Abstract
Background
Alteration in blood triglyceride levels have been found in patients with coronavirus disease 2019 (COVID-19). However, the association between hypertriglyceridemia and mortality in COVID-19 patients is unknown.
Objective
To investigate the association between alteration in triglyceride level and mortality in hospitalized COVID-19 patients.
Methods
We conducted a retrospective study of 600 hospitalized patients with COVID-19 diagnosis (ICD10CM:U07.1) and/or SARS-CoV-2 positive testing results between March 1, 2020 and December 21, 2020 at a tertiary academic medical center in Milwaukee, Wisconsin. De-identified data, including demographics, medical history, and blood triglyceride levels were collected and analyzed. Of the 600 patients, 109 patients died. The triglyceride value on admission was considered the baseline and the peak was defined as the highest level reported during the entire period of hospitalization. Hypertriglyceridemia was defined as greater than 150 mg/dl. Logistic regression analyses were performed to evaluate the association between hypertriglyceridemia and mortality.
Results
There was no significant difference in baseline triglyceride levels between non-survivors (n = 109) and survivors (n = 491) [Median 127 vs. 113 mg/dl, p = 0.213]. However, the non-survivors had significantly higher peak triglyceride levels during hospitalization [Median 179 vs. 134 mg/dl, p < 0.001]. Importantly, hypertriglyceridemia independently associated with mortality [odds ratio=2.3 (95% CI: 1.4–3.7, p = 0.001)], after adjusting for age, gender, obesity, history of hypertension and diabetes, high CRP, high leukocyte count and glucocorticoid treatment in a multivariable logistic regression model.
Conclusions
Hypertriglyceridemia during hospitalization is independently associated with 2.3 times higher mortality in COVID-19 patients. Prospective studies are needed to independently validate this retrospective analysis.
Keywords: Triglyceride, Diabetes, Obesity, COVID-19 mortality, Inflammation, CRP, Leukocyte count, glucocorticoid
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) has become a critical health threat since December 2019, affecting millions of people worldwide.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen that causes COVID-19.2 COVID-19 has a broad spectrum of clinical characteristics, ranging from asymptomatic to life-threatening conditions. In the early stages, most patients show mild clinical symptoms, including fever, dry cough, myalgia, and fatigue. However, around 20% rapidly progress to severe illness characterized by acute respiratory distress syndrome (ARDS) and multiorgan dysfunction3 and almost 10% of these critically ill patients do not survive.3,4 Overall, 2.3% of people with COVID-19 die but the risk of death is significantly influenced by age.5 Systemic dysregulation of the inflammatory response is a major contributor associated with death in patients with COVID-19.4 , 6
Accumulating evidence has suggested a potential role for triglyceride, a hydrophobic lipid molecule, in inflammation. Triglyceride promotes leukocyte activation in humans7 and triglyceride primes the aortic endothelium for an enhanced inflammatory response to cytokine stimulation.8 Blood triglyceride levels are increased in obesity and diabetes.9 , 10 Moreover, obesity and diabetes are risk factors of disease severity and mortality in patients with COVID-19.11 , 12 However, whether triglyceride levels are independently associated with either an exaggerated inflammation activity and/or increased mortality in patients with COVID-19 is unknown.
In the current study, we performed a retrospective investigation to examine the association between triglyceride levels and mortality in hospitalized patients with COVID-19.
Methods
Study design and population. We retrospectively reviewed hospitalized patients at a large urban tertiary care academic medical center in Milwaukee, Wisconsin. Subjects with COVID-19 diagnosis (ICD10CM:U07.1) and/or SARS-CoV-2 positive test between March 1, 2020 and December 21, 2020 were identified using Clinical Research Data Warehouse query tools offered by the Clinical & Translational Science Institute at the Medical College of Wisconsin (MCW). Positive SARS-CoV-2 testing results include SARS-CoV-2 RNA presence in respiratory specimen by nucleic acid amplification with probe detection, and/or SARS-CoV-2 IgG Ab presence in serum or plasma by immunoassay. 600 inpatients with age, gender, BMI (body weight index), medical history and laboratory tests, were included in the study.
