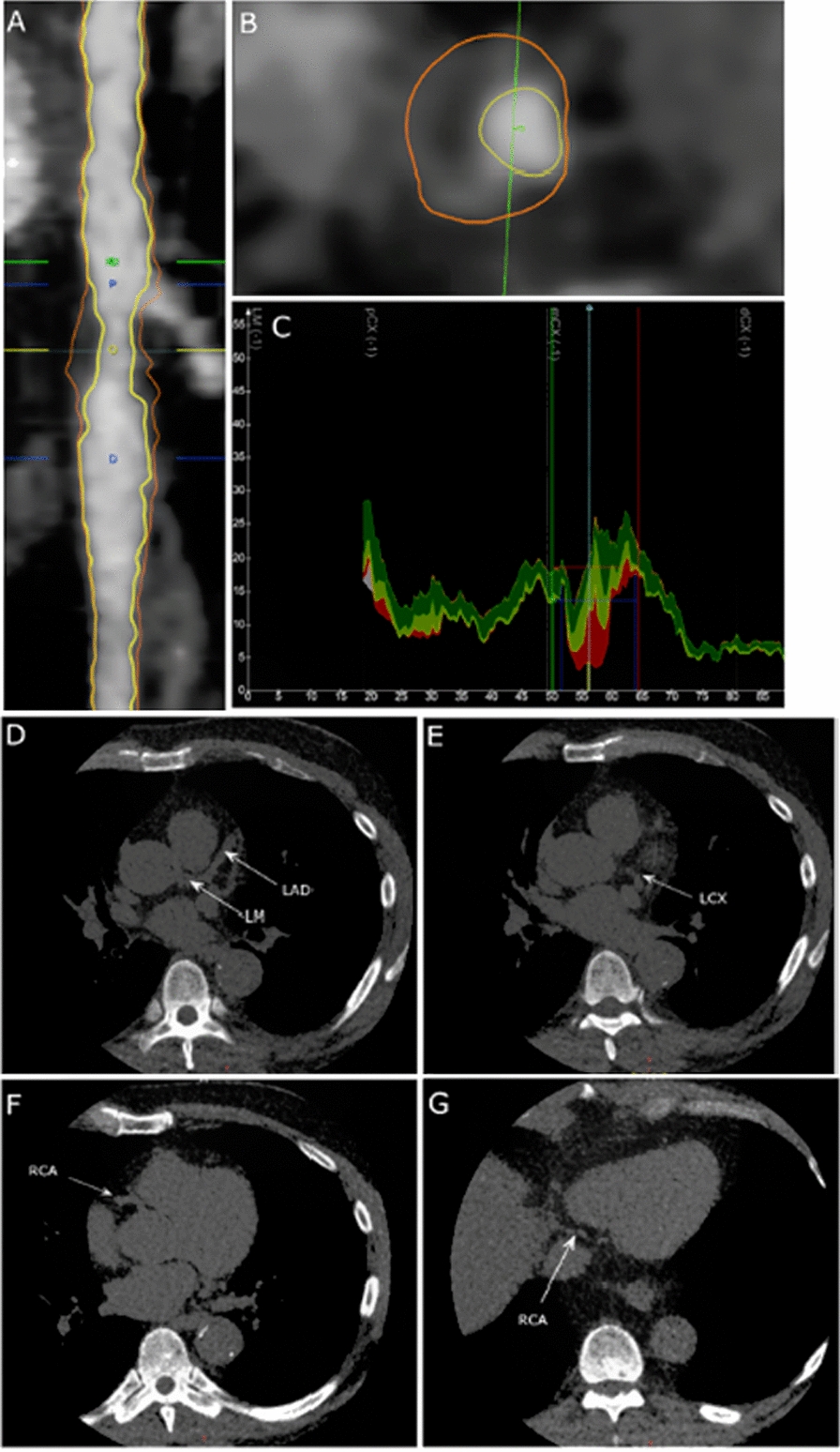
Plaque analysis with semiautomatic plaque analysis software. A Longitudinal straightened multiplanar reconstruction of left circumflex artery featuring a high-risk plaque (HRP) between the blue lines. B Transverse vessel view demonstrating HRP with positive remodeling, napkin ring sign, and low attenuation core (< 30 HU). C Graph depicting lumen and vessel areas as a function of vessel length. Plaque subtypes dense calcium, fibrous, fibrous-fatty and necrotic core are shown in grey, green, light green, and red colors. The bottom panels shows unenhanced axial images in the same patient demonstrating no coronary artery calcium present in the left main (LM) and left descending artery (LAD) (D), left circumflex artery (LCX) (E), and right coronary artery (RCA) (F, G)
Fig. 2.
