Abstract
Signal regulatory protein alpha (SIRPα) is a type I transmembrane protein that inhibits macrophage phagocytosis of tumor cells upon interaction with CD47, and the CD47‐SIRPα pathway acts as an immune checkpoint factor in cancers. This study aims to clarify the clinical significance of SIRPα expression in esophageal squamous cell carcinoma (ESCC). First, we assessed SIRPα expression using RNA sequencing data of 95 ESCC tissues from The Cancer Genome Atlas (TCGA) and immunohistochemical analytic data from our cohort of 131 patients with ESCC. Next, we investigated the correlation of SIRPα expression with clinicopathological factors, patient survival, infiltration of tumor immune cells, and expression of programmed cell death‐ligand 1 (PD‐L1). Overall survival was significantly poorer with high SIRPα expression than with low expression in both TCGA and our patient cohort (P < .001 and P = .027, respectively). High SIRPα expression was associated with greater depth of tumor invasion (P = .0017). Expression of SIRPα was also significantly correlated with the tumor infiltration of M1 macrophages, M2 macrophages, CD8+ T cells, and PD‐L1 expression (P < .001, P < .001, P = .03, and P < .001, respectively). Moreover, patients with SIRPα/PD‐L1 coexpression tended to have a worse prognosis than patients with expression of either protein alone or neither. Taken together, SIRPα indicates poor prognosis in ESCC, possibly through inhibiting macrophage phagocytosis of tumor cells and inducing suppression of antitumor immunity. Signal regulatory protein alpha should be considered as a potential therapeutic target in ESCC, especially if combined with PD‐1‐PD‐L1 blockade.
Keywords: cancer immunotherapy, esophageal cancer, immune checkpoint factor, PD‐L1, SIRPα
This article highlights the clinical significance of signal regulatory protein alpha (SIRPα) in esophageal squamous cell carcinoma (ESCC). The results showed that high SIRPα expression was a significant indicator of poor prognosis in ESCC and SIRPα/programmed cell death‐ligand 1 (PD‐L1) coexpression had a negative synergistic impact on prognosis in ESCC. Thus, SIRPα could be a novel clinical biomarker and a potential therapeutic target in ESCC, especially if combined with PD‐1‐PD‐L1 blockade.
1. INTRODUCTION
Esophageal cancer is the seventh most common cause of cancer‐related death globally because of its high malignant potential and poor prognosis.1 Esophageal cancer mainly consists of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Esophageal squamous cell carcinoma is the most common histopathological type in eastern Asia and Africa, whereas EAC predominates in European and American countries.2 Despite recent development in the treatment for esophageal cancer, including surgery, radiotherapy, cytotoxic chemotherapy, and a multimodal strategy combining these treatments, the prognosis of esophageal cancer remains poor, with an approximately 15%‐25% 5‐year survival rate.3
Cancer immunotherapy, such as immune checkpoint inhibition, has emerged as a promising therapeutic option. Inhibition of the programmed cell death‐1 (PD‐1) and programmed cell death‐ligand 1 (PD‐L1) pathway has shown remarkable clinical efficacy in many types of cancers, including esophageal cancer.4, 5 However, the overall response rate of PD‐1 inhibitor for the patients with ESCC is insufficient because resistance to T cell‐dependent adaptive immune checkpoint therapy develops through a variety of mechanisms to avoid immune surveillance, such as suppressing innate immune systems.4, 6 Therefore, combination therapy targeting both adaptive and innate immune responses should be explored to improve the response rates and overcome resistance to adaptive immune checkpoint inhibitors.
Signal regulatory protein alpha (SIRPα) is a type I transmembrane protein with three extracellular Ig‐like domains and two cytoplasmic immunoreceptor tyrosine‐based inhibition motifs that are putative phosphorylation sites and binding sites of the Src‐homology‐2 domain‐containing protein tyrosine phosphatase 1 (SHP1).8, 9, 10 Signal regulatory protein alpha is especially abundant in neurons and myeloid hematopoietic cells, such as macrophages, neutrophils, and dendritic cells.11
Macrophages take part in the innate immune response by phagocytosis of target cells. The interaction of SIRPα on macrophages with CD47 on target cells suppresses macrophage phagocytosis of target cells, and the SIRPα‐CD47 pathway acts as an innate immune checkpoint for activated macrophage phagocytosis. Tumor cells exploit this immune checkpoint pathway to evade antitumor immunity; thus, treatments targeting this pathway are promising immunotherapeutic approaches for cancers. Indeed, recent preclinical studies reveal that blockade of the CD47‐SIRPα pathway by anti‐CD47 Abs and anti‐SIRPα Abs enhances the phagocytosis of tumor cells by macrophages in vivo12, 13, 14, 15, 16, 17, 18 and effectively inhibits tumor progression in mouse models.19, 20 In contrast, several clinical trials investigating the antitumor effect of CD47‐SIRPα inhibitors against hematopoietic and solid cancers show that monotherapy with CD47 inhibitors is not significantly effective, which suggests that the in vivo and tumor models might be more immunogenic and thus too artificial compared to human cancer.7 Therefore, we believe that evaluating the clinical significance of SIRPα expression in human tumor tissue is essential to develop more effective cancer immunotherapy.
The present study aimed to clarify the clinical significance of SIRPα expression in cancer tissue using ESCC RNA sequencing (RNA‐seq) data from a public database and immunohistochemical data from our cohort of patients with ESCC. We investigated the association between SIRPα expression and clinicopathological factors and determined the prognostic value of SIRPα expression in patients with ESCC. We also assessed the relationship between SIRPα and tumor‐infiltrating immune cells in the tumor. Finally, we evaluated the correlation between SIRPα and other immune checkpoint molecules.
