Abstract
The argument concerning the exact minimum number of examined lymph nodes (ELNs) has continued for a long time among various regions, and no consensus has been reached for stratified pathological T stages for data to date. Data from 4607 pN0 patients with gastric cancer were analyzed. Kaplan‐Meier analysis showed the similar overall survival (OS) outcomes among the 3 groups (ELNs ≤ 15, 16 ≤ ELNs ≤ 29 and ELNs ≥ 30, P = .171). However, the ELNs ≥ 30 group had a better disease‐free survival (DFS) outcome compared with the others (all P < .05). An increased ELN group (ELNs ≥ 30) showed an improved OS only for pT3 patients (hazard ratio [HR] = 0.397, 95% confidence interval (CI): 0.182‐0.866, P = .020), while an improved DFS for pT3 patients (HR = 0.362, 95%CI: 0.152‐0.860, P = .021) and pT4 patients (HR = 0.484, 95%CI: 0.277‐0.844, P = .011) in the multivariate analysis. A well discriminated and calibrated nomogram was constructed to predict the probability of the OS and DFS, with the C‐index for OS and DFS prediction of 0.782 (95%CI: 0.735 to 0.829) and 0.738 (95%CI: 0.685 to 0.791), respectively. This study provides new and useful insights into the impact of ELN count on reducing stage migration and postoperative recurrence of pN0 patients with gastric cancer in 2000‐2017. In conclusion, a larger number of ELNs is suggested for surgeons to prolong the prognosis of pN0 gastric cancer, especially for pT3 patients.
Keywords: examined lymph nodes, gastric cancer, prediction model, stage migration, survival outcomes
The OS and DFS of the total pN0 patients in different ELNs group. (A) OS, P = .171, (B) DFS, P = .029.
![]()
1. INTRODUCTION
Despite its significant decline in incidence in the past decades, gastric cancer is still the third leading cause of cancer‐related mortality worldwide.1 As the most commonly used tool to determine pathologic T and N staging for resected gastric cancer at this time, the TNM system of the International Union for Cancer Control/American Joint Committee on Cancer (UICC/AJCC) requires 15 or more lymph nodes (ELNs) to be examined to guarantee the accurate prognosis of the pN category, especially for pN0 patients.2, 3 The latest National Comprehensive Cancer Network Guidelines (NCCN Guidelines) recommends the examination of not less than 16 regional lymph nodes when determining nodal metastatic status.4
Nevertheless, accumulating evidence has demonstrated that increasing the numbers of ELNs examined increases the likelihood of accurate staging, therefore stage migration could be gradually reduced or prevented (the Will Rogers phenomenon).5, 6, 7, 8 Ji et al demonstrated that ELNs ≥ 22 is an independent prognostic factor for pN0 population.6 Smith et al indicated that 25 or more lymph nodes are necessary for D2 lymphadenectomy.9 Published studies also showed that a higher number of ELNs (≥30 numbers) was associated with a better survival outcome, as nodal metastases serve as a well known prognostic factor for gastric cancer after radical treatment.7, 8, 10, 11, 12, 13 In this context, it still remains controversial how to quantitatively assess the effects of the number of ELNs on achieving an optimum reliability in stage assignment for gastric cancer. In addition, it is unknown how risk factors are associated with recurrence after gastrectomy for pN0 gastric cancer patients based on adequate ELNs.
Given these considerations, we conducted this study on 2 of the biggest Chinese gastric cancer cohorts from the China National Cancer Center, and National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, to investigate whether stage migration exists in pN0 gastric cancer patients, as well as the relationship between ELN count after gastrectomy and survival outcomes. In addition, a nomogram was developed to predict the probability of overall survival (OS) and disease‐free survival (DFS), to directly help surgeons to formulate adjuvant therapeutic and preventive strategies for pN0 gastric cancer.
2. MATERIALS AND METHODS
2.1. Patient population
The study queried data from 2000 to 2017 from the 2 large gastric cancer cohorts. The first cohort, a huge bidirectional group with gastric cancer, was sourced from the China National Cancer Center, a single but large‐scale institution, and included more than 19 000 patients from around China examined from 1997 to 2017. The second cohort was from a prospective database, which collected clinicopathologic data, biological specimens, and follow‐up information on patients who were admitted to the Xijing Hospital of Digestive Diseases. By December 2019, this database had included more than 11 000 patients who were diagnosed with gastric or gastroesophageal cancer.
Those patients who underwent curative gastrectomy and were defined pathologically as gastric adenocarcinoma (pTanyN0M0) were included in this study. The exclusion criteria were as follows: (1) patients received neoadjuvant therapy before surgery; (2) patients with signet ring cell carcinoma; (3) patients with linitis plastica; (4) patients with pathologically positive resection margin; (5) patients with a history of other cancer or tumor; and (6) patients with any missed important data, such as surgery date, pTNM stage, and ELNs. Based on these screening criteria, 4607 gastric cancer patients with pTanyN0M0 were identified. Figure 1 showed the flow diagram for selecting the patients.
FIGURE 1.
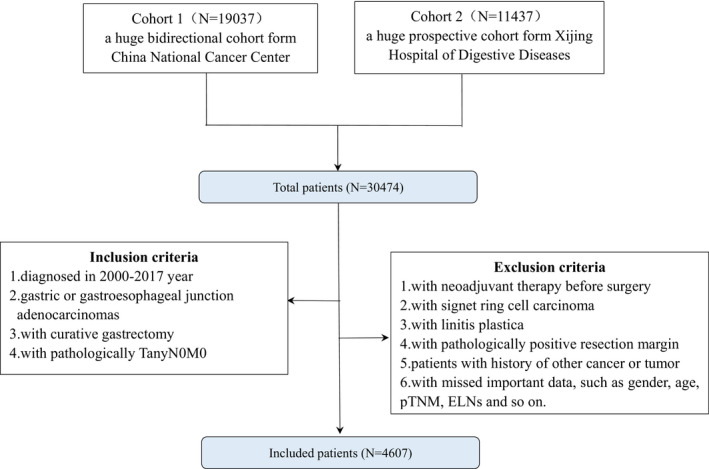
Flow diagram of the patient selection process
2.2. Statistical analyses
Comparisons between the groups were tested with t test for continuous variables and chi‐square test for categorical variables. OS and DFS analyses were performed for the entire study.
