Abstract
Pembrolizumab plus pemetrexed‐platinum significantly improved overall survival (OS) and progression‐free survival (PFS) with manageable safety compared with placebo plus pemetrexed‐platinum in patients with previously untreated metastatic nonsquamous non–small‐cell lung cancer (NSCLC) without EGFR/ALK alterations in the global, randomized, double‐blind, phase 3 KEYNOTE‐189 study. We present results of Japanese patients enrolled in the KEYNOTE‐189 global and Japan extension studies. Patients were randomized 2:1 to intravenous pembrolizumab 200 mg or placebo every 3 weeks (Q3W) for up to 35 cycles. All patients received pemetrexed 500 mg/m2 plus the investigator’s choice of cisplatin or carboplatin Q3W for four cycles, followed by maintenance pemetrexed 500 mg/m2 Q3W (all intravenous). Co–primary endpoints were OS and PFS. Forty Japanese patients enrolled (pembrolizumab, n = 25; placebo, n = 15). At data cutoff (20 May 2019; median time from randomization to data cutoff, 18.5 [range, 14.7‒38.2] months), the median OS was not reached in the pembrolizumab plus pemetrexed‐platinum arm; the median OS was 25.9 (95% confidence interval [CI], 11.9‒29.0) months in the placebo plus pemetrexed‐platinum arm (hazard ratio [HR] .29; 95% CI, .07‒1.15). The median (95% CI) PFS was 16.5 (8.8‒21.1) compared with 7.1 (4.7‒21.4) months (HR, .62; 95% CI, .27‒1.42), respectively. There were no grade 5 adverse events (AE). Grade 3/4 AE occurred in 72% vs 60% of patients in the pembrolizumab vs placebo arms; 40% vs 20% had immune‐mediated AE, and 4% vs 0% had infusion reactions. Efficacy and safety outcomes were similar to those from the global study and support first‐line therapy with pembrolizumab plus pemetrexed‐platinum in Japanese patients with nonsquamous NSCLC without EGFR/ALK alterations.
Keywords: PD‐L1 protein, Japan, non–small‐cell lung carcinoma, pembrolizumab, treatment outcome
In conclusion, consistent with the global KEYNOTE‐189 study, pembrolizumab in combination with pemetrexed and platinum improved OS, PFS, ORR, and PFS2 compared with placebo plus pemetrexed‐platinum and demonstrated a manageable safety profile in Japanese patients with previously untreated metastatic nonsquamous NSCLC. The results from this study confirm the role of pembrolizumab plus pemetrexed‐platinum as a first‐line standard‐of‐care therapy for Japanese patients with metastatic nonsquamous NSCLC.
1. INTRODUCTION
Cancer of the trachea and lung is the leading cause of cancer‐related death globally as well as in Japan, where it accounts for approximately 20% of all cancer‐related deaths.1, 2 Non–small‐cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of cases.3 Historically, first‐line treatment for advanced NSCLC without sensitizing EGFR/ALK alterations consisted primarily of platinum‐based chemotherapy.4, 5, 6 However, the introduction of programmed death 1 (PD‐1) and programmed death ligand 1 (PD‐L1) inhibitors has dramatically altered the treatment paradigm for patients with NSCLC. Accordingly, the current Japanese Lung Cancer Society guidelines recommend anti–PD‐(L)1 immunotherapy in combination with platinum‐based chemotherapy as first‐line treatment in patients with metastatic NSCLC without targetable gene alterations. In addition, pembrolizumab monotherapy is recommended as first‐line treatment in patients with metastatic NSCLC without targetable gene alterations and with PD‐L1 tumor proportion score (TPS) ≥50%.4
Pembrolizumab is a humanized monoclonal antibody that selectively binds to the inhibitory immune checkpoint receptor, PD‐1, and blocks its interactions with its ligands, PD‐L1 and PD‐L2, thus reactivating T‐cell–mediated tumor destruction.7, 8, 9 As first‐line therapy, pembrolizumab in combination with platinum‐based chemotherapy is approved (including in Japan10, 11) for the treatment of patients with metastatic squamous12 and nonsquamous NSCLC13, 14 without EGFR or ALK alterations and regardless of PD‐L1 TPS after demonstrating significant survival benefit compared with chemotherapy alone in these patients. The benefits associated with first‐line pembrolizumab plus platinum‐based chemotherapy in patients with metastatic nonsquamous NSCLC without EGFR or ALK alterations were first demonstrated in the randomized, open‐label, phase 2 cohort G of the multicohort KEYNOTE‐021 study, where the combination was associated with a significantly higher objective response rate (ORR; 55% vs 29%; P = .0016) and significantly longer progression‐free survival (PFS; hazard ratio [HR], .53; 95% confidence interval [CI], .31‐.91; P = .010) compared with chemotherapy alone.13 The randomized, double‐blind, placebo‐controlled phase 3 KEYNOTE‐189 study of pembrolizumab plus pemetrexed and platinum in 616 patients with previously untreated metastatic nonsquamous NSCLC without sensitizing EGFR or ALK alterations confirmed these findings and led to full FDA approval in these patients.14 Compared with placebo plus pemetrexed‐platinum, pembrolizumab plus pemetrexed‐platinum significantly improved overall survival (OS; HR, .49; 95% CI, .38‐.64; P < .001) and PFS (HR, .52; 95% CI, .43‐.64; P < .001) after a median time from randomization to the date of death or data cutoff of 10.5 months. These findings were extended in an updated analysis with 10 additional calendar months of follow up (HR for OS, .56 [95% CI, .45‐.70]; HR for PFS, .48 [95% CI, .40‐.58]).15 Pembrolizumab plus pemetrexed‐platinum demonstrated a manageable safety profile, with no apparent increase in the frequency of adverse events (AE) commonly associated with pemetrexed plus platinum‐based chemotherapy.14, 15
Given that Asian patients are generally underrepresented in global randomized trials with anti‒PD‐(L)1 therapies16 and because of documented ethnic differences in response to treatment for NSCLC,17, 18 it is of interest to evaluate these treatments specifically in Asian populations. Accordingly, after enrollment in the KEYNOTE‐189 global study ended, additional patients were enrolled in an extension study to further assess clinical outcomes in Japanese patients.