2. MATERIALS AND METHODS
2.1. Public dataset
Using The Cancer Genome Atlas (TCGA), we obtained RNA‐seq data from 95 cancer tissues and 11 noncancer tissues in the ESCC dataset through the Firehose pipeline at the Broad Institute (http://gdac.broadinstitute.org/runs/stddata__2016_01_28/data/ESCA/20160128/). The RNA‐seq data (fragments per kilobase million [FPKM] values, raw counts) were subjected to quantile normalization and used for in silico analysis. For clinical analysis, patients were divided into two groups based on their SIRPα mRNA expression level; the patients with more SIRPα mRNA expression above vs below the median value were designated as having high vs low expression, respectively.
2.2. Our patient cohort
A retrospective analysis was carried out at a single center. Approval for this study was obtained from the clinical research ethical committee of our Institutional Review Board (Kyushu University, IRB NO. 2019‐212). All patients included in the present study provided written informed consent. A total of 131 patients who underwent surgical resection for ESCC from January 2011 to December 2015 were eligible for this study; patients with stage IV disease were excluded. The follow‐up data and the following clinicopathological information were obtained from medical records: age at surgery; sex; tumor location; histopathology; pathological depth of tumor invasion, lymph node, and metastasis stage (UICC TNM classification, 8th edition); history of preoperative treatments; and pathological effects of preoperative treatment using the criteria from the Japanese Classification of Esophageal Cancer (11th edition).
2.3. Treatment in our patient cohort
Patients with locally advanced esophageal cancer underwent neoadjuvant chemotherapy (NAC) or chemoradiotherapy (NACRT). For NAC, chemotherapy with cisplatin and 5‐fluorouracil (5‐FU) was repeated as two cycles every 4 weeks (cisplatin 60‐80 mg/m2/d, 5‐FU 600‐800 mg/m2/d).21 Chemotherapy for NACRT consisted of either low‐dose cisplatin and 5‐FU (cisplatin 5 mg/m2/d, 5‐FU 250 mg/m2/d, with treatment given on weekdays and repeated every 3‐4 weeks) or a standard‐dose regimen (cisplatin 70 mg/m2 on days 1 and 29 and 5‐FU 700 mg/m2/d on days 1‐4 and 29‐32). Radiotherapy was delivered using equally weighted anterior‐ and posterior‐opposed beams from a 10‐MV linear accelerator in 15‐25 fractions of 1.8‐2.0 Gy (total dose, 30‐45 Gy). Esophagectomy was carried out 4‐10 weeks after completing NAC or NACRT. For definitive chemoradiotherapy (dCRT), additional radiation (total dose >60 Gy) was administered using two parallel oblique fields or multiple fields. Salvage esophagectomy was carried out for either residual or recurrent esophageal cancer after dCRT.22 For thoracic esophageal cancer, we mainly used subtotal esophagectomy (the McKeown technique) with two‐ or three‐field lymph node dissection. We did not use adjuvant chemotherapy. All patients were postoperatively followed up using a computed tomography scan every 3 months and gastrointestinal endoscopic assessment annually.
2.4. Immunohistochemical analysis
Immunohistochemistry (IHC) was undertaken on formalin‐fixed, paraffin‐embedded tumor tissue sections of 4‐μm thickness. Sections were singly stained with the following primary Abs according to the manufacturers’ recommendations: SIRPα (clone D613M, 1:100 dilution; Cell Signaling Technology), CD80 (clone B7‐1, 1:100 dilution; R&D Systems), CD163 (clone 10D6, 1:100 dilution; Leica Biosystems), CD8 (clone C8/144B, 1:100 dilution; Dako), and PD‐L1 (clone 28‐8, 1:483 dilution; Abcam). In brief, sections were deparaffinized with xylene and a descending ethanol series. Endogenous peroxidase activity was inhibited by incubation for 30 minutes with 3% H2O2 in methanol. The sections were pretreated with Target Retrieval Solution (Dako; pH 9.0 for SIRPα and PD‐L1; pH 6.0 for CD80, CD163, and CD8) in a microwave oven at 100℃ for 20 minutes for SIRPα or a decloaking chamber at 121℃ for 10‐15 minutes for others. The sections were then incubated with the primary Ab at 4℃ overnight. Bound Ab was detected using the Dako EnVision Detection System (Dako). Finally, the sections were incubated with 3,3′‐diaminobenzidine, counterstained with hematoxylin, and mounted. We used sections from human tonsils or placentas as positive controls. Stained slides were scanned using the NanoZoomer (Hamamatsu Photonics).
Specimens were independently evaluated by two of the authors in a blinded manner, and the final assessments were achieved by consensus. Evaluation of IHC was undertaken at the area including tumor cells. If the resected specimens included no viable cancer cells because of the effect of preoperative treatment, the observation of fibrosis was used to identify the area that could be regarded as the extent of the preexisting tumor and was evaluated for IHC analysis.
Staining for SIRPα was considered to be positive if IHC staining was observed in the cytoplasm and membranes of tumor cells or stromal tumor‐infiltrating cells. The staining was rated as 0‐3 based on the estimated proportion of positively stained cells within the tumor as follows: 0, no staining of tumor or stromal cells; 1, less than one‐third of cells positively stained; 2, one‐ to two‐thirds of cells positively stained, and 3, more than two‐thirds of cells positively stained. Cases rated 0 were identified as low; cases rated 1‐3 were identified as high.