Data collection. Demographic characteristics, medical histories, medication administered, and laboratory test data of de-identified participants were extracted using Froedtert Health System Honest Broker Data Extraction Tools. The Clinical Research Data Warehouse has a waiver of informed consent for fully de-identified data from the MCW Institutional Review Board. Laboratory test results including blood triglyceride, CRP and leukocyte counts were collected. The first-documented clinical and laboratory test data at admission were considered baseline. Peak triglyceride, CRP and leukocyte counts were defined as the highest levels of those reached during the entire hospitalization period. Obesity was defined as BMI ≥ 30 kg/m2.13 Hypertriglyceridemia was defined as blood triglyceride ≥ 150 mg/dL.14 , 15 High CRP was defined as CRP ≥ 3 mg/L.16 High leukocyte count was defined as ≥ 10 × 109/L.17 Information of glucocorticoids treatment during hospitalization period was collected. Logistic regression analyses were performed to evaluate the association between hypertriglyceridemia and mortality. Pearson correlation analyses were used to evaluate the relation of CRP and leukocyte counts with triglyceride. Diabetes (ICD10CM: E08-E13) and hypertension (ICD10CM: I10) were defined based on documented clinical diagnosis.
Statistical analysis. Numerical variables were expressed as “median (interquartile range, IQR)”. Categorical variables were expressed as “percentages (n numbers)”. Differences in numerical variables between groups were analyzed by the Mann-Whitney U test, and categorical variables were analyzed by the chi-square test. The Pearson's correlation and the multiple linear regression analysis were used to evaluate the association of CRP and leukocyte count with triglyceride. Logistic regression models were used to perform association analyses and Concordance statistic (C-statistic) was used to evaluate the accuracy of the logistic regression model. All statistical analyses were carried out using SPSS software (version 20.0; SPSS Inc., Cary, Chicago, USA). For all analyses, two-tailed p values < 0.05 were considered statistically significant.
Results
Baseline characteristics of study population
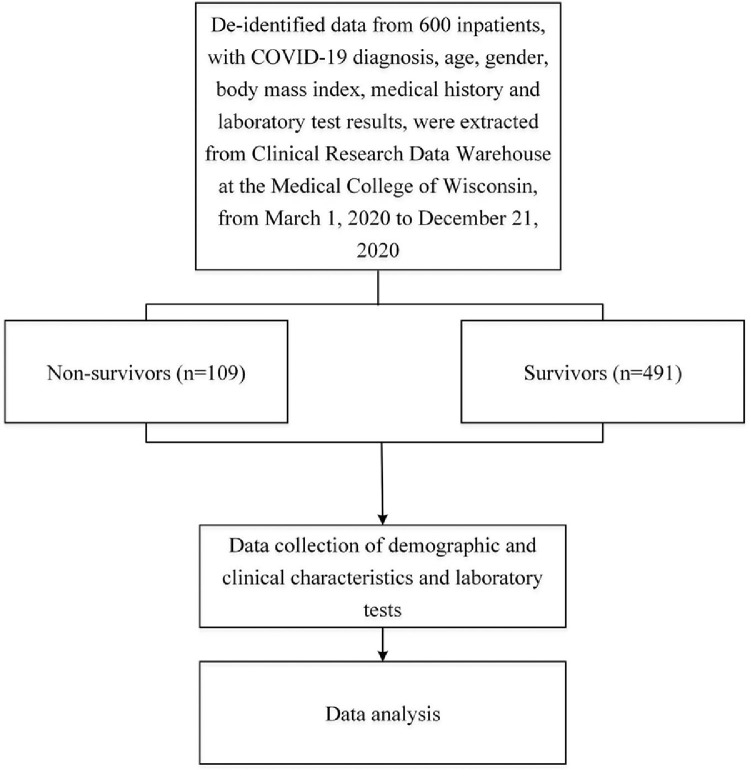
The flow diagram of study population inclusion was shown in Fig. 1 . A total of 600 inpatients with COVID-19, consisting of 307 males (51.2%), were included in the study (Fig. 1). Median age was 66 (IQR, 53–77) years and median BMI was 30.1 (IQR, 25.0–35.7) kg/m2 (Table 1 ). Baseline characteristics of all participants were shown in Table 1. The 109 patients (18.2%) who died were older than those who survived and were predominantly male. Non-survivors had higher median leukocyte count yet remained in the normal range. Blood CRP levels were higher in non-survivors, and total cholesterol levels were lower in non-survivors. All p < 0.05. There was also a higher prevalence of hypertension in non-survivors (p < 0.01).
