Abstract
Amino acids are indispensable nutrients for both normal and cancer cells. Cancer cells are unable to synthesize essential amino acids as well as some non‐essential amino acids adequately to support rapid proliferation, and must take up amino acids from the surroundings. To meet the increased demand for the amino acid needed for proliferation, high levels of amino acid transporters are expressed on the surface of cancer cells. Cancer cells utilize amino acids to synthesize proteins and nucleotides, as well as to obtain energy. In addition, amino acids are known to play pathological roles in cancer cells. Interestingly, breast cancer cells limit the use of amino acids for cell proliferation based on amino acid availability, which depends on estrogen receptor status. Here, we present a summarized literature review of novel amino acid functions in cancer cells. This review organizes the available knowledge on 2 amino acid transporters, SLC7A5 and SLC7A11, which are considered essential for breast cancer cell growth in a cell‐dependent manner. In particular, we propose the glutamine recycling model to clarify the mechanism underlying aberrant SLC7A5 activation. Finally, we overview the pathological significances of SLC7A5 and SLC7A11 in cancer tissues.
Keywords: amino acid transporter, breast cancer, cell proliferation, cystine uptake, leucine uptake
Amino acids are indispensable nutrients for cancer cells. Cancer cells are unable to synthesize essential amino acids as well as some non‐essential amino acids adequately to support rapid proliferation, and must uptake amino acids from the surroundings. In order to meet the increased demand for amino acids needed for proliferation, high levels of amino acid transporters are expressed on the surface of cancer cells. This review organizes the knowledge available on two amino acid transporters, SLC7A5 and SLC7A11, which are considered essential for breast cancer cell growth in a cell‐dependent manner.
Abbreviations
- ARE
Antioxidant response element
- CASTOR1/2
Cellular arginine sensor for mTORC1
- ER
Estrogen receptor
- GATOR2
GAP activity toward Rag GTPases2
- KEAP1
Kelch‐like ECH‐associated protein 1
- LAT1
L‐type amino acid transporter 1
- L‐DOPA
L‐3,4‐dihydroxyphenylalanine
- LLGL2
Lethal giant larvae
- mTORC1
Mechanistic targets of rapamycin complex 1
- SNARE
Soluble NSF attachment protein receptor
1. INTRODUCTION
Metabolic reprogramming, which is a hallmark of cancer, enables cancer cells to continue growing under severe environmental conditions, such as those involving a lack of nutrients.1 One aspect of metabolic reprogramming observed in cancer cells is deregulated amino acid uptake. Amino acids are indispensable nutrients for both normal and cancer cells. Cancer cells use amino acids to synthesize proteins and nucleotides, as well as to obtain energy.2 Interestingly, recent studies have revealed that some amino acids are specifically used to promote cancer cell growth. Therefore, the biological role of amino acids may vary unexpectedly in a tumor‐specific or oncogene‐dependent manner. Therefore, amino acids that exhibit tumor‐ or oncogene‐dependent functions have attracted attention as therapeutic targets.
Essential amino acids are taken in from extracellular sources. Therefore, amino acid transporters in tumor cells are frequently found to be aberrantly expressed. Overexpression of amino acid transporters in breast cancer tissues is strongly associated with amino acid dependency.
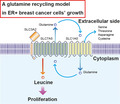
Here, we review the current understanding of newly discovered biological characteristics of amino acids in cancer cells. Furthermore, we summarize the role played by amino acid transporters, SLC7A5 and SLC7A11, in breast cancer cell growth. We briefly explain the new systemic flow “glutamine recycling” model to clarify the aberrant activation of SLC7A5 in addition to the reported SLC7A11 activation model. Finally, we present an overview of the pathological roles played by SLC7A5 and SLC7A11 in other cancer tissues.
2. THE PATHOLOGICAL ROLES OF AMINO ACID IN CANCER
Diversity in amino acid metabolism among tumors has been observed at the same time in various cancers. Recent studies have shown that some amino acids are preferentially used by cancer cells to promote cellular growth or metastasis. For instance, pancreatic cancer cells, in which alanine functions as a carbon source, utilize autophagic alanine secretion from stellate cells to promote pancreatic tumor growth.3 Leucine depletion from culture media reportedly induced apoptosis in RAS‐MEK‐activated melanoma cells.4 Restriction of dietary methionine significantly improved the therapeutic response in KRAS‐mutated colorectal cancer cells.5 Asparagine was indispensable for forming metastases in metastatic triple‐negative breast cancer cell models.6 These reports suggested that amino acids exert a cell–context‐dependent effect to regulate cell proliferation or metastasis in cancer cells. However, the molecular mechanisms underlying the regulation of cancer cell growth by amino acids remain unclear.
3. AMINO ACIDS DIRECTLY AFFECT PROTEIN FUNCTION AS AN EFFECTOR MOLECULE
Amino acid‐derived metabolites exhibit numerous tumorigenic functions. However, regulation of cell proliferation or metastasis in cancer cells by amino acid‐derived metabolites is beyond the scope of this review. Instead, we focus on the functions of amino acids as effector molecules.