Programmed cell death‐ligand 1 expression was evaluated based on the frequency of positive membrane‐stained tumor cells (TCs) and tumor‐infiltrating immune cells (ICs) and characterized by the combined positive score (CPS), which was defined as the number of PD‐L1 positive cells (TCs and ICs) divided by the total number of TCs × 100. Specimens were considered PD‐L1 positive if CPS was 1% or higher and PD‐L1 negative if CPS was less than 1%,23 with the 1% cut‐off value being based on a previous study.24 The numbers of CD80+, CD163+, and CD8+ cells were manually counted under ×400 magnification in five high‐powered fields (0.04 mm2/high‐powered fields; NanoZoomer) in each specimen. The median numbers of CD80+ and CD163+ cells were calculated. The cases with more than the median number of CD80+ and CD163+ cells were identified as CD80‐high and CD163‐high, respectively.
2.5. Analysis of CIBERSORT
CIBERSORT (https://cibersort.stanford.edu) is an analytical tool to estimate the relative abundance of 22 types of immune cells in a mixed cell population using gene expression data. Gene expression data for 95 ESCC samples collected from TCGA were used for the CIBERSORT analysis.
2.6. Statistics and survival analysis
Continuous variables were summarized using descriptive statistics and compared using Student’s t test or Mann‐Whitney U test. The proportions of categorical variables were summarized as frequency (number of patients and % of the population) and compared using the χ2 test.
Both disease‐free survival (DFS) and overall survival (OS) rates were estimated with the Kaplan‐Meier method and compared using the log‐rank test. Survival was defined as the interval from the date of the surgery until the date of either death (due to any cause) or the first recurrence detected by radiologic imaging. The impacts of the factors on survival rates or SIRPα expression were described with hazard ratio or odds ratio and 95% confidence interval. The prognostic factors for survival and disease recurrence were analyzed using a Cox proportional hazards model; the significant factors for SIRPα expression were analyzed using a logistic regression model. All variables that were P < .10 in the univariable analysis and intended to evaluate were included in the multivariable analysis. Differences were considered to be significant if P < .05. The data were analyzed using JMP14 software (SAS Institute) and R software version 3.3.2 (The R Foundation).
3. RESULTS
3.1. Signal regulatory protein alpha expression was a prognostic factor of ESCC in TCGA dataset
We addressed the question of whether SIRPα could have an impact on the prognosis of patients with ESCC. To this end, we investigated the association between SIRPα expression and patient prognosis in ESCC using both the public dataset and our patient cohort. First, we estimated the mRNA expression level of SIRPα in 95 patients with ESCC from TCGA dataset. The expression of SIRPα mRNA in cancer tissue was significantly higher than in noncancer tissue (P < .001, Figure 1A). The 95 patients were divided into high‐ (n = 48) and low‐ (n = 47) expression groups according to their SIRPα mRNA expression level. The OS in the high‐expression group was significantly worse than in the low‐expression group (P < .001, log‐rank test; Figure 1B).
FIGURE 1.
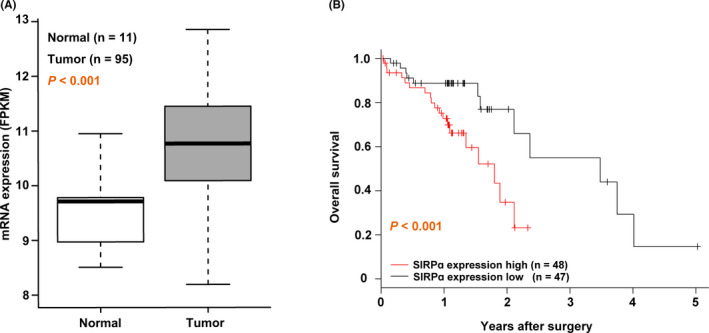
Overall survival in the signal regulatory protein α (SIRPα) high‐expression group was worse than in the low‐expression group using a public dataset of esophageal squamous cell carcinoma (ESCC). A, The mRNA expression level of SIRPα in tumor tissue compared with normal tissue using The Cancer Genome Atlas ESCC dataset. FPKM, fragments per kilobase million. B, Overall survival of the high‐ (red line; n = 48) and low‐ (black line; n = 47) expression groups of SIRPα was analyzed by the Kaplan‐Meier method. Statistical analysis was undertaken using the log‐rank test
3.2. Clinicopathological and prognostic significance of SIRPα expression in our patient cohort
Next, we analyzed the protein expression level of SIRPα in our patient cohort using IHC analysis. A total of 131 patients with ESCC who underwent esophagectomy were included in this cohort. Figure 2A,B shows representative IHC staining of SIRPα in surgically resected specimens from patients with ESCC. Immunohistochemical staining of SIRPα was detected in both the membranes and cytoplasm of tumor cells and stromal tumor‐infiltrating cells. The patients were divided into high‐ (n = 84) and low‐ (n = 47) expression groups according to IHC staining for SIRPα.
FIGURE 2.