The current study reports on the efficacy and safety of pembrolizumab plus pemetrexed and platinum as first‐line therapy in Japanese patients with metastatic nonsquamous NSCLC enrolled in the KEYNOTE‐189 global and Japan extension studies.
2. MATERIALS AND METHODS
2.1. Study design and patients
The study design of the phase 3, global, randomized, double‐blind, placebo‐controlled KEYNOTE‐189 trial (NCT02578680) has been published previously.14, 15 The design of the KEYNOTE‐189 Japan extension study (NCT03950674) was identical to the global KEYNOTE‐189 study, with the exception that it only enrolled patients in Japan. Briefly, eligible patients had untreated stage IV nonsquamous NSCLC without sensitizing EGFR or ALK alterations, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, no symptomatic brain metastases, and no history of noninfectious pneumonitis requiring systemic steroids. Patients with active autoimmune disease and those receiving systemic immunosuppressive therapy were not eligible for enrollment. Eligible patients were required to provide a tumor sample from either archival or recently obtained tissue for PD‐L1 assessment. All patients provided written informed consent before participation in the study. The study protocols and all amendments were approved by an institutional review board or independent ethics committee at each study site, and the studies were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Eligible patients were randomized (2:1) to receive intravenous pembrolizumab 200 mg or saline placebo every 3 weeks (Q3W) for up to 35 cycles. All patients received pemetrexed 500 mg/m2 plus the investigator’s choice of cisplatin 75 mg/m2 or carboplatin area under the concentration–time curve 5 mg/mL/min Q3W for four cycles, followed by maintenance therapy with pemetrexed 500 mg/m2 Q3W (all administered intravenously). Treatment continued for the prespecified number of cycles or until documented disease progression, unacceptable AE, intercurrent illness, investigator’s decision, withdrawal of consent, pregnancy, or noncompliance, or due to administrative reasons. Randomization was stratified by PD‐L1 TPS (<1% vs ≥1%), choice of platinum (cisplatin vs carboplatin), and smoking history (never vs former/current). Patients randomized to placebo could cross over to pembrolizumab monotherapy at the time of disease progression (as verified by a blinded, independent central radiologic review [BICR]) if they met all eligibility criteria.
2.2. Assessments
Tumor tissue samples obtained by biopsy at the time of diagnosis were fixed in formalin and assessed for PD‐L1 expression at a central laboratory using the PD‐L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA, USA), and PD‐L1 expression was characterized by TPS (the percentage of tumor cells with membranous PD‐L1 staining). Tumor imaging was performed at weeks 6 and 12, followed by every 9 weeks through to week 48, and then every 12 weeks for patients remaining on treatment beyond week 48. Tumor response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by BICR. Following discontinuation of study treatment, survival was assessed every 12 weeks until death, withdrawal of consent, or the end of the study.
Safety and tolerability were assessed by clinical review of all relevant parameters, including AE, laboratory tests, and vital signs. AE, including those of special interest, occurring through 30 days (90 days for serious AE) after the last treatment dose or until start of new therapy were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Immune‐mediated AE and infusion reactions were assessed based on a list of preferred terms identified by the sponsor as having an immune etiology, regardless of attribution to study treatment.
2.3. Endpoints
The dual primary endpoints were OS and PFS. Secondary endpoints included ORR, duration of response (DOR), and safety. PFS2, defined as the time from randomization to objective tumor progression on next‐line treatment (including subsequent anti‒PD‐[L]1 therapy) or death from any cause, whichever occurred first, was a protocol‐specified exploratory endpoint. PFS2 events were characterized as time of investigator‐assessed disease progression leading to cessation of second‐line therapy, initiation of third‐line therapy for patients who discontinued second‐line therapy without disease progression, and time of death for those who either did not receive second‐line therapy or stopped second‐line therapy without disease progression and did not begin third‐line therapy.15, 19 Patients were censored for PFS2 at the time of last known survival if they were alive and either had not received second‐line therapy or had stopped it without disease progression and had not initiated third‐line therapy.
2.4. Statistical analysis
Overall, approximately 40 Japanese patients were planned for enrollment, including 10 in the global study and 30 in the Japan extension study. Efficacy was analyzed by assigned treatment in the intent‐to‐treat population, which included all randomized patients. Safety was analyzed in all patients who received at least one dose of treatment (as‐treated population). OS, PFS, and PFS2 were estimated using the Kaplan‐Meier method. HR and 95% CI were determined using a Cox proportional hazards model. The relatively small patient population precluded further analyses by PD‐L1 status. ORR were summarized by treatment group. DOR was summarized descriptively using the Kaplan‐Meier method. Inferential testing for statistical significance was not preplanned, and the results were descriptively presented.
3. RESULTS
3.1. Patients and treatment
Of 62 patients screened at 11 Japanese sites between 15 March 2016 to 1 March 2018, 40 were enrolled (10 in the global study; 30 in the extension study), including 25 randomly assigned to pembrolizumab plus pemetrexed‐platinum and 15 to placebo plus pemetrexed‐platinum (Figure 1). Baseline demographics and clinical characteristics were generally similar in the pembrolizumab plus pemetrexed‐platinum arm and the placebo plus pemetrexed‐platinum arm, with the exception of fewer patients with brain metastases (16% vs 33%), previous adjuvant therapy (12% vs 20%) and PD‐L1 TPS not evaluable (4% vs 20%), and a larger proportion of patients with PD‐L1 TPS <1% (56% vs 40%) (Table 1).
FIGURE 1.