Fig. 1.
The flowchart of study population inclusion. COVID-19, coronavirus disease 2019; BMI, body mass index; CRP, C-reactive protein.
Table 1.
Baseline characteristics of study population.
| Total(n = 600) | Non-survivors(n = 109) | Survivors(n = 491) | p | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 66 (53–77) | 75 (67–84) | 63 (51–75) | < 0.001 |
| Male percentage,% (n) | 51.2 (307) | 60.6 (66) | 49.1 (241) | 0.034 |
| BMI, kg/m2 | 30.1 (25.0–35.7) | 28.8 (23.6–35.5) | 30.3 (25.6–35.8) | 0.104 |
| Obesity percentage,% (n) | 50.3 (302) | 44.0 (48) | 51.7 (254) | 0.034 |
| Hypertension,% (n) | 61.0 (366) | 72.5 (79) | 58.5 (287) | 0.007 |
| Diabetes,% (n) | 36.0 (216) | 40.4 (44) | 35.0 (172) | 0.321 |
| Biochemical parameters | ||||
| Platelet, x109/L | 211 (171–259) | 208 (159–249) | 212 (171–261) | 0.239 |
| Leukocyte count, x109/L | 6.8 (5.2–9.5) | 7.6 (5.5–10.4) | 6.6 (5.1–9.1) | 0.025 |
| Total cholesterol, mmol/L * | 154 (130–188) | 144 (119–162) | 156 (131–190) | 0.039 |
| Triglyceride, mg/dl | 114 (86–167) | 127 (85–189) | 113 (87–164) | 0.213 |
| CRP, mg/L | 4.8 (1.8–10.6) | 9.6 (3.6–17.4) | 4.4 (1.6–9.4) | <0.001 |
Data are shown as median (interquartile range), or percentages (n) as appropriate. P values were from analyses of the Mann-Whitney U test, or Chi-Square test between non-survivors and survivors. Two-tailed p < 0.05 was considered statistically significant. BMI, body mass index; CRP, C-reactive protein. * Data of total cholesterol level was available for 248 subjects, including 231 survivors and 17 non-survivors.
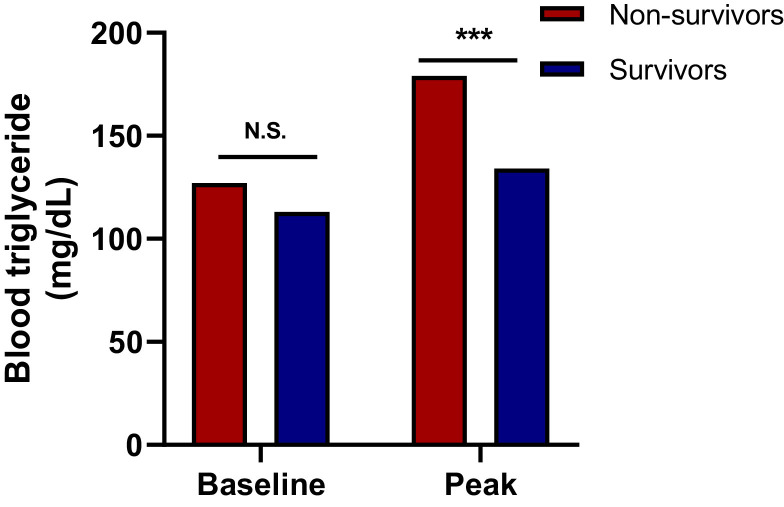
Peak triglyceride level during hospitalization
Non-survivors had higher median peak triglyceride levels than survivors (179 vs. 134 mg/dL, p < 0.001; Table 2 , Fig. 2 ), while baseline levels were not statistically different (127 vs. 113 mg/dL, p = 0.213; Table 1), suggesting a more prominent increase in blood triglyceride in non-survivors during their hospitalization.
Table 2.