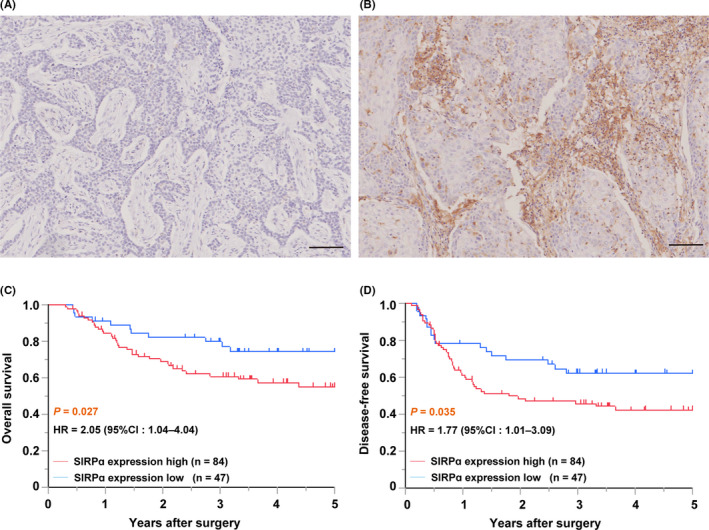
Signal regulatory protein α (SIRPα) expression was an indicator of poor prognosis for esophageal squamous cell carcinoma (ESCC) in our patient cohort. A, B, Representative immunohistochemical staining of SIRPα in surgically resected specimens from patients with ESCC. ESCC showing low (A) and high (B) SIRPα immunoactivity. Scale bar = 100 μm. C, D, Overall survival (C) and disease‐free survival (D) of high‐ (red line; n = 84) and low‐ (blue line; n = 47) expression of SIRPα were analyzed by the Kaplan‐Meier method. Statistical analysis was undertaken using the log‐rank test
The association between SIRPα expression and clinicopathological factors is described in Table 1. The high‐ and low‐expression groups were similar with respect to age, sex, tumor location, histopathology, pathological lymph node metastasis, preoperative treatment, and effects of preoperative treatment. Patients with pathological depth of tumor invasion (T) 2‐4 and pathological stage II–III were significantly more frequent in the high‐expression group than the low‐expression group (P = .0017 and P = .027, respectively).
TABLE 1.
Baseline characteristics of 131 esophageal squamous cell carcinoma patients grouped according to expression of signal regulatory protein alpha (SIRPα)
| Factor | Category | High SIRPα (n = 84) | Low SIRPα (n = 47) | P value |
|---|---|---|---|---|
| Age, years | 66.4 (±0.9) | 65.7 (±1.2) | .6700 | |
| Sex | Male | 72 (86) | 40 (85) | .9200 |
| Tumor location | Ce | 11 (13) | 9 (19) | .5100 |
| Ut | 14 (17) | 7 (15) | ||
| Mt | 41 (49) | 21 (45) | ||
| Lt | 18 (21) | 10 (21) | ||
| Histopathology | Well differentiated | 25 (30) | 6 (13) | .0600 |
| Moderately differentiated | 49 (58) | 36 (77) | ||
| Poorly differentiated | 10 (12) | 5 (11) | ||
| pT | T2‐4 | 64 (76) | 23 (49) | .0017 |
| pN | N1‐3 | 31 (37) | 17 (36) | .9300 |
| pM | M1 | 0 (0) | 0 (0) | |
| pStage | Stages II‐III | 64 (76) | 27 (57) | .0270 |
| Preoperative treatment | None | 20 (24) | 15 (32) | .2800 |
| NAC | 27 (32) | 19 (40) | ||
| NACRT | 31 (37) | 10 (21) | ||
| dCRT | 6 (7.1) | 3 (6.4) | ||
| Effects of preoperative treatment | Grade 1 | 45 (69) | 23 (74) | .7800 |
| Grade 2 | 12 (18) | 4 (13) | ||
| Grade 3 | 8 (12) | 4 (13) |
Data are shown as mean (±SD) or n (%).
Abbreviations: Ce, cervical esophagus; dCRT, definitive chemoradiotherapy; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; NAC, neoadjuvant chemotherapy; NACRT, neoadjuvant chemoradiotherapy; pM, pathological metastasis; pN, pathological lymph node metastasis; pStage, pathological stage; pT, pathological depth of tumor invasion; Ut, upper thoracic esophagus.
Survival analysis according to SIRPα expression using the Kaplan‐Meir method in our patient cohort showed that both OS and DFS were significantly worse in the high‐expression group than the low‐expression group (P = .027 and P = .035, respectively, log‐rank test; Figure 2C,D).
Univariable and multivariable analyses were undertaken to identify the prognostic predictors in our patient cohort (Table 2). High expression of SIRPα was one of the significant prognostic factors for OS on multivariable analysis (hazard ratio = 1.988; 95% confidence interval, 0.998‐3.959; P = .04).
TABLE 2.
Univariable and multivariable Cox regression analysis for disease‐free survival (DFS) and overall survival (OS) among patients with esophageal squamous cell carcinoma (n = 131)
| Factor | DFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, years | 0.991 | 0.962‐1.022 | .6000 | – | – | – | 0.995 | 0.959‐1.032 | .790 | – | – | – | |
| Sex | Male | 0.667 | 0.347‐1.278 | .2400 | – | – | – | 1.027 | 0.458‐2.302 | .940 | – | – | – |
| Tumor location | Ce and Ut | 1.508 | 0.908‐2.507 | .1100 | – | – | – | 1.171 | 0.640‐2.143 | .610 | – | – | – |
| Histopathology | Poorly differentiated | 1.487 | 0.734‐3.012 | .2900 | – | – | – | 1.754 | 0.816‐3.772 | .170 | – | – | – |
| pStage | II‐III | 2.587 | 1.378‐4.856 | .0012 | 2.811 | 1.475‐5.357 | <.001 | 1.909 | 0.948‐3.844 | .055 | 2.039 | 0.994‐4.183 | .0400 |
| Effect of preoperative treatment | Grade 2‐3 | 0.360 | 0.164‐0.790 | .0038 | 0.281 | 0.127‐0.622 | <.001 | 0.334 | 0.131‐0.847 | .008 | 0.265 | 0.102‐0.683 | .0013 |
| SIRPα expression | High | 1.775 | 1.017‐3.096 | .0350 | 1.600 | 0.907‐2.821 | .090 | 2.056 | 1.045‐4.042 | .027 | 1.988 | 0.998‐3.959 | .0400 |
| PD‐L1 expression | Positive | 1.425 | 0.867‐2.343 | .1500 | – | – | – | 1.151 | 0.649‐2.043 | .620 | – | – | – |
| SIRPα/PD‐L1 | Coexpression | 1.624 | 0.992‐2.658 | .0550 | – | – | – | 1.627 | 0.916‐2.887 | .090 | – | – | – |
Abbreviations: Ce, cervical esophagus; CI, confidence interval; HR, hazard ratio; PD‐L1, programmed cell death‐ligand 1; pStage, pathological stage; SIRPα, signal regulatory protein alpha; Ut, upper thoracic esophagus; –, not included in analysis.