Distribution of 230 patients with and without high-risk plaque (HRP) stratified by coronary artery calcium score (CACS)
CCTA findings
The measures of plaque burden stratified by the presence of HRP are presented in Table 3.
Table 3.
Total atheroma volume and plaque composition in patients with CAD with HRP and CAD without HRP. N = 203
| Variable | CAD with HRP n = 86 | CAD without HRP n = 117 | p-value |
|---|---|---|---|
| Vessel length, mm | 209.1 ± 109.0 | 187.5 ± 117.7 | 0.18 |
| TAV, mm3 | 867.5 ± 471.1 | 782.7 ± 653.1 | 0.31 |
| PAV (%) | 34.4 ± 7.0 | 31.9 ± 10.1 | 0.05 |
| Normalized TAV | 745.9 ± 250.6 | 730.7 ± 328.7 | 0.71 |
| -Fibrous volume, mm3 | 432.2 ± 152.0 | 449.6 ± 169.4 | 0.45 |
| -Fibrous-fatty volume, mm3 | 142.2 ± 63.5 | 108.4 ± 61.6 | < 0.001 |
| -Necrotic core volume, mm3 | 104.5 ± 100.1 | 43.8 ± 76.3 | < 0.001 |
| -Dense calcium volume, mm3 | 64.0 ± 69.4 | 131.1 ± 190.5 | 0.002 |
| Coronary stenosis | |||
| Mild (25–49%), n (%) | 68 (79) | 75 (64) | 0.01 |
| Moderate (50–69%), n (%) | 32 (37) | 30 (26) | 0.01 |
| Severe (≥ 70%), n (%) | 3 (3) | 3 (3) | 0.52 |
Volumes of fibrous, fibrous-fatty, necrotic core and necrotic core plaque were normalized according to vessel length
TAV total atheroma volume, PAV percent atheroma volume, NAV normalized atheroma volume
In 27 patients we did not find any evidence of coronary atherosclerosis, and they were excluded from this analysis. There was no significant difference in the length of vessels analyzed between groups (187.5 ± 117.7 mm vs. 209.1 ± 109.0 mm; p = 0.183). Patients with HRP did not have significantly higher TAV compared to patients without HRP (787.7 ± 653.1 mm3 vs. 867.5 ± 471 mm3; p = 0.18). There was no difference in the other quantitative plaque measures PAV and normalized TAV between groups either. However, patients with HRP had significantly larger normalized volumes of fibrous-fatty (142.2 ± 63.5 mm3 vs. 108.4 ± 61.6 mm3; p < 0.001) and necrotic core volumes (104.5 ± 100.1 mm3 vs. 43.8 ± 76.3 mm3; p < 0.001), while patients with no HRP presented significantly larger volumes of dense calcium (64.0 ± 69.4 mm3 vs. 131.1 ± 190.5 mm3; p = 0.002). Mild stenosis were found more frequent in patients with HRP (68 vs 75%; p = 0.01) as well as moderate stenosis (37 vs 26%; p = 0.01). Severe stenosis were found in three patients with HRP and three patients without high-risk plaque. Percent diameter stenosis could not be calculated in 19 without HRP due to extensive calcification. This was only the case in one patient with HRP.