The Kaplan‐Meier method was used to calculate OS, and differences between the survival curves were assessed using the log‐rank test. Univariate and multivariate Cox proportional hazards models were used to determine the prognostic factors for OS and DFS. Variables with a P‐value of <.10 in the univariate analysis were adopted for the multivariate analysis. Finally, the adjusted factors included age, gender, year of diagnosis, type of gastrectomy, vascular invasion, nerve invasion, adjuvant therapy, ELNs, and pathologic T stage. Hazard ratio (HR) and 95% confidence interval (CI) were used to measure the risk of death. A P‐value of less than .05 was considered to be statistically significant and all the tests were two‐sided. A nomogram was formulated based on the results of the multivariate analysis.14 We then selected the pN0 patients from the last period (2013‐2017) as a validated cohort to complete nomogram prediction, which was measured with a concordance index (C‐index) based on the regression analysis. The larger the C‐index, the more accurate the prognostic prediction.15
All statistical analyses were performed using SPSS v.25 (College Station, TX, USA) and R software v.3.6.3 (http://www.r‐project.org/).
3. RESULT
3.1. Clinicopathologic characteristics
Data from 4607 pN0 gastric cancer patients were analyzed, and the clinicopathologic characteristics of the 2 cohorts are shown in Table 1. There were 1238 patients (26.9%) with ELNs ≤ 15, 2121 patients (46.0%) with 16 ≤ ELNs ≤ 29, and 1248 patients (27.1%) with ELNs ≥ 30. Compared with the ELNs ≥ 30 group, the less lymphadenectomy group (ELNs ≤ 15) was more likely to have been diagnosed in the earlier year period (2000‐2004, 26.3% vs. 3.8%, P < .001), at the earlier pT stage (T1 + Tis, 42.2% vs. 36.3%, P < .001), and treated with proximal gastrectomy (44.3% vs. 22.0%, P < .001). More patients in the 16 ≤ ELNs ≤ 29 and ELNs ≥ 30 groups underwent total gastrectomy (5.9% vs. 15.1% vs. 17.5%, P < .001), and were diagnosed with vascular invasion (10.1% vs. 16.9% vs. 18.6%, P < .001) and nerve invasion (8.6% vs. 30.9% vs. 33.3%, P < .001) compared with the ELNs ≤15 group.
TABLE 1.
Characteristics of pN0 patients with different ELNs
| Characteristics | Total | ELNs ≤ 15 | 16 ≤ ELNs ≤ 29 | ELNs ≥ 30 | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | ||
| Total | 4607 | 100 | 1238 | 26.87 | 2121 | 46.04 | 1248 | 27.09 | |
| Age at diagnosis (y) | |||||||||
| Mean (SD) | 58.14 | 10.837 | 59.16 | 10.840 | 58.22 | 10.993 | 57.00 | 10.46 | <.001 |
| Younger (≤35) | 139 | 3.0 | 26 | 2.1 | 72 | 3.4 | 41 | 3.3 | <.001 |
| Middle‐aged (36‐65) | 3258 | 70.7 | 831 | 67.1 | 1484 | 70 | 943 | 75.6 | |
| Older (≥66) | 1210 | 26.3 | 381 | 30.8 | 565 | 26.6 | 264 | 21.2 | |
| Gender | |||||||||
| Male | 3467 | 75.3 | 970 | 78.4 | 1601 | 75.5 | 896 | 71.8 | .001 |
| Female | 1140 | 24.7 | 268 | 21.6 | 520 | 24.5 | 352 | 28.2 | |
| Year at diagnosis | |||||||||
| 2000‐2004 | 550 | 11.9 | 326 | 26.3 | 176 | 8.3 | 48 | 3.8 | <.001 |
| 2005‐2009 | 959 | 20.8 | 489 | 39.5 | 365 | 17.2 | 105 | 8.4 | |
| 2010‐2013 | 1494 | 32.4 | 288 | 23.3 | 767 | 36.2 | 439 | 35.2 | |
| 2014‐2017 | 1604 | 34.8 | 135 | 10.9 | 813 | 38.3 | 656 | 62.6 | |
| Type of gastrectomy | |||||||||
| Proximal | 1528 | 33.2 | 549 | 44.3 | 704 | 33.2 | 275 | 22.0 | <.001 |
| Distal | 2467 | 53.5 | 616 | 49.8 | 1097 | 51.7 | 754 | 60.4 | |
| Total | 612 | 13.3 | 73 | 5.9 | 320 | 15.1 | 219 | 17.5 | |