3.3. High SIRPα expression associated with tumor‐infiltrating immune cells
We examined whether SIRPα could be involved in the regulation of antitumor immunity in ESCC. To this end, we used IHC analysis to evaluate the association between SIRPα expression (Figure 3A) and the numbers of tumor‐infiltrating immune cells such as M1 macrophages, M2 macrophages, and cytotoxic T cells. We used CD80, CD163, and CD8 as cell surface markers of M1 macrophages, M2 macrophages, and cytotoxic T cells, respectively. Representative images of CD80, CD163, and CD8 staining are shown in Figure 3B‐D. Significantly greater numbers of CD80+ and CD163+ macrophages were present in the SIRPα high‐expression group than the low‐expression group (P < .001 and P < .001, respectively; Figure 3E,F). In contrast, the number of CD8+ cytotoxic T cells in the SIRPα high‐expression group was significantly smaller than in the low‐expression group (P = .03; Figure 3G). Multivariable analysis showed that high SIRPα expression was significantly associated with pathological T 2‐4, greater number of infiltrating CD163+ M2 macrophages, and smaller number of infiltrating CD8+ cytotoxic T cells (Table 3). Moreover, we globally analyzed the association between SIRPα expression and composition of infiltrating immune cells in ESCC using the CIBERSORT system. Signal regulatory protein alpha expression was significantly positively associated with the proportions of tumor‐infiltrating M0, M1, and M2 macrophages and CD4+ resting T cells (Figure S1).
FIGURE 3.
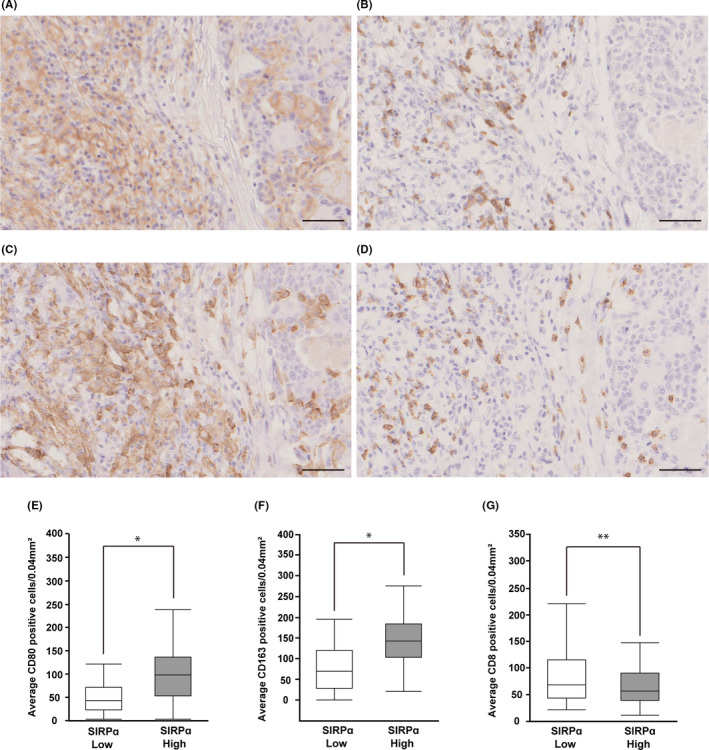
Signal regulatory protein α (SIRPα) expression was associated with tumor‐infiltrating immune cells in esophageal squamous cell carcinoma (ESCC). A‐D, Representative immunohistochemical staining of SIRPα (A), CD80 (B), CD163 (C), and CD8 (D) in a surgically resected specimen from one patient with ESCC. All of the images represent a single area of one specimen. Scale bar = 50 μm. E, F, Association between SIRPα expression and the infiltrating numbers of tumor immune cells including CD80 (E), CD163 (F), and CD8 (G) were evaluated by manual cell counts of immunohistochemically stained samples. *P < .001; **P = .03
TABLE 3.
Univariable and multivariable analysis of the relationship between signal regulatory protein alpha (SIRPα) expression and other clinicopathological factors in patients with esophageal squamous cell carcinoma (n = 131)
| Factor | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age, years | 1.009 | 0.967‐1.052 | .6600 | – | – | – | |
| Sex | Male | 1.050 | 0.382‐2.880 | .9200 | – | – | – |
| Tumor location | Ce and Ut | 0.820 | 0.382‐1.761 | .6100 | – | – | – |
| Histopathology | Poorly differentiated | 1.135 | 0.363‐3.543 | .8200 | – | – | – |
| pT | T2‐4 | 3.339 | 1.559‐7.147 | .0017 | 3.555 | 1.218‐10.37 | .0160 |
| pN | N1‐3 | 1.032 | 0.491‐2.167 | .9300 | – | – | – |
| Preoperative treatment | Yes | 1.500 | 0.678‐3.313 | .3100 | – | – | – |
| Effect of preoperative treatment | Grade 2‐3 | 1.523 | 0.612‐3.790 | .3500 | – | – | – |
| CD80 | Number of positive cells/HPF | 1.008 | 1.002‐1.015 | .0045 | 1.004 | 0.996‐1.013 | .2100 |
| CD163 | Number of positive cells/HPF | 1.019 | 1.011‐1.027 | <.001 | 1.015 | 1.005‐1.025 | <.0010 |
| CD8 | Number of positive cells/HPF | 0.994 | 0.987‐1.002 | .1500 | 0.983 | 0.971‐0.995 | .0017 |
| PD‐L1 | Positive | 4.029 | 1.872‐8.671 | <.001 | 3.766 | 1.148‐12.35 | .0240 |
Abbreviations: Ce, cervical esophagus; CI, confidence interval; HPF, high‐powered field; OR, odds ratio; PD‐L1, programmed cell death‐ligand 1; pN, pathological lymph node metastasis; pT, pathological depth of tumor invasion; Ut, upper thoracic esophagus; –, not included in analysis.