Leucine, arginine, and cysteine, in particular, modulate the activities of mTORC1, which is a master regulator that responds to environmental inputs such as nutrients. Leucine, arginine, and cysteine bind directly to amino acid sensor proteins. These sensor proteins control mTORC1 activity to regulate cell growth.7 Sestrin2 is a cytosolic leucine sensor, while CASTOR1/2 is a cytosolic arginine sensor. Intracellular leucine binds to Sestrin2, a negative regulator of mTORC1, and dissociates Sestrin2 from GATOR2, a positive regulator of mTORC1 activity (Figure 1A).8 The CASTOR1/2 protein binds to GATOR2 under arginine‐deprived conditions. Arginine binding to CASTOR disrupts the interaction between CASTOR and GATOR2, resulting in the activation of mTORC1 (Figure 1B).9
FIGURE 1.
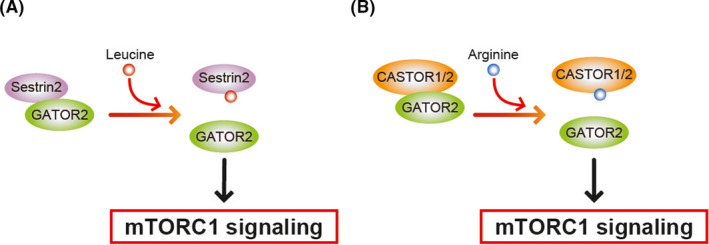
Schematic of mTORC1 regulation by amino acids. A, Leucine‐binding to Sestrin2 causes dissociation of GATOR2 from Sestrin2. B, Arginine binding to CASTOR leads to disruption of the CASTOR/GATOR2 interaction, leading to the activation of mTORC1 signaling
Furthermore, cysteine is sensed by EglN1 (also known as PHD), which regulates the accumulation of the HIF transcription factor. Decreased intracellular cysteine levels inactivate EglN1, resulting in the stabilization of HIF‐1 α.10 Therefore, intracellular amino acids function as effector molecules that modulate growth signals in cancer cells.
4. PATHOLOGICAL ROLES OF AMINO ACID IN BREAST CANCER
Amino acids in cancer cells may function in a tissue‐dependent or oncogene‐dependent manner. Next, we outline amino acid utilization in breast cancer cells. Breast cancer is characterized based on the aberrant gene expression status of ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).11 Based on gene expression patterns, breast cancer is roughly classified into 3 subclasses: ER‐positive (corresponding to luminal type); HER2‐positive; and basal (also known as triple‐negative type or ER‐negative) breast cancer. In breast cancer cells, the amount of amino acids required for cell proliferation differs according to ER status. ER+ breast cancer cells require leucine for growth, whereas ER‐negative (ER−) breast cancer cells require cystine. However, there have been no reports on the amino acid requirements for cell proliferation in HER2‐positive breast cancer cells.
The key amino acids involved in breast cancer cell proliferation are leucine and cystine. ER+ breast cancer cells use leucine, whereas ER− breast cancer cells need cystine for growth. These 2 amino acids are taken in from the extracellular environment by amino acid transporters. The amino acid transporter SLC7A5 (LAT1) plays a central role in leucine uptake by ER+ breast cancer cells, while SLC7A11 (also known as xCT and system Xc −) is used for cystine uptake in ER− breast cancer cells. Both SLC7A5 and SLC7A11 are members of the heteromeric amino acid transporter group. In the following section, we summarize the characteristics of these 2 transporters with particular reference to the involvement of these in therapeutic developments in breast and other tumor cells.
5. SLC7A5 IN BREAST CANCER CELLS
SLC7A5 is a bidirectional amino acid transporter that localizes at the plasma membrane (Figure 2). Phenylalanine, tryptophan, leucine, tyrosine, valine, histidine, methionine, isoleucine, and L‐DOPA are substrates of SLC7A5.12 SLC7A5 functions together with SLC3A2 (also known as 4F2hc or CD98hc), as a heterodimeric complex at the plasma membrane. Notably, complex formation with SLC3A2 is indispensable for the transport activity and substrate specificity of SLC7A5.13, 14 Structural analysis indicated that SLC7A5, which contains 12 transmembrane domains, interacts with SLC3A2 through a disulfide bond and hydrophobic interaction at their protein surface.14, 15
FIGURE 2.