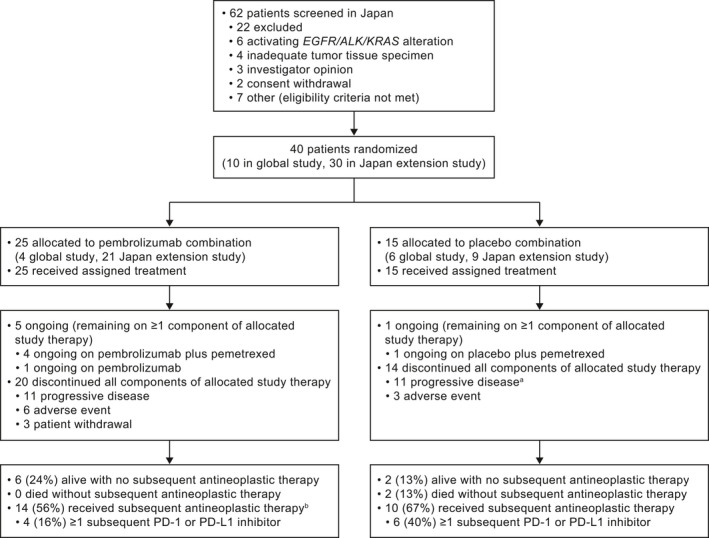
Disposition of patients in the study. PD‐1, programmed death 1; PD‐L1, programmed death ligand 1. aIncludes patients with clinical progression or progressive disease. bIncludes patients who received subsequent antineoplastic therapy. It excludes 1 patient in the pembrolizumab combination arm who received radiation (see Table S1 for further details)
TABLE 1.
Patient demographic and disease characteristics at baseline
| Characteristic | Pembrolizumab + pemetrexed‐platinum (n = 25) | Placebo + pemetrexed‐platinum (n = 15) |
|---|---|---|
| Age, median (range), y | 64.0 (34‐77) | 66.0 (34‐74) |
| Male | 19 (76) | 12 (80) |
| ECOG PS | ||
| 0 | 15 (60) | 9 (60) |
| 1 | 10 (40) | 6 (40) |
| Histology | ||
| Adenocarcinoma | 23 (92) | 14 (93) |
| NSCLC NOS | 1 (4) | 0 (0) |
| Other | 1 (4) | 1 (7) |
| Smoking status | ||
| Former/current smoker | 18 (72) | 12 (80) |
| Never | 7 (28) | 3 (20) |
| Brain metastases | 4 (16) | 5 (33) |
| PD‐L1 TPS | ||
| <1% | 14 (56) | 6 (40) |
| ≥1% | 10 (40) | 6 (40) |
| Not evaluable | 1 (4) | 3 (20) |
| Prior therapy | ||
| Neoadjuvant therapy | 0 | 0 |
| Adjuvant therapy | 3 (12) | 3 (20) |
| Prior radiationa | 8 (32) | 4 (27) |
Data are n (%) unless otherwise noted.
ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC NOS, non–small‐cell lung cancer not otherwise specified; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
No patient had prior thoracic radiation.
At data cutoff (20 May 2019), the median time from randomization to the date of database cutoff was 18.5 (range, 14.7‐38.2) months. The median treatment duration was 9 (range, 0‒21.2) months with pembrolizumab plus pemetrexed‐platinum and 6 (range, 1.2 ‒17.2) months in the placebo plus pemetrexed‐platinum arm. At data cutoff, 5 patients (20%) in the pembrolizumab plus pemetrexed‐platinum arm compared with 1 (7%) in the placebo plus pemetrexed‐platinum arm remained on ≥1 component of study therapy (Figure 1). A total of 6 patients (24%) in the pembrolizumab plus pemetrexed‐platinum arm and 2 (13%) in the placebo plus pemetrexed‐platinum arm were alive without subsequent treatment, including 4 (16%) and 1 (7%), respectively, who did not have disease progression. A total of 14 patients (56%) in the pembrolizumab plus pemetrexed‐platinum arm and 10 (67%) in the placebo plus pemetrexed‐platinum arm received subsequent antineoplastic therapy; 4 patients (27%) in the placebo plus pemetrexed‐platinum arm crossed over on study to pembrolizumab monotherapy, and 4 patients (16%) in the pembrolizumab plus pemetrexed‐platinum arm and 6 (40%) in the placebo plus pemetrexed‐platinum arm received subsequent PD‐(L)1 inhibitor therapy (Table S1 ).
3.2. Efficacy outcomes
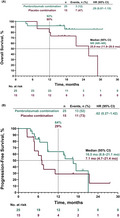
At the time of data cutoff, 3 patients (12%) in the pembrolizumab plus pemetrexed‐platinum arm and 7 (47%) in the placebo plus pemetrexed‐platinum arm had died. Median OS was not reached (NR; 95% CI, NE–NE) among patients receiving pembrolizumab plus pemetrexed‐platinum and was 25.9 (95% CI, 11.9‐29.0) months among those receiving placebo plus pemetrexed‐platinum (HR [95% CI], .29 [.07‐1.15]; Figure 2A). Estimated 12‐month OS rates were 92% and 80% in the pembrolizumab plus pemetrexed‐platinum and placebo plus pemetrexed‐platinum arms, respectively.
FIGURE 2.
