To the Editor:
Results of several studies have shown high efficacy of 95% to BNT162b2 mRNA COVID-19 vaccine in terms of immune response in the healthy population [1]. This is in sharp contrast to what was observed in both treatment-naive and previously treated patients with chronic lymphocytic leukemia (CLL) who have impaired response in terms of antibody production [2–4]. In addition, most of the currently available data in terms of vaccine efficacy has been assessed and reported after a short follow-up of 7–30 days post first or second shot [1, 5].
Immunological memory to SARS-CoV-2 has been assessed and reported longitudinally with a follow-up of 8 months after infection, indicating a continuous decay of IgG levels in the plasma of convalescents [5]. Similar results were recently published in healthy adults following administration of the Moderna mRNA-1273 vaccine [6, 7].
In the current prospective study, we describe a cohort of 84 patients with CLL who have been followed in three medical centers in Israel. All patients received two doses of BNT162b2 mRNA Covid-19 vaccine (21 days apart) and were tested twice for the presence of the spike antibodies: the first test was taken at a median of 22 days (17–36) after the second dose of the vaccine and the second test was performed after a median of 100 (74–131) days following the second shot. The study was approved by the respective Ethical Committee of all three medical centers.
Anti-spike antibody tests were performed in two medical centers using a commercial kit: The Architect AdviseDx SARS-CoV-2 IgG II (Abbot, Lake Forest, IL, USA), with a positive cutoff of >50 U/mL. In the third medical center test was performed at the Central Virology Laboratory, Ministry of Health and Sheba Medical Center using enzyme-linked immunosorbent assay (ELISA) that detects IgG antibodies against the RBD of SARS-CoV-2 (positive value >1.1; range 1.1–10) [8]
After data collection, statistical analysis was performed to evaluate the differences in the positive response rate between the first and second serology tests using McNemar’s test. P values for differences in the titer values were calculated using the Wilcoxon signed-rank test. In all cases, the significance level was set at 5%, and a result was considered significant if the estimated p value (p) was below the significance level.
As in the study by Rose et al. [6] the antibody decay rates, and half-life were calculated using an exponential model described in detail in supplement data (Supplementary Table 1).
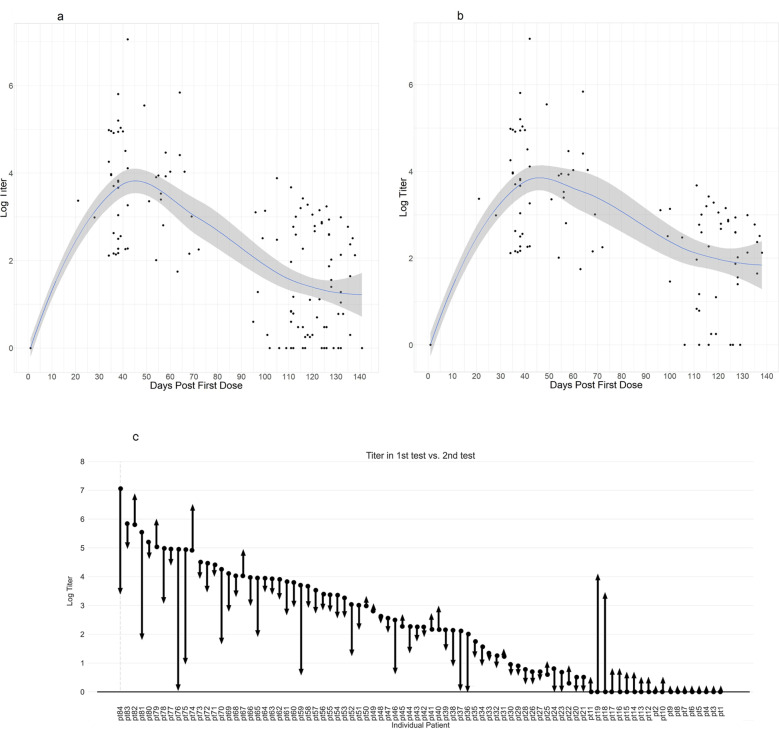
Of the 49 (58%) patients who tested positive in the first serology test, 36 (73%) maintained their positive status (Fig. 1a). On the other hand, of 35 (42%) patients who had tested negative in the first serology test, two patients (6%) developed sufficient antibodies to be considered positive in the second test. (Fig. 1b–d).
Fig. 1. Antibody levels following BNT162b mRNA Covid19 vaccine after a median of 22 and 100 days after the second dose in patients with CLL.
a Time course following BNT162b mRNA Covid19 vaccine, each dot represents immune response level for the whole cohort. b Time course following BNT162b mRNA Covid19 vaccine, each dot represents immune response level only for patients with CLL who were tested positive in the first serology test. c Arrow diagram presenting the dynamics of antibody value along 100 days of follow-up.