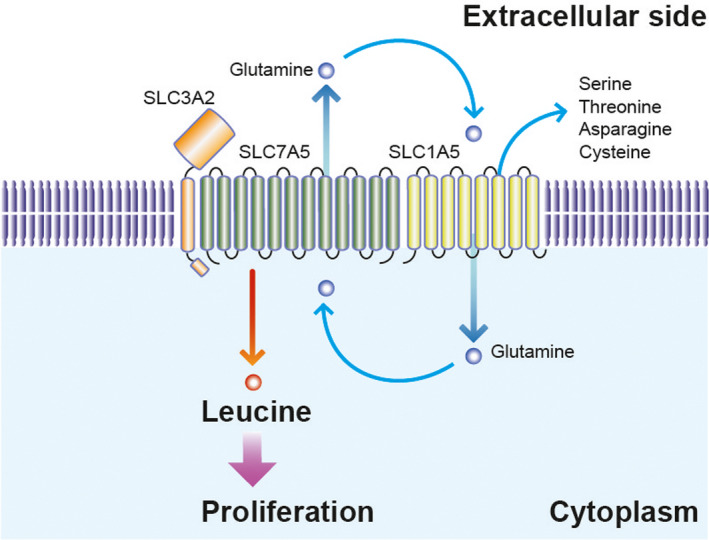
Schematic of amino acid flux by SLC7A5/SLC3A2 in ER+ breast cancer cells. The bidirectional amino acid transporter, SLC7A5, interacts with SLC3A2 and SLC1A5 on the plasma membrane. In ER+ breast cancer cells, leucine uptake by SLC7A5 accelerates cell proliferation. The glutamine cycle model supports the view that high consumption of glutamine, due to the activation of SLC7A5, is retrieved by SLC1A5
SLC7A5 is an antiporter that exports intracellular glutamine to facilitate uptake of its substrate leucine. The growth of ER+ breast cancer cells depends highly on intracellular leucine and SLC7A5, as mentioned below. Therefore, intracellular glutamine must be aberrantly excreted from the cell to allow leucine‐dependent cell proliferation to take place in ER+ breast cancer cells. Curiously, the SLC7A5/SLC3A2 heterodimeric complex contains SLC1A5, a glutamine transporter that regulates glutamine uptake into cells.16, 17 Overexpression of SLC1A5 is frequently observed in breast cancer tissues. Therefore, we believe that “glutamine recycling” between SLC7A5 and SLC1A5, enables aberrant leucine uptake into ER+ breast cancer cells (Figure 2).
SLC7A5 functions at the plasma membrane in cancer cells. Membrane localization of SLC7A5 is modulated by the polarity protein LLGL2 (mammalian orthologue lgl, lethal giant larvae), and the trafficking protein YKT6, a SNARE protein. Briefly, LLGL2 shuttles SLC7A5 from the cytosol to the plasma membrane, whereupon the LLGL2‐SLC7A5 complex interacts with YKT6 at the plasma membrane, resulting in the translocation of SLC7A5 to the plasma membrane.
The cellular surface protein level of SLC7A5 is higher in ER+ breast cancer cells compared with that in ER− breast cancer cells. In addition, treatment with a low dose of leucine/glutamine culture medium or a SLC7A5 inhibitor suppresses cell proliferation in ER+ breast cancer cells, whereas this treatment does not affect cell proliferation in ER− breast cancer cells. These results indicated that, although ER+ breast cancer cells rely on leucine uptake by SLC7A5 to proliferate, ER− breast cancer cell growth is less affected by leucine uptake by SLC7A5.18 LLGL2 and SLC7A5 are direct transcriptional targets of the ER in ER+ breast cancer cells.19 Furthermore, SLC7A5 expression is a marker of the Mammostat test that predicts clinical outcomes for ER+ breast cancer patients.20 Considering that ER+ breast cancer cells depend significantly on leucine uptake to grow, the LLGL2‐SLC7A5 pathway plays an important role in the growth of ER+ breast cancer cells, and therefore SLC7A5 presents a potential target for cancer therapy in ER+ breast cancer.
6. SLC7A5 IN CANCER TISSUES
The pathological relevance of SLC7A5 to tumorigenesis is not restricted to ER+ breast cancer cells. Overexpression of SLC7A5 is broadly observed in many cancer tissues, and high SLC7A5 mRNA expression levels are known to be correlated with poor clinical prognoses for ER+ breast cancer, as well as a variety of other cancer types (Table 1). Therefore, inhibitors targeting SLC7A5 in these cancer cells are extensively evaluated for the purpose of clinical application. The efficacies of the SLC7A5 inhibitors, BCH and JPH203, in cancer cells are listed (Table 1). Inhibition of SLC7A5 by BCH or JPH203 suppresses the growth of breast and other cancer cell types (Table 1). Interestingly, high SLC7A5 expression levels are associated with therapeutic resistance in breast, prostate, pancreas, ovary, and uterine cancer cells, suggesting that SLC7A5 inhibitors may be valuable as therapeutic agents for cancer even against some drug‐resistant tumors (Table 1).
TABLE 1.