| Grade | |||||||||
| Well | 318 | 7.3 | 95 | 8.4 | 163 | 8.0 | 60 | 5.0 | <.001 |
| Well‐Moderately | 294 | 6.8 | 68 | 6.0 | 146 | 7.2 | 80 | 6.7 | |
| Moderately | 1107 | 25.4 | 314 | 27.9 | 536 | 26.5 | 257 | 21.4 | |
| Poorly‐Moderately | 1038 | 23.8 | 241 | 21.4 | 493 | 24.3 | 304 | 25.3 | |
| Poorly | 1596 | 36.7 | 408 | 36.2 | 688 | 34.0 | 500 | 41.6 | |
| Vascular invasion | |||||||||
| Yes | 693 | 15.7 | 114 | 10.1 | 350 | 16.9 | 229 | 18.6 | <.001 |
| No | 3734 | 84.3 | 1014 | 89.9 | 1719 | 83.1 | 1001 | 81.4 | |
| Nerve invasion | 4427 | ||||||||
| Yes | 1144 | 25.9 | 96 | 8.6 | 639 | 30.9 | 409 | 33.3 | <.001 |
| No | 3276 | 74.1 | 1025 | 91.4 | 1432 | 69.1 | 819 | 66.7 | |
| Pathologic T stage | |||||||||
| T1 + Tis | 1670 | 36.2 | 523 | 42.2 | 694 | 32.7 | 453 | 36.3 | <.001 |
| T2 | 939 | 20.4 | 250 | 20.2 | 449 | 21.2 | 240 | 19.2 | |
| T3 | 862 | 18.7 | 125 | 10.1 | 447 | 21.1 | 290 | 23.2 | |
| T4 | 1136 | 24.7 | 340 | 27.5 | 531 | 25.0 | 265 | 21.2 | |
| Adjuvant therapy | |||||||||
| Yes | 1510 | 59.2 | 335 | 58.5 | 759 | 60.6 | 416 | 57.5 | .352 |
| No | 1039 | 40.8 | 238 | 41.5 | 493 | 39.4 | 308 | 42.5 | |
| ELNs | |||||||||
| Mean (SD) | 23.61 | 12.935 | 9.92 | 3.880 | 22.15 | 3.903 | 39.68 | 11.349 | <.001 |
The mean (±SD) age of the pN0 patients was 59.16 ± 10.840 in the ELNs ≤ 15 group, 58.22 ± 10.993 in the 16 ≤ ELNs ≤ 29 group, and 57.00 ± 10.46 in the ELNs ≥ 30 group, respectively. The mean (±SD) number of pathologically proven ELNs of these pN0 patients was 9.92 ± 3.880 in the ELNs ≤ 15 group, 22.15 ± 3.903 in the 16 ≤ ELNs ≤ 29 group, and 39.68 ± 11.349 in the ELNs ≥ 30 group, respectively.
3.2. Stage migration
For analysis of stage migration, the pN0 gastric cancer patients with ELNs ≤ 15 (n = 1239) and pN1 patients with ELNs ≥ 16 (n = 1176) were included using long‐rank test. We hypothesized that an increasing number of ELNs may transform the pN0 patients to pN1 patients at the same pT stage. Based on this definition, we compared several groups (Figure 2A‐D): (A) pT1N1M0 (ELNs ≥ 16) vs. pT1N0M0 (ELNs ≤ 15), P = .889; (B) pT2N1M0 (ELNs ≥ 16) vs. pT2N0M0 (ELNs ≤ 15), P = .691; (C) pT3N1M0 (ELNs ≥ 16) vs. pT3N0M0 (ELNs ≤ 15), P = .570; and (D) pT4N1M0 (ELNs ≥ 16) vs. pT4N0M0 (ELNs ≤ 15), P = .889. There was no significant difference between these groups. Therefore, stage migration may be proven and the pT1N0M0 patients were classified as Stage pT1N1M0 with an increased number of ELNs. Similarly, pT2N0M0 may migrate to pT2N1M0 with an increased number of ELNs, pT3N0M0 may migrate to pT3N1M0 with an increased number of ELNs, and pT4N0M0 may migrate to pT4N1M0 with an increased number of ELNs.
FIGURE 2.
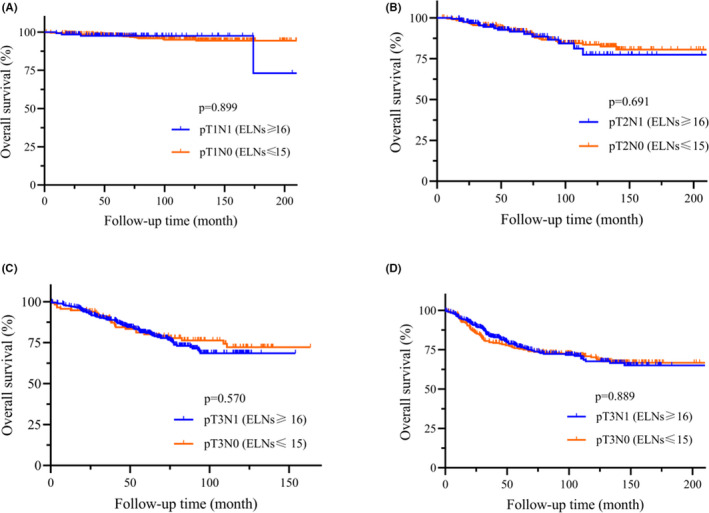
OS of pN0 vs. pN1. A, pT1N0 (ELNs ≤ 15, n = 523) vs. pT1N1 (ELNs ≥ 16, n = 144). B, pT2N0 (ELNs ≤ 15, n = 250) vs. pT2N1 (ELNs ≥ 16, n = 189). C, pT3N0 (ELNs ≤ 15, n = 862) vs. pT3N1 (ELNs ≥ 16, n = 390). D, pT4N0 (ELNs ≤ 15, n = 1136) vs. pT4N1 (ELNs ≥ 16, n = 454)
3.3. OS and DFS analysis
Figure 3 shows the Kaplan‐Meier curves for OS (Figure 3A) and DFS (Figure 3B) of 2 large population‐based cohorts with different ELNs groups. The analysis showed the similar OS outcomes among the 3 groups (ELNs ≤ 15, 16 ≤ ELNs ≤ 29 and ELNs ≥ 30, P = .171). However, the ELNs ≥ 30 group had a better DFS outcome compared with both the ELNs ≤ 15 and 16 ≤ ELNs ≤ 29 groups (P = .029).
FIGURE 3.