When we compared our calculated decay of IgG levels in patients with CLL (provided they were tested positive in the first serology test), to that published in healthy controls [6], we observed that the decay among patients with CLL is comparable to the decay reported for healthy controls that are 70 years old or older. Nevertheless, since patients with CLL tend to demonstrate lower IgG titer all along, it should be noted that 27% of the patients (13 out of 49) had titer values below the required threshold to be considered positive (RBD-IgG titer value greater than 50 U/mL is considered positive). In contrast, none of the healthy adults has titer values below the threshold even after 209 days [6].
Table 1 summarizes the clinical and demographic characteristics of the patients studied and their corresponding results in terms of positive/negative rate and the titer values (in log scale). Additional demographic data are available in Supplementary Table 2.
Table 1.
clinical and demographic characteristics of the patients and their corresponding results in terms of positive/negative and the titer values (in log scale).
| Variable | Entire cohort n = 84 |
Positive response rate (positive cutoff of >50 U/mL) | Anti covid19 RBD-IgG titer values (in log scale) | ||||
|---|---|---|---|---|---|---|---|
| Test 1 58.3% |
Test 2 47.6% |
p value <0.01 | Test 1 2.311 (1.9) |
Test 2 1.935 (1.7) |
p value <0.01 | ||
| Age [years], median | 69 (44–87) | ||||||
| ≤70 | 45 (53.6%) | 27 (60.0%) | 23 (51.1%) | <0.1 | 2.627 (0.699, 3.955) | 2.020 (0.602, 3.240) | <0.05 |
| >70 | 39 (46.4%) | 22 (56.4%) | 17 (43.6%) | <0.05 | 2.115 (0.301, 3.317) | 1.398 (0.125, 2.865) | <0.01 |
| Sex | |||||||
| Female | 29 (35%) | 15 (51.7%) | 9 (31.0%) | <0.05 | 1.748 (0.301, 3.008) | 0.845 (0.000, 2.369) | <0.01 |
| Male | 53 (65%) | 33 (62.3%) | 30 (56.6%) | 0.13 | 2.627 (0.699, 4.111) | 2.272 (0.602, 3.240) | <0.1 |
| Revised CIRS Score, median | |||||||
| <6 | 40 (50%) | 26 (65.0%) | 20 (50.0%) | <0.05 | 2.718 (0.699, 3.968) | 1.713 (0.571, 3.015) | <0.01 |
| ≥6 | 40 (50%) | 20 (50.0%) | 17 (42.5%) | 0.13 | 1.658 (0.000, 3.913) | 1.338 (0.288, 3.209) | <0.05 |
| Binet Stage at vaccination | |||||||
| A | 59 (72%) | 37 (62.7%) | 28 (47.5%) | <0.01 | 2.272 (0.452, 3.938) | 1.643 (0.602, 3.129) | <0.01 |
| B | 17 (21%) | 8 (47.1%) | 7 (41.2%) | 0.479 | 1.568 (0.301, 3.668) | 1.041 (0.000, 2.774) | 0.127 |
| C | 6 (7%) | 3 (50.0%) | 4 (66.7%) | NA | 2.046 (0.626, 4.158) | 3.467 (1.121, 4.134) | NA |
| Therapy status | |||||||
| Therapy naive | 21 (25%) | 16 (76.2%) | 13 (61.9%) | 0.13 | 3.394 (2.222, 4.383) | 2.477 (1.135, 3.094) | <0.05 |
| Previously treated | 42 (50%) | 26 (61.9%) | 22 (52.4%) | 0.16 | 2.418 (1.892, 2.945) | 2.258 (1.752, 2.763) | 0.15 |
| Currently treated | 21 (25%) | 6 (28.6%) | 4 (19.0%) | 0.31 | 1.362 (0.478, 2.245) | 0.943 (0.296, 1.590) | 0.12 |
| Lines of therapy | |||||||
| 1 | 30 (36%) | 13 (43.3%) | 10 (33.3%) | 0.13 | 1.243 (0.000, 3.597) | 0.812 (0.263, 2.945) | <0.05 |
| 2 | 13 (15%) | 7 (53.8%) | 6 (46.2%) | 0.48 | 2.115 (0.301, 4.031) | 1.114 (0.301, 2.791) | <0.1 |
| ≥3 | 20 (24%) | 12 (60%) | 10 (50%) | 0.15 | 2.608 (1.345, 3.718) | 2.169 (1.138, 3.150) | <0.05 |
| Treatment | |||||||
| Not on ongoing treatment | 63 (75%) | 43 (68.3%) | 36 (57.1%) | <0.05 | 2.808 (1.067, 3.965) | 2.369 (0.810, 3.220) | <0.05 |
| BTKi | 11 (13%) | 4 (36.4%) | 2 (18.2%) | 0.25 | 0.954 (0.000, 2.194) | 0.602 (0.250, 1.335) | <0.01 |
| BCL2i | 6 (7%) | 1 (16.7%) | 1 (16.7%) | NA | 0.151 (0.000, 0.433) | 0.074 (0.000, 0.226) | NA |
| Other | 4 (5%) | 1 (25.0%) | 1 (25.0%) | NA | 0.349 (0.000, 1.985) | 0.389 (0.226, 1.654) | NA |
| Previous anti CD20 Ab | |||||||
| No | 40 (48%) | 31 (77.5%) | 23 (57.5%) | <0.01 | 3.197 (2.042, 4.051) | 2.250 (1.032, 3.073) | <0.01 |
| Yes | 44 (52%) | 18 (40.9%) | 17 (38.6%) | 0.48 | 1.092 (0.000, 3.374) | 0.818 (0.288, 3.116) | 0.255 |