Peak values of triglyceride, C-reactive protein, and leukocyte count in COVID-19 patients during hospitalization.
| Total | Non-survivors | Survivors | p | |
|---|---|---|---|---|
| (n = 600) | (n = 109) | (n = 491) | ||
| Triglyceride, mg/dl | 139 (100–216) | 179 (108–381) | 134 (99–199) | < 0.001 |
| CRP, mg/L | 8.9 (3.8–16.8) | 17.5 (11.8–28.9) | 7.3 (3.3–14.0) | < 0.001 |
| Leukocyte count, x109/L | 11.4 (8.1–16.1) | 17.2 (11.8–25.6) | 10.6 (7.8–14.6) | < 0.001 |
Data are shown as median (interquartile range). P values were from analyses of the Mann-Whitney U test between non-survivors and survivors group. Two-tailed p < 0.05 was considered statistically significant. CRP, C-reactive protein.
Fig. 2.
Blood levels of triglyceride in the study population (Table 2). The first-documented clinical and laboratory test data at admission were considered baseline. Peak triglyceride values were defined as the highest levels of those reached during the entire hospitalization period. Results are shown as median. Asterisks represent statistical significance at *p < 0.05 or ***p < 0.001; N.S., non-significant, using Mann-Whitney U test.
Association between hypertriglyceridemia and mortality
In a univariate logistic regression analysis, presence of hypertriglyceridemia after hospital admission was associated with 2 times higher mortality [odds ratio (OR) = 2.0, 95% confidential interval (CI), 1.3–3.0, p = 0.001] (Table 3 ). Moreover, the co-morbidity of hypertension along with high CRP and high leukocyte counts were associated with higher mortality (Table 3). Pre-existing obesity and diabetes are risk factors for severity/mortality in patients with COVID-19,11 , 12 and hypertriglyceridemia is often seen in these conditions.9 We therefore used multivariable logistic regression analysis to test whether hypertriglyceridemia associated with mortality independently of diabetes, obesity, age, gender, hypertension, high CRP, high leukocyte count and glucocorticoid treatment. We found thathypertriglyceridemia associated with 2.3 times higher mortality after adjusting for those factors (OR = 2.3, 95% CI, 1.4–3.7, p = 0.001, C-statistic = 0.796) (Table 3). In summary, these data indicated that the association between hypertriglyceridemia and mortality was independent of diabetes and obesity, the known contributing factors to hypertriglyceridemia. Moreover, the association was independent of other risk factors of mortality including hypertension, high CRP, and high leukocyte count (Table 3). However, we found that the presence of hypertriglyceridemia at baseline was not associated with mortality, suggesting that increased triglyceride levels during hospitalization may be useful in assessing mortality risk in patients with COVID-19.
Table 3.
Logistic analysis of the association between hypertriglyceridemia during hospitalization and mortality.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Presence of hypertriglyceridemia | 2.0 (1.3–3.0) | 0.001 | 2.3 (1.4–3.7) | 0.001 |
| Age | 1.0 (1.0–1.1) | < 0.001 | 1.0 (1.0–1.1) | < 0.001 |
| Male gender | 1.6 (1.0–2.4) | 0.031 | 1.6 (1.0–2.6) | 0.050 |
| Obesity | 0.7 (0.5–1.1) | 0.147 | 1.0 (0.6–1.7) | 0.854 |
| Presence of hypertension | 1.9 (1.2–3.0) | 0.007 | 1.5 (0.9–2.5) | 0.166 |
| Presence of diabetes | 1.3 (0.8–1.9) | 0.294 | 0.8 (0.5–1.3) | 0.342 |
| Presence of high CRP | 6.5 (2.6–16.3) | < 0.001 | 4.1 (1.5–10.6) | 0.005 |
| Presence of high leukocyte count | 5.0 (2.8–8.7) | < 0.001 | 4.2 (2.3–7.7) | < 0.001 |
| Glucocorticoid treatment | 1.3 (0.8–1.9) | 0.286 | 1.0 (0.6–1.5) | 0.889 |
P values from analysis of the logistic regression analysis. Two-tailed p < 0.05 was considered statistically significant. BMI, body mass index; CRP, C-reactive protein; OR, odds ratio; CI, confidence interval. Obesity was defined as BMI ≥ 30 kg/m2. Hypertriglyceridemia was defined as blood triglyceride ≥ 150 mg/dL. High CRP was defined as CRP ≥ 3 mg/L. High leukocyte count was referred to leukocyte counts ≥ 10 × 109/L.