Inter- and intra-observer variability
Repeatability was good, and the mean bias for intra-observer and inter-observer variability for total plaque were 5.0 mm3, 95% LOA (− 89.7; 99.7) and 8.5 mm3, 95% LOA (− 176.1; 193.1), respectively. A strong correlation was detected between both intra-observer analysis (r = 0.98) and inter-observer analysis (r = 0.91). Inter- and intra-observer agreement and correlation for additional plaque components are shown in Table 4.
Table 4.
Inter- and intra-observer variability
| Variable | Intra-observer variability | Inter-observer variability | ||
|---|---|---|---|---|
| Mean diff. [95% LOA] | r | Mean diff. [95% LOA] | r | |
| Total plaque volume | 5.0 [− 89.7; 99.7] | 0.98 | 8.5 [− 176.1; 193.1] | 0.91 |
| Dense plaque | 1.6 [− 22.9; 26.1] | 0.99 | 0.5 [− 28.9; 29.9] | 0.99 |
| Fibrous plaque | 5.9 [− 11.5; 23.2] | 0.95 | 5.7 [− 13.5; 24.9] | 0.95 |
| Fibrous-fatty plaque | 1.1 [ − 28.7; 30.9] | 0.96 | 5.3 [− 44.9; 55.4] | 0.87 |
| Necrotic core plaque | − 3.9 [− 45.7; 37.9] | 0.91 | − 3.1 [− 55.7; 49.4] | 0.86 |
Mean diff Mean difference between paired observations, LOA Limits of agreement calculated as the mean difference ± 1.96 standard deviation; r: Pearson’s coefficient of correlation between paired observations
Discussion
The main findings of this study are the following: (1) HRP was identified in 86 asymptomatic patients with T2D. (2) Men were more likely to have HRP compared to women. (3) HbA1c was the only diabetes-specific risk factor which remained significantly associated with HRP. (4) HRP was most frequently seen in patients with “mild CAD” (CACS = 11–100) but was identified in all strata including patients with “No CAD” (CACS = 0).
A limited number of studies have assessed plaque morphology in asymptomatic diabetes using varying definitions of HRP. Using a similar definition of HRP as our study, Kamimura et al. reported that HRP was only present in 17% of patients with asymptomatic diabetes but included patients with lower BMI and hb1Ac compared to our study [23]. Nezarat et al. examined subclinical CAD in patients with diabetes younger than 40 years and reported that non-calcified plaque were present in 28% of the population [24]. In a population similar to the present study, Halon et. al. found 376 high-risk plaque in 499 patients with asymptomatic diabetes [25]. This number HRP seems to be comparable to our study though using a slightly different definition of HRP. In the study by Halon, events during 9-years follow-up were reported and the authors found that the majority of events were caused by HRP while events in non-HRP were rare. The risk of acute events in the study increased by the number of high-risk plaque features, but also with the degree of stenosis. In addition, more densely calcified plaque was considered a protective factor.
In our study, male gender was associated with a four time greater odds of having a HRP compared to females, which could indicate a higher risk for males. This finding contradicts several meta-analyses that have demonstrated that diabetes equalizes the risk of CAD between men and women [26, 27]. We detected several differences in the risk profile between men and women. Men had a greater exposure to smoking, a higher HbA1c, and a higher burden of late diabetic complications compared to women, which could in part explain the higher proportion of HRP in men. Another explanation could be the differences in plaque phenotypes between men and women observed in autopsy studies. In patients with sudden cardiac death from coronary thrombosis, plaque erosion is more common in women than in men [28, 29]. In plaque erosion, the plaques tend to be more rich in smooth muscle cells, lack the superficial lipid core, and tend to be less calcified [30]. Because of these morphological differences, plaques prone to erosion may not be detected as HRP by CCTA explaining an underestimation of HRP in women.