Summary of SLC7A5 involvement in cancer tissues
| Tissue | Type of cancer cells | SLC7A5 inhibitors used in | Patient data (IHC or K‐M plot) | Relationship to therapeutic resistance | References |
|---|---|---|---|---|---|
| Breast | Estrogen receptor (ER)‐positive breast cancer | BCH, JPH203 | Yes | Yes | [16] |
| Luminal type of breast cancer | ‐ | Yes | Yes | [29] | |
| Luminal type of breast cancer | ‐ | Yes | ‐ | [30] | |
| Endocrine therapy‐treated patients | ‐ | Yes | Yes | [31] | |
| Lung | NSCLC | ‐ | Yes | ‐ | [32] |
| NSCLC | BCH | ‐ | ‐ | [33] | |
| Prostate | Prostate cancer | ‐ | Yes | Yes | [34] |
| Biliary tract | Cholangiocarcinoma | BCH | Yes | ‐ | [35] |
| Cholangiocarcinoma | JPH203 | ‐ | ‐ | [36] | |
| Biliary tract cancer | ‐ | Yes | ‐ | [37] | |
| Gastrointestinal cells | Gastric cancer cell line/colon cancer cell line | JPH203 | ‐ | ‐ | [38] |
| Gastric cancer cell line | Knockdown | Yes | ‐ | [39] | |
| Esophageal squamous cell carcinoma | ‐ | Yes | ‐ | [40] | |
| Colon | KRAS‐mutant CRC | ‐ | ‐ | ‐ | [41] |
| Pancreas | Pancreatic ductal adenocarcinoma | ‐ | Yes | ‐ | [42] |
| Pancreatic ductal adenocarcinoma | ‐ | Yes | Yes | [43] | |
| Pancreatic ductal adenocarcinoma | ‐ | Yes | ‐ | [44] | |
| Skin | Basal cell carcinoma | ‐ | Yes | ‐ | [45] |
| Melanoma | ‐ | Yes | ‐ | [46] | |
| Ovary | Ovarian cancer | ‐ | Yes | Yes | [47] |
| Ovarian cancer | BCH | ‐ | ‐ | [48] | |
| Uterine | Endometrioid carcinoma | ‐ | Yes | Yes | [49] |
| Endometrioid carcinoma | BCH | Yes | ‐ | [50] | |
| Thyroid | Anaplastic thyroid cancer | JPH203 | Yes | ‐ | [51] |
| Anaplastic thyroid cancer | JPH203 | Yes | ‐ | [52] | |
| Papillary thyroid cancer | ‐ | Yes | ‐ | [53] | |
| Liver | hepatocellular carcinoma | ‐ | Yes | ‐ | [54] |
| Neuroendocrine tissue | Pheochromocytoma/medullary thyroid carcinoma | ‐ | Yes | ‐ | [55] |
| Medulloblastoma | JPH203 | ‐ | ‐ | [56] | |
| Blood | T‐cell acute lymphoblastic leukemia/T‐cell lymphoblastic lymphoma | BCH, JPH203 | Yes | ‐ | [57] |
| Kidney | Renal cell carcinoma | JPH203 | Yes | ‐ | [58] |
| Bone | Rhabdomyosarcoma, synovial sarcoma, Ewing sarcoma, epithelioid sarcoma, and angiosarcoma | ‐ | Yes | ‐ | [59] |
| Brain | Glioma | BCH | Yes | ‐ | [60] |
| Bladder | Bladder cancer | JPH203 | Yes | ‐ | [61] |
| Head and neck | Oral cancer | BCH, JPH203 | ‐ | [62] |
7. SLC7A11 IN BREAST CANCER CELLS
SLC7A11 is a bidirectional amino acid transporter. SLC7A11 localizes to the plasma membrane and mediates cystine uptake with a simultaneous efflux of glutamate (Figure 3). In addition, cystathionine was identified as a novel substrate of SLC7A11.21 Glutamine auxotroph analysis in breast cancer cells revealed that inhibition of SLC7A11 suppressed cell proliferation, while cystine depletion in culture medium attenuated cell proliferation in ER− breast cancer cells. Furthermore, the expression levels of SLC7A11 mRNA and the cystine consumption were higher in ER− breast cancer cells compared with in ER+ breast cancer cells,22 suggesting that cystine is specifically required for the proliferation of ER− breast cancer cells.
FIGURE 3.