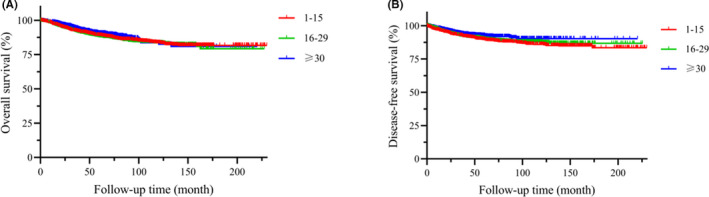
OS and DFS of the total pN0 patients in different ELN group. A, OS, P = .171. B, DFS, P = .029
Considering the clinicopathological differences among the groups, we conducted subgroup analysis based on pT stage. In the stratified analysis of patients with Stages pT1‐2 and pT4, the OS and DFS of the gastric cancer patients were comparable in these 3 groups (P > .05) (Figure 4A‐D,G,H). For the gastric cancer patients with Stage pT3 (Figure 4E,F), the ELNs ≥ 30 group had better OS and DFS results compared with the other 2 groups, respectively (P = .031 and P = .019, respectively).
FIGURE 4.
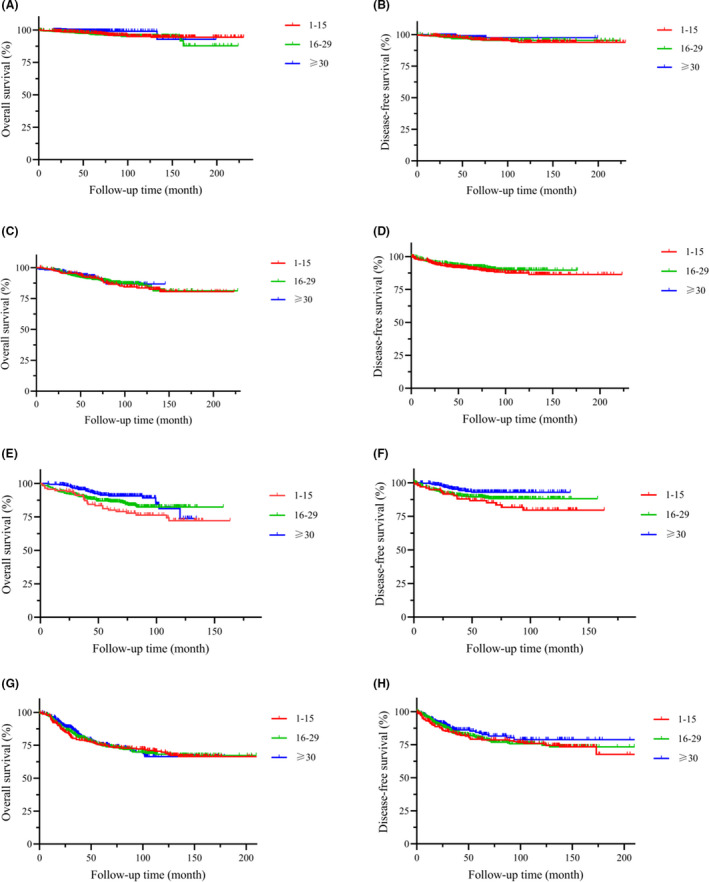
OS and DFS of pN0 patients in different ELN groups. A, OS of pT1 and pTis patients, P = .156. B, DFS of pT1 and pTis patients, P = .927. C, OS of pT2 patients, P = .930. D, DFS of pT2 patients, P = .154. E, OS of pT3 patients, P = .031. F, DFS of pT3 patients, P = .019. G, OS of pT4 patients, P = .970. H, DFS of pT4 patients, P = .458
A linear ELN count‐to‐survival correlation model provided the best fit in each pT stage subgroup (Figure 5A‐D). A superior 5‐y survival rate is depicted in the higher ELNs groups (31 ≤ ELNs ≤ 44, or ELNs ≥ 45) for all 4 stage subgroups.
FIGURE 5.
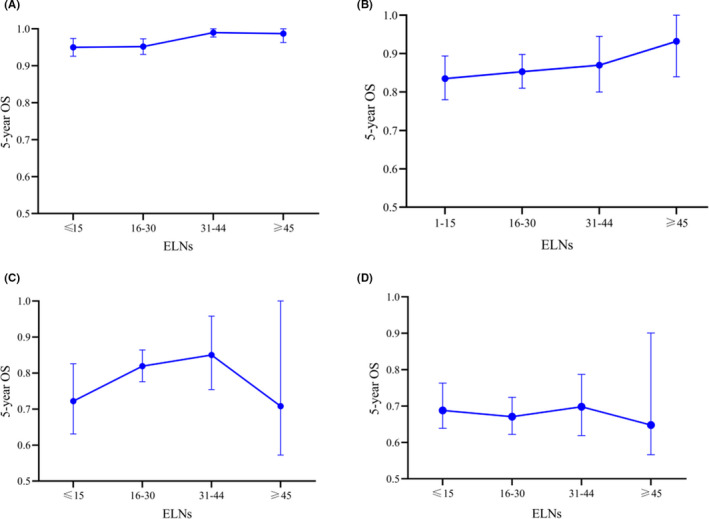
A linear ELNs to survival correlation model for each pT stage subgroup. A, pT1 and pTis. B, pT2. C, pT3. D, pT4
Furthermore, the univariate and multivariate Cox proportional hazards models were used to determine the prognostic factors for OS and DFS (Tables 2 and 3). Variables with a P‐value of less than .10 in the univariate analysis were involved in the multivariate analysis, including age, gender, year of diagnosis, type of gastrectomy, vascular invasion, nerve invasion, adjuvant therapy, pT stage, and ELNs. For all the patients, the independent predictor for OS included distal gastrectomy (HR = 0.632, 95%CI: 0.469‐0.853, P = .003), nerve invasion (HR = 0.614, 95%CI: 0.455‐0.828, P = .001), and increasing pT stage (P < .05). However, the increased ELNs group (ELNs ≥ 30) showed an improved survival only for pT3 patients (HR = 0.397, 95%CI: 0.182‐0.866, P = .020) (Table 2). For the DFS analysis in the multivariate analysis (Table 3), there were significant differences among the different ELNs groups for all the patients (HR = 0.635, 95%CI: 0.431‐0.935, P = .021), pT3 patients (HR = 0.362, 95%CI: 0.152‐0.860, P = .021), and pT4 patients (HR = 0.484, 95%CI: 0.277‐0.844, P = .011).