We analyzed the contribution of CD80+ and CD163+ macrophages as well as CD8+ cytotoxic T cells to patient survival. None of these was a significant prognostic factor for OS or DFS of patients with ESCC (Table S1).
3.4. Prognostic significance of coexpression of SIRPα and PD‐L1
We investigated the correlation between SIRPα expression and immune checkpoint molecule PD‐L1 expression in ESCC using IHC analysis. In our ESCC patient cohort, 67 patients (51%) were positive for PD‐L1 (CPS ≥ 1%). Significantly more patients positive for PD‐L1 were included in the SIRPα high‐expression group than the low‐expression group (P < .001, χ2 test; Table S2). Moreover, multivariable analysis revealed that PD‐L1 positivity was significantly associated with high expression of SIRPα (Table 3).
We analyzed the association between the coexpression of SIRPα and PD‐L1 and patient survival using the Kaplan‐Meier method. Representative images of the coexpression of SIRPα and PD‐L1 are shown in Figure 4A,B. The OS and DFS were analyzed in patients categorized as SIRPα–/PD‐L1–, SIRPα–/PD‐L1+, SIRPα+/PD‐L1–, and SIRPα+/PD‐L1+. Patients in the SIRPα+/PD‐L1+ category tended to have worse OS and DFS than any other categories, although not significantly (Figure 4C,D).
FIGURE 4.
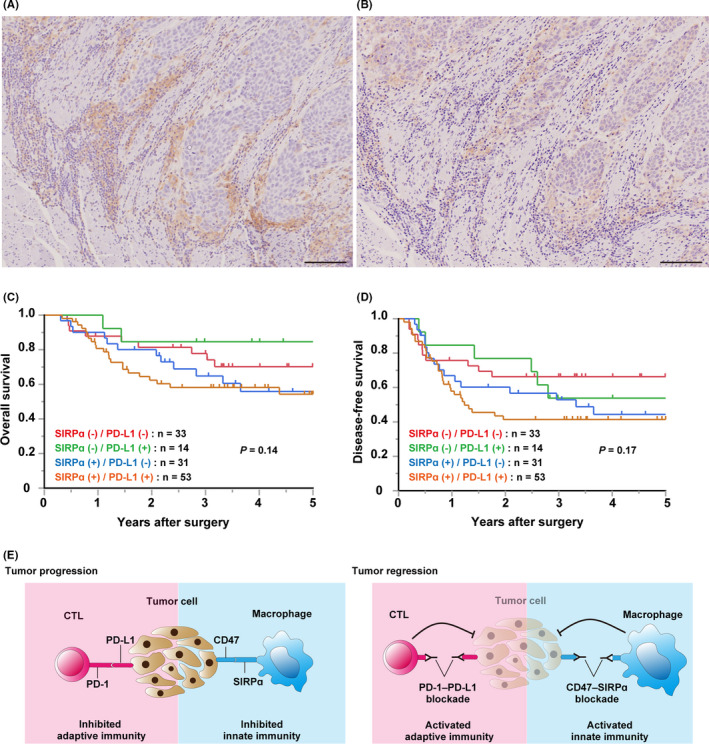
Coexpression of signal regulatory protein α (SIRPα) and programmed cell death‐ligand 1 (PD‐L1) was prognostically significant in esophageal squamous cell carcinoma (ESCC). A, B, Representative images of SIRPα (A) and PD‐L1 (B) in a surgically resected specimen from one patient with ESCC. The images represent a single area of one specimen. Scale bar = 100 μm. C, D, Kaplan‐Meier curves showing overall survival (C) and disease‐free survival (D) of patients with ESCC according to the SIRPα and PD‐L1 expression patterns. Red, green, blue, and brown curves represent SIRPα–/PD‐L1– (n = 33), SIRPα–/PD‐L1+ (n = 14), SIRPα+/PD‐L1– (n = 31), and SIRPα+/PD‐L1+ (n = 53), respectively. Statistical analysis was undertaken using the log‐rank test. E, Schematic model of combination therapy targeting both the CD47‐SIRPα and PD‐1‐PD‐L1 axes
4. DISCUSSION
In this study, we identified SIRPα as an indicator of poor prognosis in ESCC. Signal regulatory protein alpha expression was significantly associated with infiltration of M1 macrophages, M2 macrophages, CD8+ T cells, and PD‐L1, indicating that SIRPα could induce a poor prognosis partly through inhibiting macrophage phagocytosis of tumor cells and regulating the antitumor immune response. This is clinically important because SIRPα should be considered as a potential therapeutic target in ESCC.