Smoking is a well-established risk factor for cardiovascular events. The exact role of smoking in plaque formation is less clear. In observational studies using intravascular ultrasound or optical coherence tomography, smoking was associated with increased lipid content in coronary plaque in patients with symptomatic CAD [31–33]. A strong association between the number of cigarettes smoked in pack years and the presence of HRP was found. We suggest, that smoking may have an important role in the formation of high-risk plaques, and that pack years may be a superior risk marker compared to smoking as simple dichotomous risk factor.
We examined several diabetes-specific factors including HbA1c, diabetes duration, and presence of any diabetic complications in relation to HRP. The only significant association was found between HbA1c and HRP. It has been demonstrated in large registry trials, that increased levels of fasting blood glucose is associated with increased risk of coronary heart disease [11]. However, several studies intervening on blood glucose levels to improve patient-outcome have failed [34–36]. In observational studies higher levels of HbA1c have been associated with an increased lipid content in coronary plaques [37] and impaired regression of atheroma volume during statin therapy [38]. In addition, autopsy studies have demonstrated that HbA1c is an independent predictor of necrotic core size [39]. In our study patients with HRP were well treated with a mean low-density lipoprotein cholesterol of 2.1 mmol/L. However, triglycerides and triglyceride-rich lipoproteins, associated with the metabolic syndrome, T2D and insulin resistance, are less affected by statin treatment [40]. These highly atherogenic lipoproteins may account for some of the residual risk in T2D with well-treated LDL-cholesterol and could explain the association of higher hbA1c in patients with HRP. The coronary artery calcium score (CACS) has been proposed as a relevant method to risk stratify asymptomatic high-risk patients due to widespread availability and low cost. While large registries like the MESA-study [41] found higher CACS in patients with diabetes more recent propensity matched studies did not find any difference in the extent of coronary artery calcium in patients with T2D compared to patients without T2D[42, 43]. In addition, several studies using CCTA found non-calcified plaque in patients with zero CACS [23, 44]. In our study HRP was most frequently seen in patients with “mild CAD” (CACS = 11–100). More concerning, in 23% of the patients with zero CACS. HRP was present. These findings are similar to the findings by Min et. al. how found that atherosclerosis was present in one third of asymptomatic patients with diabetes a CAS of zero, and that 10% of these patients had obstructive CAD [45]. In patients with no or minimal CACS who had HRP, the duration of lipid-lowering treatment was significantly shorter, and fewer of these patients were in fact treated with lipid-lowering medication. This finding may also reflect that the early atherosclerotic plaque formation does not involve calcification, or at most microcalcifications, that does not show on CT. The increased risk of acute events arising from non-calcified plaque compared to calcified plaque [3] suggests that the progressive plaque calcification could be a stabilizing factor. In this respect, statins may play an important role facilitating plaque stabilization through plaque calcification [46, 47].Despite these findings, the incidence of events in asymptomatic T2D with no CAD defined by CACS has been very low in several prognostic studies [48, 49]. However, the utility of CACS as screening tool in asymptomatic patients with diabetes has not yet been examined in a randomized trial and should therefore be used with caution.
The atherosclerotic disease burden is a well-established predictor of major cardiovascular events in patients with established CAD [50, 51]. It could be hypothesized that patients with HRP have an increased risk due to a greater global disease burden rather than from the HRP itself. Our results did not demonstrate a significant difference in TAV between patients with and without HRP. However, there was a significant difference in plaque composition, and patients with HRP had larger volumes of non-calcified plaques and less dense calcium, which could be an indicator of more unstable plaques [46]. Overall, our findings suggest that HRP could be an important feature of coronary artery disease independent of the global disease burden.
Asymptomatic diabetes is a very heterogeneous group, and the challenge is finding the true high-risk patients. There were no evidence of CAD in 12% of the patients in our study, which indicate an excellent prognosis and a low risk of cardiovascular events. CCTA screening has not yet demonstrated any prognostic benefits. However, there will be in increased demand for individualized treatment, and CCTA could play an important role in the future. In the PROSPECT ABSORB trial, preventive percutaneous coronary intervention (PCI) of non-culprit, non-obstructive high-risk lesions were neither beneficial nor hazardous compared to medical treatment. Sealing of high-risk plaque using a coronary stent may be a way forward but larger studies are needed to demonstrate clinical endpoints [52]. It has been suggested that coronary artery plaque undergo rapid progression prior to MI [53]. Accepting this thesis, lipid control becomes paramount as cholesterol levels is the major modifiable factor in plaque progression. Emerging pharmacological therapies with effects beyond statins are becoming available and allow for an escalation of treatment in patients with HRP and could be the focus of future randomized trials.