TABLE 2.
Multivariate analyses in OS of pN0 patients with gastric cancer
| Prognostic factors | Total (n = 4607) | pT1 (n = 1670) | pT2 (n = 939) | pT3 (n = 862) | pT4 (n = 1136) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age at diagnosis (y) | |||||||||||||||
| Younger (≤35) | 1 | – | 1 | – | 1 | ||||||||||
| Middle‐aged (36‐65) | 1.293 | 0.570‐2.933 | .539 | 1.339 | 0.180‐9.942 | .775 | 0.961 | 0.383‐2.413 | .932 | ||||||
| Older (≥66) | 2.236 | 0.974‐5.131 | .058 | 4.140 | 0.548‐31.271 | .169 | 1.299 | 0.500‐3.371 | .591 | ||||||
| Gender | |||||||||||||||
| Male | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Female | 0.973 | 0.745‐1.270 | .838 | 0.363 | 0.077‐1.706 | .199 | 0.937 | 0.456‐1.925 | .858 | 0.914 | 0.514‐1.626 | .759 | 1.156 | 0.815‐1.640 | .415 |
| Year at diagnosis | |||||||||||||||
| 2000‐2004 | 1 | 1 | – | 1 | – | ||||||||||
| 2005‐2009 | 1.349 | 0.684‐2.663 | .388 | 0.984 | 0.166‐5.817 | .985 | 0.674 | 0.299‐1.517 | .341 | ||||||
| 2010‐2013 | 1.502 | 0.764‐2.953 | .238 | 0.698 | 0.101‐4.800 | .698 | 0.488 | 0.199‐1.197 | .488 | ||||||
| 2014‐2017 | 1.088 | 0.536‐1.376 | .815 | 0.352 | 0.031‐4.022 | .352 | |||||||||
| Type of gastrectomy | |||||||||||||||
| Proximal | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Distal | 0.632 | 0.469‐0.853 | .003 | 0.787 | 0.247‐2.508 | .685 | 0.449 | 0.249‐0.811 | .008 | 0.633 | 0.341‐1.175 | .147 | 0.757 | 0.467‐1.126 | .257 |
| Total | 1.268 | 0.926‐1.736 | .139 | – | 0.500 | 0.22‐1.135 | .098 | 1.366 | 0.768‐2.431 | .288 | 1.760 | 1.080‐2.869 | .023 | ||
| Vascular invasion | |||||||||||||||
| Yes | 1 | 1 | 1 | 1 | 1 | ||||||||||
| No | 0.992 | 0.760‐1.293 | .951 | 0.211 | 0.049‐0.915 | .038 | 0.874 | 0.450‐1.698 | .691 | 0.974 | 0.575‐1.650 | .922 | 1.111 | 0.775‐1.591 | .568 |
| Nerve invasion | |||||||||||||||
| Yes | 1 | – | 1 | 1 | 1 | ||||||||||
| No | 0.614 | 0.455‐0.828 | .001 | 0.753 | 0.412‐1.377 | .357 | 0.642 | 0.363‐1.133 | .126 | 0.621 | 0.391‐0.986 | .043 | |||
| Adjuvant therapy | |||||||||||||||
| Yes | 1 | 1 | 1 | 1 | 1 | ||||||||||
| No | 1.105 | 0.833‐1.465 | .489 | 3.312 | 1.050‐10.449 | .041 | 1.051 | 0.601‐1.836 | .862 | 1.136 | 0.648‐1.990 | .656 | 1.488 | 0.977‐2.265 | .064 |
| ELNs | |||||||||||||||
| ELNs ≤ 15 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 16 ≤ ELNs ≤ 29 | 0.985 | 0.697‐1.393 | .933 | 1.992 | 0.596‐6.663 | .264 | 1.634 | 0.686‐3.889 | .267 | 0.576 | 0.289‐1.148 | .117 | 0.879 | 0.534‐1.447 | .613 |
| ELNs≥30 | 0.820 | 0.560‐1.199 | .306 | 0.609 | 0.060‐6.617 | .675 | 2.092 | 0.798‐5.482 | .133 | 0.397 | 0.182‐0.866 | .020 | 0.772 | 0.453‐1.316 | .342 |
| Pathologic T stage | |||||||||||||||
| T1 + Tis | 1 | – | |||||||||||||
| T2 | 3.384 | 1.867‐6.135 | <.001 | ||||||||||||
| T3 | 4.119 | 2.254‐7.528 | <.001 | ||||||||||||
| T4 | 8.644 | 4.829‐15.473 | <.001 | ||||||||||||
Adjusted factors: age, gender, year at diagnosis, type of gastrectomy, vascular invasion, nerve invasion, adjuvant therapy, ELNs, pathologic T stage.
TABLE 3.