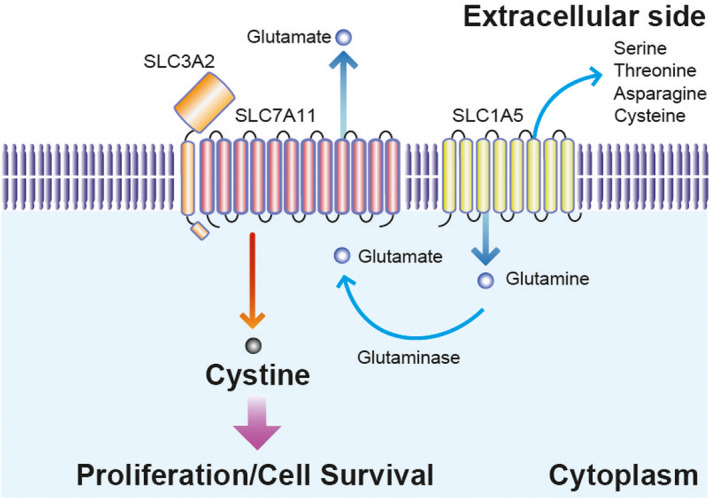
Schematic of amino acid flux by SLC7A11/SLC3A2 in ER− breast cancer cells. The bidirectional amino acid transporter, SLC7A11, interacts with SLC3A2 on the plasma membrane. In ER− breast cancer cells, cystine uptake by SLC7A11 is required for cell proliferation. Highly activated cystine uptake requires glutamate consumption to activate SLC7A11. Glutamine taken in by SLC1A5 is catabolized to glutamate by glutaminase, resulting in the activation of SLC7A11 in ER− breast cancer cells
Structural analyses have indicated that SLC7A11 contains 12 transmembrane domains and binds to SLC3A2 by disulfide bonding and hydrophobic interactions at the protein surface (Figure 3).23 Although the membrane trafficking mechanism underlying SLC7A11 function remains unresolved, membrane localization of SLC7A11 is reportedly stabilized by association with the CD44 variant, CD44v, and mucin 1 (MUC1) in ER− breast cancer cells.24
With regard to the cystine/glutamate antiporter, SLC7A11, intracellular glutamate is needed for cystine uptake. Glutamine catabolism supports glutamate supplementation of continuous cystine transport in ER− breast cancer cells. ER− breast cancer cells express high levels of the glutamine transporter, SLC1A5, and glutaminase, leading to upregulated glutamine metabolism.22, 25, 26 ER− breast cancer cells uptake glutamine via SLC1A5. Intracellular glutamine is catabolized to glutamate by glutaminase. Then, increased intracellular glutamate accelerates cystine uptake into cells, leading to aberrant proliferation of ER− breast cancer cells (Figure 3).
Intriguingly, SLC7A11 expression in ER− breast cancer cells is regulated by oxidative stress.27, 28 Oxidative stress induces the dissociation of NRF2, an antioxidant transcription factor, from KEAP1, an E3 ubiquitin ligase adaptor that triggers the protein degradation of NRF2. The accumulated NRF2 translocates to the nucleus and binds to the ARE in the promoter of its target SLC7A11.
8. SLC7A11 IN CANCER TISSUES
Overexpression of SLC7A11 has been reported in many cancer tissues, while high expression levels of SLC7A11 mRNA have been found to be associated with poor clinical prognoses in various forms of cancer (Table 2). Interestingly, SLC7A11 overexpression is associated with the acquisition of chemoresistance in small‐cell lung cancer and prostate cancer cells (Table 2).
TABLE 2.
Summary of SLC7A11 involvement in cancer tissues
| Tissue | Type of cells | SLC7A11 inhibitors used | Patient data (IHC or K‐M plot) | Relationship to therapeutic resistance | References |
|---|---|---|---|---|---|
| Breast | Basal type of breast cancer | SASP | Yes | ‐ | [22] |
| Esophagus | Squamous cell carcinoma | SASP | ‐ | ‐ | [63] |
| Colon | Colorectal cancer | SASP | Yes | ‐ | [64] |
| Lung | Non–small‐cell lung cancer (NSCLC) | SASP | Yes | ‐ | [65] |
| KRAS‐mutant lung adenocarcinoma | SASP | Yes | ‐ | [66] | |
| Small‐cell lung cancer | SASP | Yes | Yes | [67] | |
| Prostate | Advanced prostate cancer | Erastine | Yes | Yes | [68] |
| Pancreas | Pancreatic ductal adenocarcinoma | Erastine | ‐ | ‐ | [69] |
| Liver | Hepatocellular carcinoma | SASP | Yes | ‐ | [70] |
| Hepatocellular carcinoma | ‐ | Yes | ‐ | [71] | |
| Head and Neck | Squamous cell carcinoma | SASP | Yes | ‐ | [72] |
| Oral cavity squamous cell carcinoma | ‐ | Yes | ‐ | [73] | |
| Brain | Glioblastoma | ‐ | ‐ | [74] | |
| Glucose‐depleted glioma cells | SASP | ‐ | ‐ | [75] | |
| Blood | Multiple myeloma | ‐ | Yes | ‐ | [76] |
| Lymphoma | SASP | ‐ | ‐ | [77] | |
| Thyroid | Papillary thyroid carcinoma | ‐ | Yes | ‐ | [53] |
Sulfasalazine (SASP) and erastin inhibit the transport activity of SLC7A11. The tumor‐suppressive effect of SASP and erastin in various types of cancer cells is summarized (Table 2). Overall, inhibition of SLC7A11 induces a non‐apoptotic form of cell death termed ferroptosis in cancer cells, suggesting that SLC7A11 may be an attractive target in some cancer tissues.
9. CONCLUSION
We presented a summarized view of the effect exerted by the overexpression of amino acid transporters, SLC7A5 and SLC7A11, on many cancer tissues. These 2 contributed significantly to the proliferation of breast cancer cells, depending on ER status. However, overexpression of both SLC7A5 and SLC7A11 has also been reported in non–small‐cell lung cancer, pancreatic cancer, hepatocellular carcinoma, and esophageal cancer. Therefore, further investigations may be needed to better comprehend the pathological significance of amino acid transporters and amino acid usage in these tumors.