| Time since last anti-cd20 treatment [month], median | |||||||
| More than 12 months | 22 (50%) | 18 (56.2%) | 16 (50.0%) | 0.248 | 2.211 (0.675, 3.954) | 1.994 (0.571, 3.219) | 0.182 |
| Less than 12 months | 22 (50%) | 0 (0.0%) | 1 (10.0%) | NA | 0.000 (0.000, 0.226) | 0.000 (0.000, 0.433) | 0.245 |
We feel that special attention should be paid to the three patients who had increased their antibodies levels in the second test following the vaccine. Two of them have switched from a negative to a positive status. Both patients are male that were previously treated with anti-CD20 Ab (12 and 120 months ago). The third patient is a treatment-naive male whose titer values increased from 143 (positive borderline) in the first test to 323 in the second test. The table summarizes also the vaccine response according to therapy status.
Therapy naive patients yield the highest response rate (76.2% and 61.9% in the first and second test, respectively) followed by previously treated patients (61.9% and 52.4% in the first and second test, respectively). A clear drop in the measured titer values is seen virtually across all therapy groups.
We had 22 patients (26.2%) who achieved complete response (CR) following therapy for their disease. 6/22 (27.2%) developed positive Vaccine Response Rate in Test 1 and in all 6 patients positive response was maintained.
Taken together our longer follow-up, we can summarize that treatment-naive patients or those achieving CR post therapy are those who may best benefit from vaccination.
Of note, at the time of writing this letter, none of the patients have developed Covid-19 infection following vaccination. We observed four factors associated with the inability to sustain IgG levels above the threshold: patients still receiving therapy for their disease (odds ratio of 0.2987), patients who developed adverse side effects to the vaccine (odds ratio 0.3598), female gender (odds ratio of 0.4231) and having IgM levels below 40 mg/dL during the first serology test (odds ratio of 0.4481).
We need to emphasize that it is necessary to take our reported results with caution and remember that the FDA and other organizations recommend not using seroconversion levels after SARS-Cov2 vaccination to measure its effectiveness nor modifying preventive measures (FDA May 2021) (https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety). Indeed, the exact value of antibody titer is still waiting for a longer follow-up and further studies. In conclusion, this is the first longitudinally report of patients with CLL who received BNT162b mRNA Covid19 vaccine. We demonstrated that after a median of 100 days, decay of IgG levels is similar to that of the elderly healthy controls indicating that although the amount of produced antibodies is lower, patients who respond to the vaccine are able to maintain their immune response.
Supplementary information
Author contributions
TT, OB and LR designed the study and wrote the letter. TT, OB, AB and GR contribute patient’s data. LR performed statistical analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tamar Tadmor and Ohad Benjamini.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-021-01380-5.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv [Preprint]. 2021. 10.1101/2021.04.06.21254949
- 3.Herishanu Y, Avivi A, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood. 2021;137:3165–73. [DOI] [PMC free article] [PubMed]
- 4.Roeker LE, Lindsey E, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021:1–3.
- 5.Dan JM, Mateus J, Kato Y, Hastie AM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–61. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–2. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kfir O, Olmer L, Shemer-Avni Y, Wolf T, Supino-Rosin L, Prajgrod G, et al. “Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation”. E Clin Med. 2020;29:1–10. doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.