Signal regulatory protein alpha induces the signaling cascade inhibiting phagocytosis of target cells by interacting with its ligand CD47 and also functions as a negative regulator of antitumor immune responses.13 Several previous studies have shown that CD47 is overexpressed and associated with poor prognosis in ESCC and other malignancies.12, 13, 25 However, the clinical significance of SIRPα in ESCC is unknown. Therefore, we focused our investigation on the effect of SIRPα expression on the prognosis of ESCC. Our data showed that more SIRPα was expressed in ESCC tissues than in noncancer tissues, by in silico analysis. Moreover, high SIRPα expression was positively associated with the depth of invasion and tumor stage and significantly associated with a poor prognosis in ESCC, suggesting that SIRPα might contribute to patient survival by enhancing tumor invasiveness. These findings support the hypothesis that the CD47‐SIRPα system could inhibit macrophage phagocytosis of tumor cells, resulting in a poor prognosis in ESCC. Indeed, many preclinical studies have shown that blocking of the CD47‐SIRPα pathway, whether by anti‐CD47 Ab, anti‐SIRPα Ab, or SIRPα‐Fc fusion proteins that act as effective decoy receptors, has an antitumor effect against several solid and hematopoietic cancers.14, 17, 19, 26, 27, 28, 29 Several clinical trials are underway to evaluate these CD47‐SIRPα targeting inhibitors.28, 30, 31 Taken together, our findings provided a rationale to investigate the therapeutic effects of the blockade of CD47‐SIRPα on ESCC in a further study.
We showed that SIRPα expression was significantly correlated with the infiltration of CD163+ macrophages in ESCC, suggesting that CD163+ M2 macrophages might have high SIRPα expression. Macrophages in malignant tumors have been classified into activated macrophages (M1 macrophages) involved in the responses of type 1 T helper (Th1) cells to pathogens, which facilitate antitumor immunity, and alternatively activated macrophages (M2 macrophages) that are involved in Th2‐type responses and play a role in suppressing the antitumor immune response.32, 33 Previous studies indicate that tumor‐associated macrophages (TAMs) predominantly consist of M2 macrophages, which push the tumor microenvironment towards an immunosuppressive and tumor‐progressive condition, resulting in poor prognoses for patients with malignancies, including ESCC.34, 35, 36, 37, 38 Moreover, it is reported that SIRPα has a pivotal role in regulation of the phenotype of macrophages in tumor sites.20, 39, 40 Thus, our results suggest that SIRPα could be associated with macrophage differentiation and regulate antitumor immunity through TAM activity in ESCC.
Our IHC data also showed that fewer CD8+ T cells infiltrated the tumors in the SIRPα high‐expression group than in the low‐expression group, supporting the premise that the antitumor immune response might be inhibited in patients with high SIRPα expression. The CIBERSORT analysis did not reveal a significant correlation between SIRPα expression and CD8+ T cells, but the size of the CIBERSORT cohort was small. Signal regulatory protein alpha expression appears to reflect the innate immune response to malignancy, whereas CD8+ T cells are major effector cells of adaptive immunity. Thus, we consider the correlation between SIRPα and CD8+ T cells to be weak and indirect.
Interestingly, the present study showed that PD‐L1 expression was significantly associated with SIRPα expression in ESCC. Previous studies showed that PD‐L1 is expressed in TAMs and plays a role in regulating their phagocytosis.41, 42, 43 Our IHC analysis suggested that SIRPα could be expressed in TAMs. Taken together, the correlation between SIRPα and PD‐L1 suggests that SIRPα and PD‐L1 could be coexpressed in TAMs and control their activity independently or cooperatively. Moreover, our results showed that SIRPα/PD‐L1 coexpression in ESCC tended to be correlated with poor prognosis compared with the expression of either protein alone or neither. The additive influence of SIRPα and PD‐L1 on prognosis indicates that the suppression of both innate and adaptive antitumor immunity could result in the worst prognosis in the SIRPα/PD‐L1 coexpression group. Therefore, combination therapy targeting CD47‐SIRPα and PD‐1‐PD‐L1 could improve the prognosis of patients with ESCC by reactivating both the innate and adaptive immune responses involving macrophages and T cells, respectively (Figure 4E). Indeed, several preclinical studies reported that the combined blockade of CD47‐SIRPα and PD‐1‐PD‐L1 axes had synergistic antitumor effects in murine colon cancer and melanoma models in vivo.19, 44, 45, 46 Taken together, our findings support the idea that dual‐targeted therapy could be an effective treatment option for ESCC, whose efficacy should be investigated in further preclinical and clinical studies.
In conclusion, SIRPα is associated with poor prognosis in ESCC, possibly through inhibiting macrophage phagocytosis of tumor cells and inducing the suppression of antitumor immunity. Signal regulatory protein alpha should be considered as a potential therapeutic target in ESCC, especially if combined with PD‐1‐PD‐L1 blockade.
DISCLOSURE
The authors declare no conflicts of interest for this article.
Supporting information
Fig S1
Table S1
Table S2
ACKNOWLEDGMENT
The authors would like to thank Paul Dolber, PhD and JAM Post Inc for English language editing.