The findings in this study as well as new markers of high-risk plaque morphology need to be validated in larger trials. This could lead to a better selection of asymptomatic individuals with diabetes who could benefit from CCTA screening.
Limitations
This was a small observational study and is therefore prone to confounding and bias by design. We did not include a control group to compare asymptomatic patients with diabetes to individuals without diabetes. Furthermore, we did not report any prognostic information to back up the claim, that HRP is in fact associated with cardiovascular events, although this point has been supported by several trials in recent years. A general problem in plaque morphology studies is the lack of consensus on how to define high-risk plaque, which makes comparison of results between studies problematic. Finally, the proportion of patients with evidence of CAD and HRP was high in our study compared to other studies of asymptomatic diabetes [23, 54] which could indicate that this was in fact a selected high-risk population. The majority of patients were followed at the Endocrinology Outpatient Clinic rather than by their GP, which could reflect that these patients were more complex and challenging to treat. For this reason, the findings of the present study should be interpreted with caution. A major strength of this study is the thorough collection of patient characteristics collected “hands on” by the authors, which increases the reliability and uniformity of data compared to register based data.
Conclusions
We found a high frequency of high-risk plaques in asymptomatic patients with T2D despite effective lipid-lowering treatment. HRP was significantly associated with male gender, increased number of pack years, and an increased HbA1c while the absence of coronary artery calcium did not exclude HRP. We believe CCTA could be used to risk stratify asymptomatic patient with T2D based upon the presence of HRP. Whether this improves patient outcome needs further investigation.
Acknowledgements
The authors are grateful for the support from the entire staff at the Cardiovascular Research Unit and the CT-radiographers from the Department of Radiology, Svendborg Hospital, Denmark.
Abbreviations
- MI
Acute myocardial infarction
- CAD
Coronary artery disease
- T2D
Type 2 diabetes mellitus
- CCTA
Coronary computed tomography angiography
- HRP
High-risk plaque
- LAP
Low-attenuated plaque
- PR
Positive remodeling
- CACS
Coronary artery calcium score
Appendix 1
See Table 5.
Baseline characteristics were compared between men and women. A p < 0.05 was considered significant
BMI body mass index, LDL low density lipoprotein, HDL high density lipoprotein, CACS coronary artery calcium score.
Authors' contributions
LJH and GP designed the study, and carried out subject recruitment. LJH wrote the manuscript and performed the data analysis with TRA. All co-authors gave significant contributions for the study design, data interpretation, and manuscript revisions. All authors read and approved the final manuscript.
Funding
The study was funded by grants from the Region of Southern Denmark, University of Southern Denmark, and the Cardiovascular Research Unit, Svendborg Hospital.
Availiability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Regional Ethics Committee (ID S-20150029) as well as the Danish Data Protection agency (ID. 2008-58-0035) gave permission for the present study.
Consent for publications
Consent for publication was obtained for all participant in this study.