Multivariate analyses in DFS of pN0 patients with gastric cancer
| Prognostic factors | Total (n = 4607) | pT1 (n = 1670) | pT2 (n = 939) | pT3 (n = 862) | pT4 (n = 1136) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age at diagnosis (y) | |||||||||||||||
| Younger (≤35) | 1 | – | – | – | 1 | ||||||||||
| Middle‐aged (36‐65) | 1.286 | 0.567‐2.918 | .548 | 0.716 | 0.307‐1.670 | .439 | |||||||||
| Older (≥66) | 2.041 | 0.886‐4.699 | .094 | 1.069 | 0.441‐2.592 | .882 | |||||||||
| Gender | |||||||||||||||
| Male | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Female | 0.999 | 0.759‐1.316 | .995 | 0.355 | 0.132‐0.952 | .040 | 1.166 | 0.559‐2.430 | .682 | 0.704 | 0.355‐1.396 | .315 | 1.289 | 0.896‐1.856 | .172 |
| Year at diagnosis | |||||||||||||||
| 2000‐2004 | 1 | 1 | 1 | – | – | ||||||||||
| 2005‐2009 | 0.362 | 0.222‐0.589 | <.001 | 0.166 | 0.051‐0.544 | .003 | 0.347 | 0.097‐1.237 | .103 | ||||||
| 2010‐2013 | 0.452 | 0.291‐0.700 | <.001 | 0.174 | 0.059‐0.510 | .001 | 0.275 | 0.078‐0.971 | .045 | ||||||
| 2014‐2017 | 0.357 | 0.221‐0.578 | <.001 | 0.212 | 0.062‐0.733 | .014 | 0.145 | 0.037‐0.562 | .005 | ||||||
| Type of gastrectomy | |||||||||||||||
| Proximal | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Distal | 0.571 | 0.428‐0.763 | <.001 | 0.504 | 0.233‐1.091 | .082 | 0.300 | 0.158‐0.569 | <.001 | 0.391 | 0.196‐0.778 | .007 | 0.843 | 0.523‐1.359 | .483 |
| Total | 0.892 | 0.639‐1.243 | .499 | – | 0.341 | 0.127‐0.916 | .033 | 0.841 | 0.456‐1.552 | .580 | 1.347 | 0.801‐2.266 | .262 | ||
| Vascular invasion | |||||||||||||||
| Yes | 1 | 1 | 1 | 1 | 1 | ||||||||||
| No | 1.054 | 0.792‐1.402 | .72 | 0.755 | 0.238‐2.392 | .633 | 0.875 | 0.423‐1.810 | .719 | 1.157 | 0.645‐2.075 | .626 | 1.229 | 0.829‐1.823 | .305 |
| Nerve invasion | |||||||||||||||
| Yes | 1 | 1 | 1 | 1 | 1 | ||||||||||
| No | 0.676 | 0.499‐0.915 | .011 | 0.433 | 0.088‐2.132 | .304 | 1.053 | 0.532‐2.085 | .882 | 0.874 | 0.481‐1.588 | .658 | 0.451 | 0.274‐0.742 | .002 |
| Adjuvant therapy | |||||||||||||||
| Yes | 1 | 1 | 1 | 1 | 1 | ||||||||||
| No | 1.610 | 1.177‐2.203 | .003 | 4.761 | 2.252‐10.065 | <.001 | 1.603 | 0.862‐2.982 | .136 | 2.470 | 1.102‐5.536 | .028 | 1.053 | 0.639‐1.744 | .841 |
| ELNs | |||||||||||||||
| ELNs≤15 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 16 ≤ ELNs ≤ 29 | 0.870 | 0.624‐1.214 | .413 | 1.839 | 0.797‐4.244 | .153 | 0.593 | 0.555‐2.802 | .593 | 0.582 | 0.274‐1.238 | .160 | 0.689 | 0.423‐1.120 | .133 |
| ELNs ≥ 30 | 0.635 | 0.431‐0.935 | .021 | 1.130 | 0.344‐3.716 | .841 | 1.590 | 0.612‐4.133 | .341 | 0.362 | 0.152‐0.860 | .021 | 0.484 | 0.277‐0.844 | .011 |
| Pathologic T stage | |||||||||||||||
| T1 + Tis | 1 | ||||||||||||||
| T2 | 0.986 | 0.609‐1.597 | .955 | ||||||||||||
| T3 | 1.142 | 0.698‐1.868 | .597 | ||||||||||||
| T4 | 2.303 | 1.464‐3.623 | <.001 | ||||||||||||
Adjusted factors: age, gender, year at diagnosis, type of gastrectomy, vascular invasion, nerve invasion, adjuvant therapy, ELNs, pathologic T stage.
3.4. Nomogram analysis of gastric cancer patients with pN0 stage
To predict the OS and DFS of pN0 patients with gastric cancer, a nomogram was established for predicting 3‐y and 5‐y OS and DFS by incorporating the following parameters: age, gender, year of diagnosis, type of gastrectomy, vascular invasion, nerve invasion, adjuvant therapy, pT stage, and ELNs (Figure 6A,B). The C‐index for OS and DFS prediction was 0.782 (95CI: 0.735 to 0.829) and 0.738 (95CI: 0.685 to 0.791), respectively.
FIGURE 6.