No correlation between glucocorticoid use and mortality in COVID-19 patients during hospitalization
SARS-CoV-2 infection-induced hyper-inflammatory response is associated with disease severity and mortality in COVID-19 patients.18 As anti-inflammation agents, glucocorticoids have been used to treat COVID-19 patients with hyper-inflammatory phenotype.19, 20, 21, 22 Increased blood triglyceride is a side effect associated with glucocorticoid treatment.23, 24, 25 In order to investigate whether glucocorticoid treatment affected the association between hypertriglyceridemia and mortality in our study, we collected information on glucocorticoid treatment during hospitalization period, including the use of betamethasone, dexamethasone, hydrocortisone, methylprednisolone, prednisone and budesonide. In our study population, 47.7% (286/600) of COVID-19 patients received glucocorticoid therapy (Table 4 ). However, there was no significant difference in the percentage of glucocorticoid usage between non-survivors (52.3%, 57/109) and survivors (46.6%, 229/491) (p = 0.291) (Table 4). Furthermore, no association was found between the use of glucocorticoids and mortality in both the univariable and multivariable logistic regression analyses (Table 3). Moreover, the association between hypertriglyceridemia and mortality remained significant after adjusting the glucocorticoid usage in the logistic regression analysis (Table 3), indicating the association between hypertriglyceridemia and morality was not dependent on the glucocorticoid use in our study population.
Table 4.
Medications of glucocorticoid in COVID-19 patients during hospitalization.
| Total | Non-survivors | Survivors | p | |
|---|---|---|---|---|
| (n = 600) | (n = 109) | !(n = 491) | ||
| Glucocorticoid, percentage,% (n) | 47.7 (286) | 52.3 (57) | 46.6 (229) | 0.291 |
Data are shown as percentages (n). P values were from analyses of the Chi-Square test between non-survivors and survivors group. Two-tailed p < 0.05 was considered statistically significant.
Correlation of CRP and leukocyte count with triglyceride
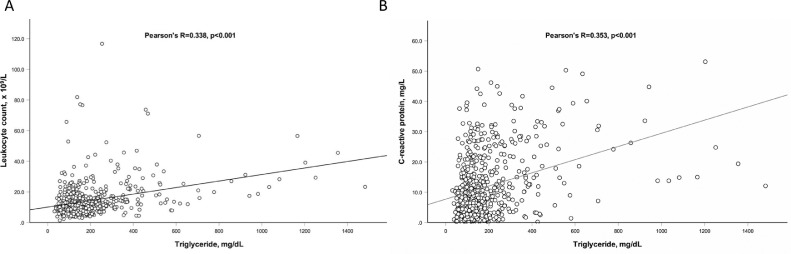
To evaluate whether increased blood triglyceride was associated with enhanced inflammatory activity, we first analyzed the correlation of the peak values of CRP and leukocyte count with the peak value of triglyceride using Pearson's correlation analysis and found both peak CRP (R = 0.353, p < 0.001) and peak leukocyte count (R = 0.338, p < 0.001) positively correlated with peak triglyceride (Fig. 3 ), suggesting the close relation between blood triglyceride and inflammation. Moreover, after adjusting glucocorticoid use, the association of CRP or leukocyte count and triglyceride level remained significant in a multiple linear regression model (β = 0.273 for CRP, β = 0.255 for leukocyte count, both p < 0.001), indicating the correlation of CRP or leukocyte count with triglyceride is independent on glucocorticoid use in our study population (Table 5 ).
Fig. 3.
Correlation of peak leukocyte counts (A) and peak C-reactive protein (B) with peak triglyceride during hospitalization. Peak triglyceride, C-reactive protein and leukocyte counts were defined as the highest levels of those reached during the entire hospitalization period. Pearson's correlation analysis was used to evaluate the association of peak C-reactive protein and peak leukocyte count with peak triglyceride levels.
Table 5.
Multiple linear regression analysis of the correlation of blood CRP and leukocyte count with triglyceride adjusting glucocorticoids use.
| Variables | Standardized coefficients, β | p |
|---|---|---|
| CRP | 0.273 | < 0.001 |
| Leukocyte count | 0.255 | < 0.001 |
P values from analysis of the multiple linear regression analysis. Two-tailed p < 0.05 was considered statistically significant. CRP, C-reactive protein.
Discussion
In the current study, we found that hypertriglyceridemia was independently associated with mortality in hospitalized COVID-19 patients. Furthermore, CRP and leukocyte counts were positively correlated with blood triglyceride levels. Our findings provided new evidence about the potential effects of hypertriglyceridemia in the assessment mortality risk in COVID-19 patients.