Competing interests
The authors declare that no competing interests were present.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 2.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 3.Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2018;11(1):e006973. doi: 10.1161/CIRCIMAGING.117.006973. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Maehara A, Mintz GS, Kubo T, Xu K, Choi SY, et al. Virtual histology intravascular ultrasound analysis of non-culprit attenuated plaques detected by grayscale intravascular ultrasound in patients with acute coronary syndromes. Am J Cardiol. 2010;105(1):48–53. doi: 10.1016/j.amjcard.2009.08.649. [DOI] [PubMed] [Google Scholar]
- 5.Prati F, Romagnoli E, Gatto L, La Manna A, Burzotta F, Ozaki Y, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J. 2020;41(3):383–391. doi: 10.1093/eurheartj/ehz520. [DOI] [PubMed] [Google Scholar]
- 6.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 7.Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18(7):772–779. doi: 10.1093/ehjci/jew167. [DOI] [PubMed] [Google Scholar]
- 8.Conte E, Annoni A, Pontone G, Mushtaq S, Guglielmo M, Baggiano A, et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18(10):1170–1178. doi: 10.1093/ehjci/jew200. [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 10.Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–54. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 11.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health O. HEARTS D: diagnosis and management of type 2 diabetes. Geneva: World Health Organization; 2020 2020. Contract No.: WHO/UCN/NCD/20.1.
- 13.Das A, Stroud S, Mehta A, Rangasamy S. New treatments for diabetic retinopathy. Diabetes Obes Metab. 2015;17(3):219–230. doi: 10.1111/dom.12384. [DOI] [PubMed] [Google Scholar]
- 14.Masters N TC. Masters N, Tutt C. Smoking pack years calculator. [DOI] [PMC free article] [PubMed]
- 15.Lambrechtsen J, Egstrup K. Pre-treatment with a sinus node blockade, ivabradine, before coronary CT angiography: a retrospective audit. Clin Radiol. 2013;68(10):1054–1058. doi: 10.1016/j.crad.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP, et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging. 2013;29(5):1177–1190. doi: 10.1007/s10554-013-0194-x. [DOI] [PubMed] [Google Scholar]
- 17.Wu FZ, Wu MT. 2014 SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2015;9(2):e3. doi: 10.1016/j.jcct.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Schlett CL, Ferencik M, Celeng C, Maurovich-Horvat P, Scheffel H, Stolzmann P, et al. How to assess non-calcified plaque in CT angiography: delineation methods affect diagnostic accuracy of low-attenuation plaque by CT for lipid-core plaque in histology. Eur Heart J Cardiovasc Imaging. 2013;14(11):1099–1105. doi: 10.1093/ehjci/jet030. [DOI] [PubMed] [Google Scholar]
- 19.Dalager MG, Bøttcher M, Andersen G, Thygesen J, Pedersen EM, Dejbjerg L, et al. Impact of luminal density on plaque classification by CT coronary angiography. Int J Cardiovasc Imaging. 2011;27(4):593–600. doi: 10.1007/s10554-010-9695-z. [DOI] [PubMed] [Google Scholar]
- 20.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101(6):598–603. doi: 10.1161/01.CIR.101.6.598. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Kunita E, Oka T, et al. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J Cardiovasc Comput Tomogr. 2013;7(3):192–199. doi: 10.1016/j.jcct.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 23.Kamimura M, Moroi M, Isobe M, Hiroe M. Role of coronary CT angiography in asymptomatic patients with type 2 diabetes mellitus. Int Heart J. 2012;53(1):23–28. doi: 10.1536/ihj.53.23. [DOI] [PubMed] [Google Scholar]
- 24.Nezarat N, Budoff MJ, Luo Y, Darabian S, Nakanishi R, Li D, et al. Presence, characteristics, and volumes of coronary plaque determined by computed tomography angiography in young type 2 diabetes mellitus. Am J Cardiol. 2017;119(10):1566–1571. doi: 10.1016/j.amjcard.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Halon DA, Lavi I, Barnett-Griness O, Rubinshtein R, Zafrir B, Azencot M, et al. Plaque morphology as predictor of late plaque events in patients with asymptomatic type 2 diabetes: a long-term observational study. JACC Cardiovasc Imaging. 2019;12(7 Pt 2):1353–1363. doi: 10.1016/j.jcmg.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37(3):830–838. doi: 10.2337/dc13-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368(9529):29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 28.Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82(3):269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93(7):1354–63. doi: 10.1161/01.CIR.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 30.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;34(10):719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai S, Amano T, Takashima H, Waseda K, Kurita A, Ando H, et al. Impact of cigarette smoking on coronary plaque composition. Coron Artery Dis. 2015;26(1):60–65. doi: 10.1097/MCA.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 32.Bolorunduro O, Cushman C, Kapoor D, Alexander K, Cuellar-Silva J, Giri S, et al. Comparison of coronary atherosclerotic plaque burden and composition of culprit lesions between cigarette smokers and non-smokers by in vivo virtual histology intravascular ultrasound. J Invasive Cardiol. 2015;27(8):354–358. [PubMed] [Google Scholar]