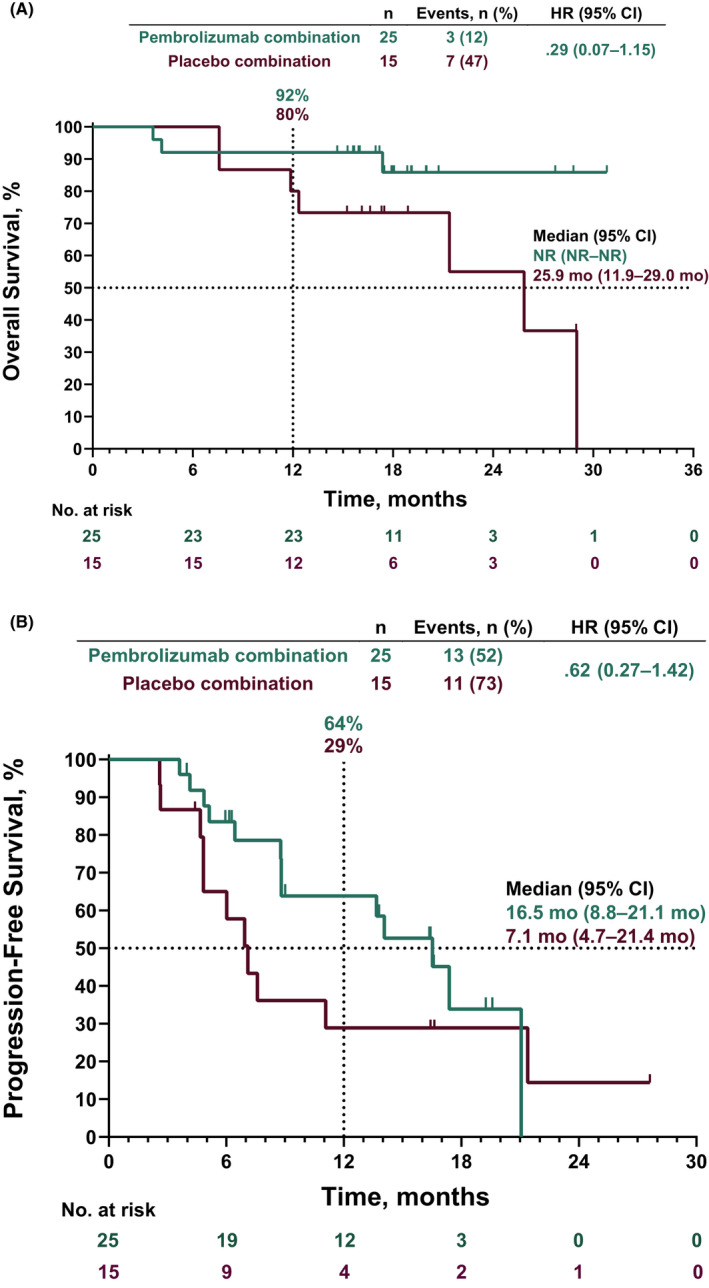
Kaplan‐Meier analysis of (A) OS and (B) PFS in the intent‐to‐treat population. HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression‐free survival
At data cutoff, disease progression or death had occurred in 13 patients (52%) in the pembrolizumab plus pemetrexed‐platinum arm and 11 (73%) in the placebo plus pemetrexed‐platinum arm. Median (95% CI) PFS was 16.5 (8.8‐21.1) months in the pembrolizumab plus pemetrexed‐platinum arm and 7.1 (4.7‐21.4) months in the placebo plus pemetrexed‐platinum arm (HR [95% CI], .62 [.27‐1.42]; Figure 2B). Estimated 12‐month PFS rates were 64% and 29%, respectively.
Confirmed ORR was 56% (1 with complete response; 13 with partial response [PR]) in the pembrolizumab plus pemetrexed‐platinum arm and 33% (all 5 with PR) in the placebo plus pemetrexed‐platinum arm (Table 2). At data cutoff, no patient had progressive disease in the pembrolizumab plus pemetrexed‐platinum arm compared with 13% (n = 2) in the placebo plus pemetrexed‐platinum arm (Table 2). Median (range) DOR was 13.6 (3.3+ to 19.6) months in the pembrolizumab plus pemetrexed‐platinum arm and 9.7 (3.4 to 26.4+) months in the placebo plus pemetrexed‐platinum arm (+ indicates no progressive disease by the time of last assessment; Figure 3). Overall, 62% of patients in the pembrolizumab plus pemetrexed‐platinum arm compared with 40% in the placebo plus pemetrexed‐platinum arm had estimated DOR ≥12 months.
TABLE 2.
Summary of tumor response per RECIST Version 1.1 by BICR
| Pembrolizumab +pemetrexed‐platinum (n = 25) | Placebo + pemetrexed‐platinum (n = 15) | |
|---|---|---|
| Objective response rate, % (95% CI) | 56 (34.9 to 75.6) | 33 (11.8 to 61.6) |
| Complete response | 1 (4) | 0 (0) |
| Partial response | 13 (52) | 5 (33) |
| Stable diseasea | 9 (36) | 8 (53) |
| Progressive disease | 0 (0) | 2 (13) |
| Not evaluableb | 2 (8) | 0 (0) |
| Time to response, median (range), monthsc | 1.4 (1.2 to 3.0) | 1.4 (1.2 to 4.9) |
| Duration of response, median (range), months c , d | 13.6 (3.3+ to 19.6) | 9.7 (3.4 to 26.4+) |
| Kaplan‐Meier estimate of patients with extended duration of response, % c , d | ||
| ≥12 mo | 61.9 | 40.0 |
Data are n (%) unless otherwise noted.
+, no progressive disease at time of last assessment.
BICR, blinded, independent central radiologic review; RECIST, Response Evaluation Criteria in Solid Tumors.
Includes stable disease and noncomplete response/nonprogressive disease.
Postbaseline assessments were not evaluable or complete response/partial response/stable disease was <6 wk from randomization.
Assessed in patients with a best objective response of confirmed complete or partial response.
Estimated using product‐limit (Kaplan‐Meier) method for censored data.
FIGURE 3.