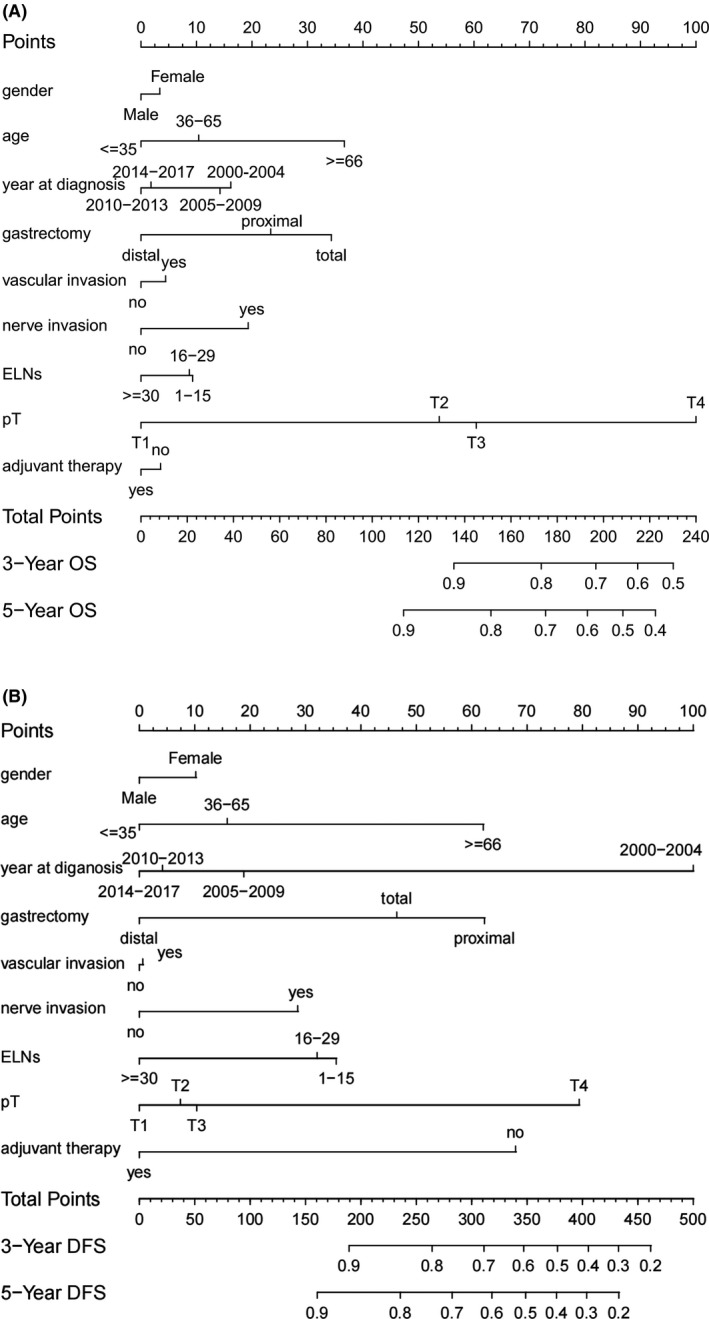
Prognostic nomogram for pN0 gastric cancer patients. A, OS nomogram. B, DFS nomogram
This result was similar to the multivariate outcome that adjuvant therapy was an independent factor for DFS but not for OS in patients with pN0 gastric cancer. We can see that adjuvant therapy had a longer line in the DFS nomogram compared with the OS nomogram.
4. DISCUSSION
This multicenter study investigated systematically how ELNs following gastrectomy affected the prognosis in patients with gastric cancer. To the best of our knowledge, our analysis represents the largest evaluation of ELN count‐to‐survival outcomes in patients with gastric cancer. A primary finding was that the higher the number of ELNs following gastrectomy, the better the possibility of long‐term survival in pN0 patients. The effect was also observed in the pT3 stage subgroup in the multivariate analysis, in which the ELNs ≥ 30 group had improved OS and DFS outcomes. Therefore, the exact minimum number of ELNs deserves further discussion before a considered conclusion is given.
Notably, there were 1238 patients (26.87%) with the minimum of 16 ELNs in our study, which meant that more than 1/4 gastric cancer patients received inappropriate lymphadenectomy based on the AJCC TNM stage. The main reasons for this phenomenon are as follows. Firstly, the ELNs ≤ 15 group had a greater proportion of earlier year diagnosis (during 2000‐2004), while the D2 lymphadenectomy of gastric cancer is not yet fully mature then.16 Secondly, the ELNs ≤ 15 group presented to be more frequent in proximal gastrectomy compared with the ELNs ≥ 30 group, while total gastrectomy did enable a more complete nodal dissection as previously reported than proximal gastrectomy.17
However, gastric cancer could be staged incorrectly because of an insufficient number of ELNs, which is called “stage migration.”2 To confirm the aforementioned speculation, we initially designed the study to investigate whether stage migration existed by examining those subgroups: pT1N1M0 (ELNs ≥ 16) vs. pT1N0M0 (ELNs ≤ 15), pT2N1M0 (ELNs ≥ 16) vs. pT2N0M0 (ELNs ≤ 15), pT3N1M0 (ELNs ≥ 16) vs. pT3N0M0 (ELNs ≤ 15), and pT4N1M0 (ELNs ≥ 16) vs. pT4N0M0 (ELNs ≤ 15), which indicated that a significant portion of patients classified as pN0 had been understaged in the ELNs ≤ 15 group. Because the number of ELNs could be controlled by surgeons with pathological diagnostic biases, it is necessary for surgeons to perform standard lymphadenectomy during surgery. Luckily, the stage migration of pN0 patients has been gradually reduced as most Chinese medical centers can achieve D2 lymphadenectomy successfully.18
In the analysis of survival trend and ELNs, a better 5‐y survival rate was depicted in the higher ELNs group for all 4 stage subgroups in Figure 5. However, the lessening of the curve with pT3 and pT4 patients was possibly because the number of patients with ELNs ≥ 45 was limited. This result is similar to that of 1 published study, namely, a significant 5‐y OS improvement for pT3 patients by up to 11% for every 10 extra ELNs.9 Both studies indicated than more ELNs were strongly recommended for pN0 patients.
In addition, a superior OS in pT3 patients was showed in the multivariate analysis based on ELNs ≥ 30. This is possibly not just because of a low probability of stage migration in the ELNs ≥ 30 group, and regional disease control is another important factor. In our study, the ELNs ≥ 30 patients showed a better DFS outcome compared with the other groups (ELNs ≤ 15 and 16 ≤ ELNs ≤ 29 groups). Based on the stratification by prognostic factors, ELNs ≥ 30 was defined as an independent predictor for improved DFS in pT3 and pT4 patients. Given these findings, pT3 patients with gastric cancer may constitute a special population attracting attention for the optimal number of ELNs following gastrectomy. In addition, Smith et al reported that 20 or 25 ELNs were advised for the examination for gastrectomy for pT3 and pT4 patients.9 A recent published study indicated that 31 ELNs are required for an accurate evaluation of pT4bN0 patients.13 This was comparable with our research. Therefore, we concluded that ELNs ≥ 30 is a prerequisite for reducing the postoperative recurrence of pT3‐4N0 gastric cancer patients. The present results still need to be validated in the future with larger prospective studies.