ER− breast cancer cells overexpress both SLC7A5 and SLC7A11. The cell surface levels of SLC7A5 are lower in ER− breast cancer cells, thereby minimizing the effect of SLC7A5 on the growth of ER− breast cancer cells. Therefore, the molecular basis of complex formation between SLC7A5 and SLC3A2 or between SLC7A11 and SLC3A2 may help to better understand the preferential role played by SLC7A11 in ER− breast cancer cells.
Finally, an understanding of the mechanism underlying the trafficking of SLC7A5/SLC3A2 and SLC7A11/SLC3A2 to the plasma membrane may enhance the knowledge needed to develop transporter‐targeting therapies in breast cancers.
DISCLOSURE
YS is funded by The Mochida Memorial Foundation, The Yasuda Medical Foundation, The Astellas Foundation, The Kanae Foundation, The Uehara Memorial Foundation, and The Naito Foundation. TS have no conflict of interest.
ACKNOWLEDGMENTS
The work conducted by YS was supported by a Grant‐in‐Aid for Scientific Research (C) and Grant‐in‐Aid for Research Activity Start‐up from the Japan Society for the Promotion of Science (JSPS), The Mochida Memorial Foundation, The Yasuda Medical Foundation, Astellas Foundation for Research on Metabolic Disorders, Kanae Foundation, The Uehara Memorial Foundation, The Naito Foundation, and the foundation from Yamagata prefectural government and the City of Tsuruoka.
Saito Y, Soga T. Amino acid transporters as emerging therapeutic targets in cancer. Cancer Sci. 2021;112:2958–2965. 10.1111/cas.15006
Funding information
Naito Foundation, Uehara Memorial Foundation, Japan Society for the Promotion of Science, (Grant/Award Number: 19K23896, 20K07620) Yasuda Memorial Medical Foundation, Astellas Foundation for Research on Metabolic Disorders, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kanae Foundation for the Promotion of Medical Science
Contributor Information
Yasuhiro Saito, Email: ysaito@ttck.keio.ac.jp.
Tomoyoshi Soga, Email: soga@sfc.keio.ac.jp.
REFERENCES
- 1.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa CM, Biancur DE, Wang X, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheen J‐H, Zoncu R, Kim D, Sabatini DM. Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell. 2011;19:613‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X, Sanderson SM, Dai Z, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddocks ODK, Athineos D, Cheung EC, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372‐376. [DOI] [PubMed] [Google Scholar]
- 7.Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017;26:301‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfson RL, Chantranupong L, Saxton RA, et al. Sestrin2 is a leucine sensor for the mTROC1 pathway. Science. 2016;351:43‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantranupong L, Scaria SM, Saxton RA, et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell. 2016;165:153‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggs KJ, Koivunen P, Cao S, et al. Paracrine induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell. 2016;166:126‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagida O, Kanai Y, Chairoungdua A, et al. Human L‐type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291‐302. [DOI] [PubMed] [Google Scholar]
- 13.Kantipudi S, Jeckelmann J‐M, Ucurum Z, Bosshart PD, Fotiadis D. The heavy chain 4F2hc modulates the substrate affinity and specificity of the light chains LAT1 and LAT2. Int J Mol Sci. 2020;21:7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature. 2019;568:127‐130. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Wiriyasermkul P, Jin C, et al. Cryo‐EM structure of the human L‐type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol. 2019;26:510‐517. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Li L, Coyaud E, et al. LLGL2 rescues nutrient stress by promoting leucine uptake in ER+ breast cancer. Nature. 2019;569:275‐279. [DOI] [PubMed] [Google Scholar]
- 17.Xu D, Hemler ME. Metabolic activation‐related CD147‐CD98 Complex* S. Mol Cell Proteomics. 2005;4:1061‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruvberger S, Ringnér M, Chen Y, et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979‐5984. [PubMed] [Google Scholar]
- 19.Xu J, Fan S, Rosen EM. Regulation of the estrogen‐inducible gene expression profile by the breast cancer susceptibility gene BRCA1. Endocrinology. 2005;146(4):2031‐2047. [DOI] [PubMed] [Google Scholar]
- 20.Arpino G, Generali D, Sapino A, et al. Gene expression profiling in breast cancer: a clinical perspective. Breast. 2013;22(2):109‐120. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Sato M, Kasakoshi T, et al. Cystathionine is a novel substrate of cystine/glutamate transporter implications for immune function. J Biol Chem. 2015;290:8778‐8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmerman LA, Holton T, Yuneva M, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple‐negative breast tumor therapeutic target. Cancer Cell. 2013;24:450‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oda K, Lee Y, Wiriyasermkul P, et al. Consensus mutagenesis approach improves the thermal stability of system xc− transporter, xCT, and enables cryo‐EM analyses. Protein Sci. 2020;29:2398‐2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa M, Takahashi H, Rajabi H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1‐C oncoprotein in triple‐negative breast cancer cells. Oncotarget. 2016;7:11756‐11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geck RC, Toker A. Non‐essential amino acid metabolism in breast cancer. Adv Biol Regul. 2016;62:11‐17. [DOI] [PubMed] [Google Scholar]