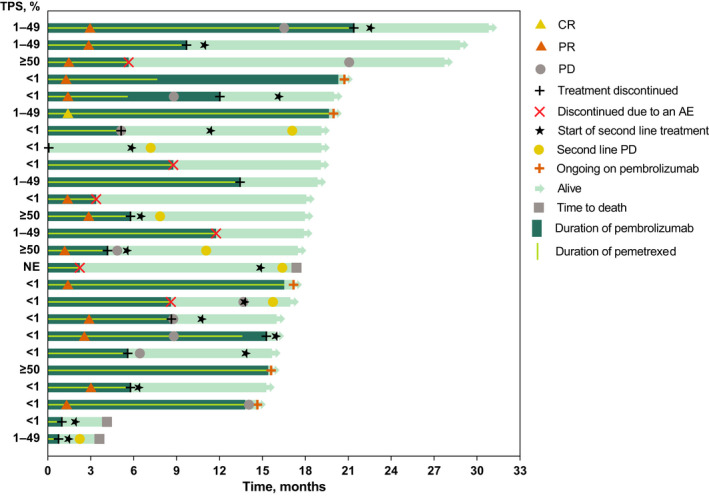
Treatment duration and time to response among patients in the pembrolizumab plus pemetrexed‐platinum group. Light green bars indicate the months of follow up. AE, adverse event; CR, complete response; PD, progressive disease; PR, partial response
Median PFS2 was not reached (95% CI, 16.4 months–NE) in the pembrolizumab plus pemetrexed‐platinum arm and was 12.4 (95% CI, 7.6‐20.5) months in the placebo plus pemetrexed‐platinum arm (HR [95% CI], .34 [.14‐.86]). The estimated 12‐month PFS2 rate was 80% in the pembrolizumab plus pemetrexed‐platinum arm and 53% in the placebo plus pemetrexed‐platinum arm (Figure 4).
FIGURE 4.
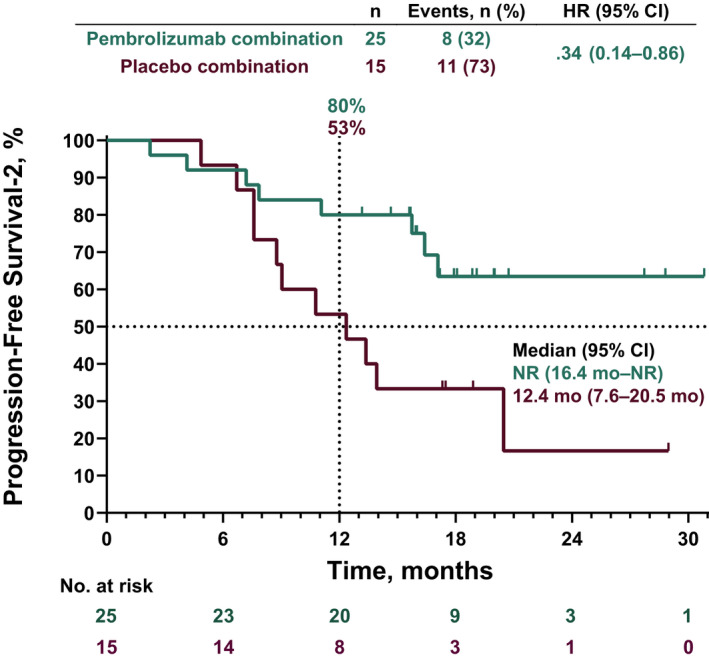
Kaplan‐Meier analysis of progression‐free survival‐2 (PFS2) in the intent‐to‐treat population. PFS2 was defined as the time from randomization to objective tumor progression on next‐line treatment (including subsequent anti–PD‐[L]1 therapy) or death from any cause, whichever occurred first.19 HR, hazard ratio; NR, not reached; PD‐(L)1, programmed death 1 or programmed death ligand 1
3.3. Safety
All patients in both treatment arms experienced at least one AE (Table 3; Table S2). The most commonly reported AE were nausea, constipation, decreased appetite, and anemia. Grade 3/4 AE occurred in 18 patients (72%) in the pembrolizumab plus pemetrexed‐platinum arm and in 9 patients (60%) in the placebo plus pemetrexed‐platinum arm; there were no grade 5 AE in either treatment arm. There were three AE associated with renal toxicity in the pembrolizumab plus pemetrexed‐platinum arm (blood creatinine increased, n = 2 [8%]; creatinine renal clearance decreased, n = 1 [4%]) and four in the placebo plus pemetrexed‐platinum arm (acute kidney injury, renal impairment, blood creatinine increased and creatinine renal clearance decreased, n = 1 each [7%]). In the pembrolizumab plus pemetrexed‐platinum arm, in 1 patient with increased blood creatinine both pembrolizumab and pemetrexed were discontinued; in the second patient with increased blood creatinine treatment with both pembrolizumab and pemetrexed was interrupted; and in the patient with decreased creatinine renal clearance, only pemetrexed was discontinued. In the placebo plus pemetrexed‐platinum arm, only treatment with pemetrexed was interrupted in the patient with decreased creatinine renal clearance.
TABLE 3.