Koga N, Hu Q, Sakai A, et al. Clinical significance of signal regulatory protein alpha (SIRPα) expression in esophageal squamous cell carcinoma. Cancer Sci. 2021;112:3018–3028. 10.1111/cas.14971
REFERENCES
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rustgi A, El‐Serag HB. Esophageal carcinoma. N Engl J Med. 2015;372:1472‐1473. [DOI] [PubMed] [Google Scholar]
- 3.Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician. 2017;95:22‐28. [PubMed] [Google Scholar]
- 4.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:1506‐1517. [DOI] [PubMed] [Google Scholar]
- 5.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE‐181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–4148. [DOI] [PubMed] [Google Scholar]
- 6.Moynihan KD, Irvine DJ. Roles for innate immunity in combination immunotherapies. Cancer Res. 2017;77:5215‐5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalil AR, Andrechak JC, Discher DE. Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47‐SIRPalpha structure‐function. Antib Ther. 2020;3:80‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47‐SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72‐80. [DOI] [PubMed] [Google Scholar]
- 9.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25‐50. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Saito Y, Kotani T, Matozaki T. Blockade of CD47 or SIRPalpha: a new cancer immunotherapy. Expert Opin Ther Targets. 2020;24:945‐951. [DOI] [PubMed] [Google Scholar]
- 11.Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47‐SIRPalpha signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155:335‐344. [DOI] [PubMed] [Google Scholar]
- 12.Chao MP, Alizadeh AA, Tang C, et al. Anti‐CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non‐Hodgkin lymphoma. Cell. 2010;142:699‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47‐signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662‐6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao XW, van Beek EM, Schornagel K, et al. CD47‐signal regulatory protein‐alpha (SIRPalpha) interactions form a barrier for antibody‐mediated tumor cell destruction. Proc Natl Acad Sci USA. 2011;108:18342‐18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim J, Sockolosky JT, Sangalang E, et al. Discovery of high affinity, pan‐allelic, and pan‐mammalian reactive antibodies against the myeloid checkpoint receptor SIRPalpha. MAbs. 2019;11:1036‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho CC, Guo N, Sockolosky JT, et al. "Velcro" engineering of high affinity CD47 ectodomain as signal regulatory protein alpha (SIRPalpha) antagonists that enhance antibody‐dependent cellular phagocytosis. J Biol Chem. 2015;290:12650‐12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ring NG, Herndler‐Brandstetter D, Weiskopf K, et al. Anti‐SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114:E10578‐E10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata Y, Tanaka D, Hazama D, et al. Anti‐human SIRPalpha antibody is a new tool for cancer immunotherapy. Cancer Sci. 2018;109:1300‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagita T, Murata Y, Tanaka D, et al. Anti‐SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2:e89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvey CM, Spinler KR, Irianto J, et al. SIRPA‐inhibited, marrow‐derived macrophages engorge, accumulate, and differentiate in antibody‐targeted regression of solid tumors. Curr Biol. 2017;27:2065‐2077.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68‐74. [DOI] [PubMed] [Google Scholar]
- 22.Ishida K, Ando N, Yamamoto S, Ide H, Shinoda M. Phase II study of cisplatin and 5‐fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 2004;34:615‐619. [DOI] [PubMed] [Google Scholar]
- 23.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet (London, England). 2019;394:1915‐1928. [DOI] [PubMed] [Google Scholar]
- 24.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand‐1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330‐337. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Yokobori T, Tanaka N, et al. CD47 expression regulated by the miR‐133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28:465‐472. [DOI] [PubMed] [Google Scholar]
- 26.Chao MP, Alizadeh AA, Tang C, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto H, Kojima Y, Matsuda K, et al. Efficacy of anti‐CD47 antibody‐mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer. 2014;50:1836‐1846. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Wang L, Zhao F, et al. Pre‐clinical development of a humanized anti‐CD47 antibody with anti‐cancer therapeutic potential. PLoS One. 2015;10:e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Fan J, Wang S, et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non‐small cell lung cancer. Cancer Immunol Res. 2017;5:363‐375. [DOI] [PubMed] [Google Scholar]
- 30.Petrova PS, Viller NN, Wong M, et al. TTI‐621 (SIRPαFc): a CD47‐blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068‐1079. [DOI] [PubMed] [Google Scholar]
- 31.Russ A, Hua AB, Montfort WR, et al. Blocking "don't eat me" signal of CD47‐SIRPα in hematological malignancies, an in‐depth review. Blood Rev. 2018;32:480‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor‐associated macrophages in solid tumor: a meta‐analysis of the literature. PLoS One. 2012;7:e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549‐555. [DOI] [PubMed] [Google Scholar]
- 36.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti‐cancer therapy. Eur J Cancer. 2006;42:717‐727. [DOI] [PubMed] [Google Scholar]
- 37.Miyashita T, Tajima H, Shah FA, et al. Impact of inflammation‐metaplasia‐adenocarcinoma sequence and inflammatory microenvironment in esophageal carcinogenesis using surgical rat models. Ann Surg Oncol. 2014;21:2012‐2019. [DOI] [PubMed] [Google Scholar]
- 38.Shigeoka M, Urakawa N, Nakamura T, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1112‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YP, Kim HJ, Wu H, et al. SIRPα expression delineates subsets of intratumoral monocyte/macrophages with different functional and prognostic impact in follicular lymphoma. Blood Cancer J. 2019;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan YF, Tan YX, Wang M, et al. Signal regulatory protein alpha is associated with tumor‐polarized macrophages phenotype switch and plays a pivotal role in tumor progression. Hepatology. 2013;58:680‐691. [DOI] [PubMed] [Google Scholar]
- 41.Gordon SR, Maute RL, Dulken BW, et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD‐L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6:1260‐1273. [DOI] [PubMed] [Google Scholar]
- 43.Papalampros A, Vailas M, Ntostoglou K, et al. Unique spatial immune profiling in pancreatic ductal adenocarcinoma with enrichment of exhausted and senescent T cells and diffused CD47‐SIRPα expression. Cancers. 2020;12:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Guo H, Xu J, et al. Elimination of tumor by CD47/PD‐L1 dual‐targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10:315‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sockolosky JT, Dougan M, Ingram JR, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA. 2016;113:E2646‐E2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Liu L, Ren Z, et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018;24:2101‐2111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2