- 33.Abtahian F, Yonetsu T, Kato K, Jia H, Vergallo R, Tian J, et al. Comparison by optical coherence tomography of the frequency of lipid coronary plaques in current smokers, former smokers, and nonsmokers. Am J Cardiol. 2014;114(5):674–680. doi: 10.1016/j.amjcard.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 34.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 35.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 37.Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, et al. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv. 2012;5(11):1150–1158. doi: 10.1016/j.jcin.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Daida H, Takayama T, Hiro T, Yamagishi M, Hirayama A, Saito S, et al. High HbA1c levels correlate with reduced plaque regression during statin treatment in patients with stable coronary artery disease: results of the coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS) Cardiovasc Diabetol. 2012;11:87. doi: 10.1186/1475-2840-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24(7):1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 40.Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. A VOYAGER meta-analysis of the impact of statin therapy on low-density lipoprotein cholesterol and triglyceride levels in patients with hypertriglyceridemia. Am J Cardiol. 2016;117(9):1444–1448. doi: 10.1016/j.amjcard.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5(4):358–366. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim U, Leipsic JA, Sellers SL, Shao M, Blanke P, Hadamitzky M, et al. Natural history of diabetic coronary atherosclerosis by quantitative measurement of serial coronary computed tomographic angiography: results of the PARADIGM study. JACC Cardiovasc Imaging. 2018;11(10):1461–1471. doi: 10.1016/j.jcmg.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Milzi A, Burgmaier M, Burgmaier K, Hellmich M, Marx N, Reith S. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovasc Diabetol. 2017;16(1):152. doi: 10.1186/s12933-017-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park GM, Lee SW, Cho YR, Kim CJ, Cho JS, Park MW, et al. Coronary computed tomographic angiographic findings in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;113(5):765–771. doi: 10.1016/j.amjcard.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Min JK, Labounty TM, Gomez MJ, Achenbach S, Al-Mallah M, Budoff MJ, et al. Incremental prognostic value of coronary computed tomographic angiography over coronary artery calcium score for risk prediction of major adverse cardiac events in asymptomatic diabetic individuals. Atherosclerosis. 2014;232(2):298–304. doi: 10.1016/j.atherosclerosis.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auscher S, Heinsen L, Nieman K, Vinther KH, Logstrup B, Moller JE, et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: Assessment with serial coronary CT angiography. Atherosclerosis. 2015;241(2):579–587. doi: 10.1016/j.atherosclerosis.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27(6):713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 49.Yeboah J, Erbel R, Delaney JC, Nance R, Guo M, Bertoni AG, et al. Development of a new diabetes risk prediction tool for incident coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;236(2):411–417. doi: 10.1016/j.atherosclerosis.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puri R, Nissen SE, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J. 2013;34(41):3182–3190. doi: 10.1093/eurheartj/eht260. [DOI] [PubMed] [Google Scholar]
- 51.Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55(21):2399–2407. doi: 10.1016/j.jacc.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Stone GW, Maehara A, Ali ZA, Held C, Matsumura M, Kjøller-Hansen L, et al. Percutaneous coronary intervention for vulnerable coronary atherosclerotic plaque. J Am Coll Cardiol. 2020;76(20):2289–2301. doi: 10.1016/j.jacc.2020.09.547. [DOI] [PubMed] [Google Scholar]
- 53.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(12):1608–1617. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Muhlestein JB, Lappé DL, Lima JA, Rosen BD, May HT, Knight S, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312(21):2234–2243. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.