Summary of all‐cause AE in the as‐treated populationa
| AE, n (%) | Pembrolizumab + pemetrexed‐platinum (n = 25) | Placebo + pemetrexed‐platinum (n = 15) | ||
|---|---|---|---|---|
| Any grade | Grade 3‐5b | Any grade | Grade 3‐5b | |
| Experienced ≥1 AEc | 25 (100) | 18 (72) | 15 (100) | 9 (60) |
| Led to discontinuation of any treatment component | 9 (36) | 4 (16) | 3 (20) | 2 (13) |
| Led to death | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Occurring in ≥15% of patients in either treatment arm | ||||
| Nausea | 18 (72) | 0 (0) | 8 (53) | 1 (7) |
| Constipation | 16 (64) | 0 (0) | 11 (73) | 0 (0) |
| Decreased appetite | 14 (56) | 0 (0) | 9 (60) | 0 (0) |
| Anemia | 11 (44) | 2 (8) | 10 (67) | 3 (20) |
| White blood cell count decreased | 10 (40) | 4 (16) | 3 (20) | 1 (7) |
| Alanine aminotransferase increased | 9 (36) | 0 (0) | 5 (33) | 0 (0) |
| Diarrhea | 9 (36) | 1 (4) | 3 (20) | 1 (7) |
| Dry skin | 9 (36) | 0 (0) | 2 (13) | 0 (0) |
| Aspartate aminotransferase increased | 8 (32) | 0 (0) | 5 (33) | 0 (0) |
| Hiccups | 7 (28) | 0 (0) | 5 (33) | 0 (0) |
| Lymphocyte count decreased | 7 (28) | 6 (24) | 1 (7) | 0 (0) |
| Neutrophil count decreased | 7 (28) | 5 (20) | 2 (13) | 0 (0) |
| Stomatitis | 7 (28) | 0 (0) | 4 (27) | 0 (0) |
| Insomnia | 6 (24) | 0 (0) | 1 (7) | 0 (0) |
| Rash | 6 (24) | 0 (0) | 1 (7) | 0 (0) |
| Face edema | 5 (20) | 0 (0) | 1 (7) | 0 (0) |
| Malaise | 5 (20) | 0 (0) | 4 (27) | 0 (0) |
| Nasopharyngitis | 5 (20) | 0 (0) | 1 (7) | 0 (0) |
| Pyrexia | 5 (20) | 0 (0) | 1 (7) | 0 (0) |
| Alopecia | 4 (16) | 0 (0) | 1 (7) | 0 (0) |
| Back pain | 4 (16) | 0 (0) | 2 (13) | 0 (0) |
| Fatigue | 4 (16) | 0 (0) | 5 (33) | 0 (0) |
| Dysgeusia | 3 (12) | 0 (0) | 5 (33) | 0 (0) |
| Neutropenia | 3 (12) | 1 (4) | 3 (20) | 2 (13) |
| Peripheral edema | 2 (8) | 0 (0) | 5 (33) | 0 (0) |
| Vomiting | 2 (8) | 0 (0) | 4 (27) | 0 (0) |
| Anxiety | 1 (4) | 0 (0) | 3 (20) | 0 (0) |
| Thrombocytopenia | 1 (4) | 0 (0) | 4 (27) | 0 (0) |
| Leukopenia | 0 (0) | 0 (0) | 7 (47) | 0 (0) |
| Immune‐mediated AE and infusion reactionsd | 10 (40) | 4 (16) | 3 (20) | 2 (13) |
| Adrenal insufficiency | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Colitis | 1 (4) | 1 (4) | 0 (0) | 0 (0) |
| Hyperthyroidism | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Hypothyroidism | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Infusion reactions | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Pneumonitis | 2 (8) | 1 (4) | 2 (13) | 2 (13) |
| Severe skin reactions | 2 (8) | 2 (8) | 0 (0) | 0 (0) |
| Thyroiditis | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
AE, adverse event; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Includes all randomized patients who received at least one dose of study treatment, according to the treatment received.
No patient in either treatment arm had grade 5 AE.
AE were graded using NCI CTCAE version 4.0.
Immune‐mediated AE and infusion reactions are listed irrespective of attribution to study treatment by the investigator.
Immune‐mediated AE occurred in 10 patients (40%) in the pembrolizumab plus pemetrexed‐platinum arm and 3 (20%) in the placebo plus pemetrexed‐platinum arm, and infusion reactions occurred in 1 patient (4%) in the pembrolizumab plus pemetrexed‐platinum arm and none in the placebo plus pemetrexed‐platinum arm (Table 3). No patient experienced a grade 5 immune‐mediated AE or infusion reaction. Grade 3/4 immune‐mediated AE occurred in 4 patients (16%) in the pembrolizumab plus pemetrexed‐platinum arm (severe skin reaction, n = 2; colitis, n = 1; pneumonitis, n = 1) and 2 (13%) in the placebo plus pemetrexed‐platinum arm (pneumonitis, n = 2).
4. DISCUSSION
These results from the KEYNOTE‐189 Japan study are, to our knowledge, the first published findings from a phase 3 placebo‐controlled trial evaluating clinical outcomes with first‐line anti–PD‐(L)1 therapy in combination with platinum‐based chemotherapy in Japanese patients with advanced nonsquamous NSCLC. Treatment with pembrolizumab plus pemetrexed and platinum was associated with prolonged OS, PFS, and PFS‐2 compared with placebo plus pemetrexed and platinum, with manageable toxicity. These data were generally consistent with those of the global population from this study and support the use of pembrolizumab plus pemetrexed‐platinum as first‐line treatment for Japanese patients with metastatic nonsquamous NSCLC without sensitizing EGFR or ALK alterations.
Baseline characteristics in the Japan study were largely consistent with the global population, with the exceptions of a greater percentage of male patients (76%–80% vs 53%–62% in the global study), patients with PD‐L1 TPS <1% (40%–56% vs 31%), and patients with previous thoracic radiotherapy (27%–32% vs 7%–9%).15 At the time of data cutoff, median OS was not reached in the Japan study, but, the HR (95% CI) for OS (.29 [.07 − 1.15]) and PFS (.62 [.27 − 1.42]) in the Japan study suggest prolonged survival with pembrolizumab plus pemetrexed‐platinum compared with the placebo plus pemetrexed‐platinum arm, consistent with the global patient population (HR for OS, .56 [.45 − .70]; PFS, .48 [.40 − .58]). The 12‐month OS and PFS rates were higher in the Japan study for pembrolizumab plus pemetrexed‐platinum compared with placebo plus pemetrexed‐platinum (12‐month OS, 92% vs 80%; PFS, 64% vs 29%), similar to the global population (12‐month OS, 70% vs 48%; PFS, 39% vs 17%),15 despite a higher proportion of patients with PD‐L1 TPS <1% in the pembrolizumab plus pemetrexed‐platinum arm. These trends were also in line with the prespecified final analysis from the global KEYNOTE‐189 study, which reported HR (95% CI) for OS of .56 (.46‐.69) and for PFS of .49 (.41‐.59).20 The small patient population in the Japan study, however, precludes further analyses by PD‐L1 status. Similar to the global KEYNOTE‐189 study, the HR (95% CI) for PFS2 in the Japan study, defined as the time from randomization to objective tumor progression on next line of therapy or death from any cause, whichever occurred first, also favored pembrolizumab plus pemetrexed‐platinum compared with placebo plus pemetrexed‐platinum. This PFS2 outcome indicates that treatment effects observed in the first‐line setting in Japanese patients with metastatic NSCLC were maintained into the second line of therapy and thereby supports the use of pembrolizumab in the first‐line setting.