Nomograms have been widely used as a visualization tool for predicting the prognosis of patients with various types of cancers.19 To the best of our knowledge, the present study is the first one to construct a nomogram for predicting OS and DFS following gastrectomy based on the risk factors of survival and recurrence. The C‐index (0.782 for OS and 0.738 for DFS) demonstrated that the nomogram developed in the present study was a reliable prognostic prediction model. More importantly, the DFS nomogram showed that adjuvant therapy plays an important role in preventing recurrence following gastrectomy in pN0 patients. As Haejin et al reported, the addition of adjuvant therapy may be beneficial even for pN0 patients.20
Several limitations need to be considered in this study. Firstly, it was not just a total prospective study, and furthermore clinical trials are needed to clarify our conclusion in the future. Secondly, we included patients monitored over a long time period of 17 y, and significant differences in OS and DFS were observed between different operative periods. Thirdly, the number of lymph nodes removed during surgery for gastric cancer cannot be controllable by the surgeon's intent, and various background factors in the patient may affect the number of lymph nodes removed. It may be a challenge during the surgical process. However, it is an advantage that should not be ignored that the volume of pN0 patients was large and the source of patients usually came from northern and eastern China in the China National Cancer Center, while another medical center, the National Clinical Research Center for Digestive Diseases, was the biggest gastric cancer center in northwestern China. Therefore, the data in our study could serve as a reference for a large population‐based study in China.
The present study provides new and useful insights into the impact of ELNs count on reducing stage migration and postoperative recurrence of pN0 patients with gastric cancer during 2000‐2017. ELNs ≥ 30 is an independent predictor for improved DFS in pT3 and pT4 patients, as well as OS in pT3 patients with gastric cancer. Giving these findings, a larger number of ELNs is expected for surgeons to prolong the prognosis on gastric cancer, especially for pT3 patients.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
ETHICAL APPROVAL
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 17‐156/1412).
ACKNOWLEDGMENTS
All authors made substantial contributions to the intellectual content of this paper. This work was supported by a grant from National Key R&D Program of China (No. 2017YFC0908300)
Zhao L, Han W, Yang X, et al. Exceeding 30 ELNs is strongly recommended for pT3‐4N0 patients with gastric cancer: A multicenter study of survival, recurrence, and prediction model. Cancer Sci. 2021;112:3266–3277. 10.1111/cas.15003
Lulu Zhao, Weili Han, and Xisheng Yang are contributed equally to this work.
Contributor Information
Gang Ji, Email: jigang@fmmu.edu.cn.
Yingtai Chen, Email: yingtaichen@126.com.
DATA AVAILABILITY
The data used to support this findings of this study are include in tables within the article.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2.de Manzoni G, Verlato G, Roviello F, et al. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87(2):171‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24(12):3683‐3691. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines*): gastric cancer (Version 1. 2020). www.nccn.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng J, Yamashita H, Seto Y, et al. Increasing the number of examined lymph nodes is a prerequisite for improvement in the accurate evaluation of overall survival of node‐negative gastric cancer patients. Ann Surg Oncol. 2017;24(3):745‐753. [DOI] [PubMed] [Google Scholar]
- 6.Ji X, Bu ZD, Li ZY, et al. Prognostic significance of the total number of harvested lymph nodes for lymph node‐negative gastric cancer patients. BMC Cancer. 2017;17(1):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosa F, Tortorelli AP, Quero G, et al. Extended lymphadenectomy for gastroesophageal carcinoma in western patients. J Am Coll Surg. 2019;229(5):520. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZL, Zhu MH, Shi Q, et al. Prognostic value of the number of lymph nodes examined in patients with node‐negative gastric cancer. J Gastrointest Surg. 2019;23(3):460‐467. [DOI] [PubMed] [Google Scholar]
- 9.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US‐population database. J Clin Oncol. 2005;23(28):7114‐7124. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita K, Hosoda K, Ema A, et al. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur J Surg Oncol. 2016;42(9):1253‐1260. [DOI] [PubMed] [Google Scholar]
- 11.Gu P, Deng J, Wang W, et al. Impact of the number of examined lymph nodes on stage migration in node‐negative gastric cancer patients: a Chinese multi‐institutional analysis with propensity score matching. Ann Transl Med. 2020;8(15):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JY, Xing YN, Wang X, et al. The prognosis value of lymphatic vessel invasion in pn0 gastric cancer patients with insufficient examined lymph nodes. J Gastrointest Surg. 2020;24(2):299‐306. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Bai H, Deng J, et al. Impact of examined lymph node count on staging and long‐term survival of patients with node‐negative stage III gastric cancer: a retrospective study using a Chinese multi‐institutional registry with Surveillance, Epidemiology, and End Results (SEER) data validation. Ann Transl Med. 2020;8(17):1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huitzil‐Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28(17):2889‐2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188‐1195. [DOI] [PubMed] [Google Scholar]
- 16.Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396(10251):635‐648. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Ling R, Ma F, et al. Clinical outcomes of proximal gastrectomy versus total gastrectomy for locally advanced proximal gastric cancer: a propensity score matching analysis. Transl Cancer Res. 2020;9(4):2769‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3‐year disease‐free survival in patients with locally advanced gastric cancer: The CLASS‐01 randomized clinical trial. JAMA. 2019;321(20):1983‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In H, Kantor O, Sharpe SM, et al. Adjuvant therapy improves survival for T2N0 gastric cancer patients with sub‐optimal lymphadenectomy. Ann Surg Oncol. 2016;23(6):1956‐1962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support this findings of this study are include in tables within the article.