- 26.van Geldermalsen M , Wang Q, Nagarajah R, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple‐negative basal‐like breast cancer. Oncogene. 2016;35:3201‐3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib E, Linher‐Melville K, Lin H‐X, Singh G. Expression of xCT and activity of system xc− are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin C‐S, Mishra P, Watrous JD, et al. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun. 2017;8:15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Harada‐Shoji N, Toyohara T, et al. L‐type amino acid transporter 1 is associated with chemoresistance in breast cancer via the promotion of amino acid metabolism. Sci Rep. 2021;11:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansari RE, Craze ML, Miligy I, et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihály Z, Kormos M, Lánczky A, et al. A meta‐analysis of gene expression‐based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res Treat. 2013;140:219‐232. [DOI] [PubMed] [Google Scholar]
- 32.Imai H, Kaira K, Oriuchi N, et al. Inhibition of L‐type amino acid transporter 1 has antitumor activity in non‐small cell lung cancer. Anticancer Res. 2010;30:4819‐4828. [PubMed] [Google Scholar]
- 33.Takeuchi K, Ogata S, Nakanishi K, et al. LAT1 expression in non‐small‐cell lung carcinomas: analyses by semiquantitative reverse transcription‐PCR (237 cases) and immunohistochemistry (295 cases). Lung Cancer. 2010;68:58‐65. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, Sakamoto S, Matsushima J, et al. Up‐regulation of LAT1 during antiandrogen therapy contributes to progression in prostate cancer cells. J Urol. 2016;195:1588‐1597. [DOI] [PubMed] [Google Scholar]
- 35.Kaira K, Sunose Y, Ohshima Y, et al. Clinical significance of L‐type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer. 2013;13:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yothaisong S, Dokduang H, Anzai N, et al. Inhibition of l‐type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumor Biol. 2017;39:1010428317694545. [DOI] [PubMed] [Google Scholar]
- 37.Kaira K, Sunose Y, Oriuchi N, Kanai Y, Takeyoshi I. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat Dis Int. 2014;13:654‐657. [DOI] [PubMed] [Google Scholar]
- 38.Muto Y, Furihata T, Kaneko M, et al. Different response profiles of gastrointestinal cancer cells to an L‐type amino acid transporter inhibitor, JPH203. Anticancer Res. 2018;39:159‐165. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Chen X, Su L, Li P, Liu B, Zhu Z. LAT‐1 functions as a promotor in gastric cancer associated with clinicopathologic features. Biomed Pharmacother. 2013;67:693‐699. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi H, Ishii Y, Takayama T. Expression of L‐type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90:233‐238. [DOI] [PubMed] [Google Scholar]
- 41.Najumudeen AK, Ceteci F, Fey SK, et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS‐mutant colorectal cancer. Nat Genet. 2021;53:16‐26. [DOI] [PubMed] [Google Scholar]
- 42.Kaira K, Sunose Y, Arakawa K, et al. Prognostic significance of L‐type amino‐acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altan B, Kaira K, Watanabe A, et al. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2018;81:141‐153. [DOI] [PubMed] [Google Scholar]
- 44.Yanagisawa N, Ichinoe M, Mikami T, et al. High expression of L‐type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65:1019‐1023. [DOI] [PubMed] [Google Scholar]
- 45.Tina E, Prosén S, Lennholm S, Gasparyan G, Lindberg M, Eremo AG. Expression profile of the amino acid transporters SLC7A5, SLC7A7, SLC7A8 and the enzyme TDO2 in basal cell carcinoma. Br J Dermatol. 2019;180:130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu A, Kaira K, Kato M, et al. Prognostic significance of L‐type amino acid transporter 1 (LAT1) expression in cutaneous melanoma. Melanoma Res. 2015;25:399‐405. [DOI] [PubMed] [Google Scholar]
- 47.Sato K, Miyamoto M, Takano M, Furuya K, Tsuda H. Significant relationship between the LAT1 expression pattern and chemoresistance in ovarian clear cell carcinoma. Virchows Arch. 2019;474:701‐710. [DOI] [PubMed] [Google Scholar]
- 48.Fan X, Ross DD, Arakawa H, Ganapathy V, Tamai I, Nakanishi T. Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: a possible target for combination therapy with anti‐proliferative aminopeptidase inhibitors. Biochem Pharmacol. 2010;80:811‐818. [DOI] [PubMed] [Google Scholar]
- 49.Sato K, Miyamoto M, Takano M, Tsuda H. Correlation of high LAT1 expression with the prognosis of endometrioid carcinoma of the uterine corpus. Virchows Arch. 2020;477:421‐427. [DOI] [PubMed] [Google Scholar]
- 50.Marshall AD, van Geldermalsen M , Otte NJ, et al. LAT1 is a putative therapeutic target in endometrioid endometrial carcinoma. Int J Cancer. 2016;139:2529‐2539. [DOI] [PubMed] [Google Scholar]