No new safety signals were identified in this population of Japanese patients and, overall, pembrolizumab plus pemetrexed‐platinum was associated with a manageable toxicity profile, with no apparent increase in the frequency of AE commonly associated with pemetrexed‐platinum. A similar proportion of patients experienced grade 3 or 4 AE in the two treatment arms, and there were no deaths due to AE in either treatment arm. While a higher proportion of patients in the pembrolizumab plus pemetrexed‐platinum arm in the primary analysis from the global KEYNOTE‐189 study experienced acute kidney injury compared to the placebo plus pemetrexed‐platinum arm (5.2% vs .5%),14 there was no evidence of increased renal toxicity in either treatment arm in the current analysis of Japanese patients from the KEYNOTE‐189 global and extension studies. Overall, the safety profile of pembrolizumab plus pemetrexed‐platinum was similar in the Japan and the KEYNOTE‐189 global studies.
The findings from this study add to the growing body of evidence demonstrating the efficacy and safety of pembrolizumab in combination with platinum‐based chemotherapy in Japanese patients with NSCLC. The phase 1 KEYNOTE‐011 study parts B and C suggested clinical benefit of first‐line therapy with pembrolizumab plus platinum‐based chemotherapy in Japanese patients with advanced nonsquamous (n = 12) or squamous (n = 14) NSCLC.21 Patients enrolled in KEYNOTE‐011 received pembrolizumab in combination with platinum‐based chemotherapy for four cycles, followed by pembrolizumab for up to 2 years as monotherapy (squamous NSCLC) or in combination with pemetrexed (nonsquamous NSCLC). At the time of data cutoff, the ORR was 50% in patients with squamous NSCLC and 67% and 80% in patients in the cisplatin and carboplatin cohorts, respectively, with nonsquamous NSCLC. Treatment‐related deaths due to pneumonitis were reported for 2 patients in part B of KEYNOTE‐011 (none were reported in part C of the study); however, similar AE were not observed in the current analysis (1 patient in the pembrolizumab plus pemetrexed‐platinum arm and 2 patients in the placebo plus pemetrexed‐platinum arm experienced grade 3 or 4 pneumonitis). Separately, a subset analysis of data from 50 Japanese patients enrolled in the global, phase 3, placebo‐controlled KEYNOTE‐407 study demonstrated efficacy and safety of pembrolizumab plus chemotherapy as first‐line therapy in patients with metastatic squamous NSCLC.22 Median OS was 17.3 months in the pembrolizumab plus chemotherapy arm compared with 11.0 months in the placebo plus chemotherapy arm (HR, .56; 95% CI, .27‐1.15), and median PFS was 8.3 compared with 7.2 months (HR, .65; 95% CI, .35‐1.23), respectively. The safety profile of pembrolizumab plus chemotherapy was manageable in both of the above analyses.
The current study had certain limitations. Given the relatively small number of patients (N = 40), the KEYNOTE‐189 Japan study was not powered for formal statistical analysis and, as discussed above, did not allow for comparison of outcomes among key patient subgroups. In addition, it is unclear whether or how potential differences between treatment groups in clinical characteristics, including the smaller proportion of patients with brain metastases and the smaller proportion with previous adjuvant therapy in the pembrolizumab plus pemetrexed‐platinum arm compared with the placebo plus pemetrexed‐platinum arm, might have contributed to observed outcomes. Nonetheless, the trends for HR in the Japan study were consistent with those in the global study, which demonstrated survival benefits with pembrolizumab plus pemetrexed‐platinum compared with placebo plus pemetrexed‐platinum among patients with and without liver or brain metastases, suggesting that there is substantial benefit with pembrolizumab plus pemetrexed‐platinum compared with pemetrexed‐platinum alone as first‐line treatment in Japanese patients with advanced nonsquamous NSCLC. Notably, based on the results from KEYNOTE‐189, pembrolizumab in combination with pemetrexed and platinum‐based chemotherapy was approved in Japan in 2018 for first‐line treatment of unresectable, advanced or recurrent nonsquamous NSCLC regardless of PD‐L1 expression,10 and the Pan Asian adapted clinical practice guidelines recommended consideration of pembrolizumab plus pemetrexed‐platinum as first‐line treatment for this indication.23 Finally, the addition of a PD‐(L)1 inhibitor to platinum‐based chemotherapy is “strongly recommended” in the Japanese Lung Cancer Society guidelines for patients with driver oncogene‐negative stage IV NSCLC with ECOG PS 0‐1.4
In conclusion, consistent with the global KEYNOTE‐189 study, pembrolizumab in combination with pemetrexed and platinum improved OS, PFS, ORR, and PFS2 compared with placebo plus pemetrexed‐platinum and demonstrated a manageable safety profile in Japanese patients with previously untreated metastatic nonsquamous NSCLC. The results from this study confirm the role of pembrolizumab plus pemetrexed‐platinum as a first‐line standard‐of‐care therapy for Japanese patients with metastatic nonsquamous NSCLC.
DISCLOSURE
Hidehito Horinouchi: Lecture fees, honoraria, or other fees from Eli Lilly, AstraZeneca, Kyowa Kirin, MSD, Ono Pharmaceutical, and Bristol‐Myers Squibb; Research funds from Chugai Pharmaceutical, Daiichi Sankyo, AstraZeneca, MSD, Ono Pharmaceutical, Bristol‐Myers Squibb, and Genomic Health. Naoyuki Nogami: Honoraria from AstraZeneca, Chugai Pharmaceutical, Pfizer Japan Inc, Eli Lilly Japan KK, Ono Pharmaceutical, Taiho Pharmaceutical, MSD KK, Kyowa Kirin, Bristol‐Myers Squibb KK, and Nippon Boehringer Ingelheim. Hideo Saka: Nothing to disclose. Makoto Nishio: Honoraria for lectures and consulting from Ono Pharmaceutical, Bristol‐Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, and Novartis; Research support from MSD, Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb, Taiho Pharmaceutical, Eli Lilly, AstraZeneca, and Pfizer. Takaaki Tokito: Personal fees from AstraZeneca, Chugai Pharmaceutical, MSD, and Boehringer Ingelheim. Toshiaki Takahashi: Grants and personal fees from AstraZeneca KK, Chugai Pharmaceutical, Eli Lilly Japan KK, Ono Pharmaceutical, and MSD KK; Grants from Pfizer Japan Inc; Personal fees from Boehringer Ingelheim Japan, Inc and Roche Diagnostics KK. Kazuo Kasahara: Grants from Boehringer Ingelheim. Yoshihiro Hattori: Lecture fees from Taiho Pharmaceutical; Grants from Ono Pharmaceutical and MSD. Eiki Ichihara: Honoraria from Boehringer Ingelheim; Research support from MSD. Noriaki Adachi: Employee of MSD KK. Kazuo Noguchi: Employee of MSD KK. Fabricio Souza: Employee of Merck & Co., Inc. Takayasu Kurata: Personal fees from AstraZeneca, MSD, Eli Lilly, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, Boehringer Ingelheim, and Pfizer; Grants from AstraZeneca, MSD, Chugai Pharmaceutical, Takeda, and Bristol‐Myers Squibb.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing assistance was provided by Adrienne Drinkwater, PhD, and Shilpa Kamboj, PhD, of ICON plc (North Wales, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. A portion of the results from this study were presented at the 60th Annual Meeting of the Japan Lung Cancer Society; 6−8 December 2019; Osaka, Japan.
Horinouchi H, Nogami N, Saka H, et al. Pembrolizumab plus pemetrexed‐platinum for metastatic nonsquamous non–small‐cell lung cancer: KEYNOTE‐189 Japan Study. Cancer Sci. 2021;112:3255–3265. 10.1111/cas.14980
DATA AVAILABILITY STATEMENT
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data‐sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region‐specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis‐driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data‐sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
REFERENCES
- 1.Global Burden of Disease Cancer Collaboration , Fitzmaurice C, Abate D, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2019;5:1749‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Editorial Board of Cancer Statistics in Japan . Cancer Statistics in Japan ‐ 2017. 2018. https://ganjoho.jp/en/professional/statistics/brochure/2017_en.html. Accessed August 26, 2020.
- 3.Zappa C, Mousa SA. Non‐small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society guideline for non‐small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novello S, Barlesi F, Califano R, et al. Metastatic non‐small‐cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v1‐v27. [DOI] [PubMed] [Google Scholar]
- 6.Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first‐line treatment for advanced/metastatic non‐small‐cell lung cancer. Ann Oncol. 2019;30:884‐896. [DOI] [PubMed] [Google Scholar]
- 7.Hendriks L, Besse B. New windows open for immunotherapy in lung cancer. Nature. 2018;558:376‐377. [DOI] [PubMed] [Google Scholar]
- 8.Reck M. Pembrolizumab as first‐line therapy for metastatic non‐small‐cell lung cancer. Immunotherapy. 2018;10:93‐105. [DOI] [PubMed] [Google Scholar]
- 9.Silvinato A, Floriano I, Bernardo WM. Advanced non‐small cell lung cancer ‐ treatment with pembrolizumab. Rev Assoc Med Bras (1992). 2019;65:1423‐1432. [DOI] [PubMed] [Google Scholar]
- 10.Merck’s KEYTRUDA® (pembrolizumab) receives five new approvals in Japan, including in advanced non‐small cell lung cancer, as adjuvant therapy for melanoma, and in advanced microsatellite instability‐high tumors. 2019. https://www.merck.com/news/mercks‐keytruda‐pembrolizumab‐receives‐five‐new‐approvals‐in‐japan‐including‐in‐advanced‐non‐small‐cell‐lung‐cancer‐nsclc‐as‐adjuvant‐therapy‐for‐melanoma‐and‐in‐advanced‐microsa/. Accessed August 27, 2020.
- 11.Agency PaMD . Report on the Deliberation Results. 2018. https://www.pmda.go.jp/files/000231921.pdf. Accessed September 15, 2020.
- 12.Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379:2040‐2051. [DOI] [PubMed] [Google Scholar]
- 13.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17:1497‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 15.Gadgeel S, Rodriguez‐Abreu D, Speranza G, et al. Updated analysis from KEYNOTE‐189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2020;38:1505‐1517. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Wu YL. Immunotherapy in the Asiatic population: any differences from Caucasian population? J Thorac Dis. 2018;10:S1482‐S1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and Caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8:451‐462. [DOI] [PubMed] [Google Scholar]
- 18.Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non‐small cell lung cancer: results of a meta‐analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030‐1038. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency . Guideline on the evaluation of anticancer medicinal products in man. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/11/WC500238764.pdf. Accessed August 26, 2020.
- 20.Rodriguez Abreu D, Powell SF, Hochmair MJ, et al. Protocol‐specified final analysis of KEYNOTE‐189: Pemetrexed‐platinum chemotherapy with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC.American Society of Clincal Oncology (ASCO); 2020 May 29‐31; Virtual Scientific Program.
- 21.Kurata T, Nakagawa K, Satouchi M, et al. Primary results from Japanese phase I study of pembrolizumab plus chemotherapy as front‐line therapy for advanced NSCLC. Ann Oncol. 2019;30:vi114. [Google Scholar]
- 22.Okamoto I, Kato T, Imamura F, et al. Pembrolizumab plus chemotherapy in 1st line metastatic squamous NSCLC: KEYNOTE‐407 Japanese subgroup. Japan Lung Cancer Society; 2019 December 6‐8; Osaka, Japan.
- 23.Wu YL, Planchard D, Lu S, et al. Pan‐Asian adapted clinical practice guidelines for the management of patients with metastatic non‐small‐cell lung cancer: a CSCO‐ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171‐210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data‐sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region‐specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis‐driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data‐sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.