- 51.Enomoto K, Sato F, Tamagawa S, et al. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci Rep. 2019;9:14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Häfliger P, Graff J, Rubin M, et al. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J Exp Clin Cancer Res. 2018;37:234‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen L, Qian C, Cao H, Wang Z, Luo T, Liang C. Upregulation of the solute carrier family 7 genes is indicative of poor prognosis in papillary thyroid carcinoma. World J Surg Oncol. 2018;16:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Qiang J, Chen S‐F, Wang X, Fu J, Chen Y. The impact of L‐type amino acid transporter 1 (LAT1) in human hepatocellular carcinoma. Tumor Biol. 2013;34:2977‐2981. [DOI] [PubMed] [Google Scholar]
- 55.Barollo S, Bertazza L, Watutantrige‐Fernando S, et al. Overexpression of L‐type amino acid transporter 1 (LAT1) and 2 (LAT2): novel markers of neuroendocrine tumors. PLoS One. 2016;11:e0156044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cormerais Y, Pagnuzzi‐Boncompagni M, Schrötter S, et al. Inhibition of the amino‐acid transporter LAT1 demonstrates anti‐neoplastic activity in medulloblastoma. J Cell Mol Med. 2019;23:2711‐2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosilio C, Nebout M, Imbert V, et al. L‐type amino‐acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T‐cell lymphoblastic lymphoma/T‐cell acute lymphoblastic leukemia. Leukemia. 2015;29:1253‐1266. [DOI] [PubMed] [Google Scholar]
- 58.Higuchi K, Sakamoto S, Ando K, et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci Rep. 2019;9:16776‐16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koshi H, Sano T, Handa T, et al. L‐type amino acid transporter‐1 and CD98 expression in bone and soft tissue tumors. Pathol Int. 2015;65:460‐467. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi K, Ohnishi A, Promsuk J, et al. Enhanced tumor growth elicited by L‐type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62:493‐504. [DOI] [PubMed] [Google Scholar]
- 61.Maimaiti M, Sakamoto S, Yamada Y, et al. Expression of L‐type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci Rep. 2020;10:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun D‐W, Lee SA, Park M‐G, et al. JPH203, an L‐type amino acid transporter 1–selective compound, induces apoptosis of YD‐38 human oral cancer cells. J Pharmacol Sci. 2014;124:208‐217. [DOI] [PubMed] [Google Scholar]
- 63.Shiozaki A, Iitaka D, Ichikawa D, et al. xCT, component of cysteine/glutamate transporter, as an independent prognostic factor in human esophageal squamous cell carcinoma. J Gastroenterol. 2014;49:853‐863. [DOI] [PubMed] [Google Scholar]
- 64.Ma M, Chen G, Wang P, et al. Xc− inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH‐dependent mechanism. Cancer Lett. 2015;368:88‐96. [DOI] [PubMed] [Google Scholar]
- 65.Ji X, Qian J, Rahman SMJ, et al. xCT (SLC7A11)‐mediated metabolic reprogramming promotes non‐small cell lung cancer progression. Oncogene. 2018;37:5007‐5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu K, Li K, Lv J, et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS‐mutant lung adenocarcinoma. J Clin Invest. 2019;130:1752‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan J, Lo M, Dockery P, et al. The xc− cystine/glutamate antiporter as a potential therapeutic target for small‐cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. 2008;64:463. [DOI] [PubMed] [Google Scholar]
- 68.Ghoochani A, Hsu E‐C, Aslan M, et al. Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Res. 2021;81:1583‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badgley MA, Kremer DM, Maurer HC, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wada F, Koga H, Akiba J, et al. High expression of CD44v9 and xCT in chemoresistant hepatocellular carcinoma: potential targets by sulfasalazine. Cancer Sci. 2018;109:2801‐2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinoshita H, Okabe H, Beppu T, et al. Cystine/glutamic acid transporter is a novel marker for predicting poor survival in patients with hepatocellular carcinoma. Oncol Rep. 2013;29:685‐689. [DOI] [PubMed] [Google Scholar]
- 72.Okazaki S, Umene K, Yamasaki J, et al. Glutaminolysis‐related genes determine sensitivity to xCT‐targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453‐3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JR, Roh J, Lee SM, et al. Overexpression of cysteine‐glutamate transporter and CD44 for prediction of recurrence and survival in patients with oral cavity squamous cell carcinoma. Head Neck. 2018;40:2340‐2346. [DOI] [PubMed] [Google Scholar]
- 74.Polewski MD, Reveron‐Thornton RF, Cherryholmes GA, Marinov GK, Aboody KS. SLC7A11 overexpression in glioblastoma is associated with increased cancer stem cell‐like properties. Stem Cells Dev. 2017;26:1236‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung WJ, Lyons SA, Nelson GM, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101‐7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong Y, Tian F, Ma H, et al. FTY720 induces ferroptosis and autophagy via PP2A/AMPK pathway in multiple myeloma cells. Life Sci. 2020;260:118077. [DOI] [PubMed] [Google Scholar]
- 77.Gout P, Buckley A, Simms C, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633‐1640. [DOI] [PubMed] [Google Scholar]