The positive correlation of CRP and leukocyte count with triglyceride levels suggests that the hypertriglyceridemia in COVID-19 might be due to the immune-inflammatory response caused by SARS-CoV-2 infection. In fact, infection and inflammation have been shown to induce hypertriglyceridemia via inhibiting blood triglyceride clearance.26, 27, 28 The decrease in clearance of triglyceride is mostly due to lowered amount and/or activity of lipoprotein lipase (LPL), the key enzyme that catabolizes triglyceride in the circulation.26 , 29 LPL is attached to the luminal surface of endothelial cells in capillaries mostly by heparan sulfated peptidoglycans (HSPG) to facilitate its effect on triglyceride hydrolysis.30 During severe infections, HSPGs, such as syndecan, are removed from the endothelium leading to the dysfunction of LPL and subsequent hypertriglyceridemia.31 , 32 A recent clinical case report showed changes in serum syndecan-1 level closely reflected the change in disease conditions in a patient with COVID-19.33 Moreover, recent studies have also shown decreased LPL caused by the inflammatory response.28 , 34 , 35
Interestingly, recent studies also suggested an effect of triglyceride on promoting inflammation. Fat-loading-induced hypertriglyceridemia in healthy human subjects leads to activation of leukocytes with the greatest effects on neutrophils and monocytes.7 Another study demonstrated that hypertriglyceridemia promoted extravasation of monocytes and facilitated accumulation of tissue macrophages.36 Moreover, triglyceride has been shown to prime aortic endothelium for an enhanced inflammatory response to cytokine stimulation.8 These findings suggest the possibility that hypertriglyceridemia may have a more direct detrimental effect on COVID-19 patients due to leukocyte activation.
CRP and leukocyte counts are widely used as systemic inflammatory markers.16 , 17 We found higher peak CRP level and leukocyte count during hospitalization compared to baseline in patients with COVID-19, suggesting enhanced inflammatory response during hospitalization. Systemic inflammatory response and multiple organ failure are believed to be the main cause of death in patients with COVID-19.4 , 6 We found that CRP and leukocyte counts significantly associated with mortality in the current study. Importantly, after adjusting CRP and leukocyte counts, the association between hypertriglyceridemia and mortality remained significant suggesting an incremental value of hypertriglyceridemia in assessing mortality risk in COVID-19 patients.
Glucocorticoids are potent anti-inflammatory and immune-suppressive agents,37, 38, 39 and therefore have been used to treat hyper-inflammatory disorders, such as autoimmune diseases40 , 41 and virus infection.42, 43, 44 However, the effect of glucocorticoids on clinical outcome in COVID-19 patients remain controversial, which requires further investigation.19, 20, 21, 22 , 45 In our study, we found that glucocorticoid treatment during hospitalization did not associate with mortality in patients with COVID-19. Moreover, hypertriglyceridemia associated with mortality after adjusting glucocorticoid usage, indicating the association between hypertriglyceridemia and mortality is independent of glucocorticoid treatment.
Limitations of this study. Our findings that hypertriglyceridemia is independently associated with 2.3 times higher mortality rate raises new questions about disease pathogenesis in COVID-19. This single-center retrospective study was conducted in hospitalized patients from Milwaukee, Wisconsin that experienced higher rates of hospitalization in Blacks/African American patients with COVID-19.46 Although we still were able to include a good sample size of 600 hospitalized COVID-19 patients fitting the inclusion criteria, 735 hospitalized patients with COVID-19 from the MCW Clinical Research Data Warehouse were excluded because of the limited data availability of BMI, age, blood triglyceride and CRP levels (Fig. 1). Accordingly, prospective studies are needed to independently validate our retrospective analysis and further investigate the impact of race and ethnicity on disease severity and mortality.
In conclusion, hypertriglyceridemia during hospitalization is associated with 2.3 times higher mortality in patients with COVID-19, independent of pre-existing conditions including diabetes, obesity, and hypertension.
Funding
This study was funded by a COVID-19 Rapid Response Grant from the Cullen Run Foundation and the Medical College of Wisconsin Cardiovascular Center (to ZZ), a startup fund from the Medical College of Wisconsin and Blood Research Institute through the Advancing a Healthier Wisconsin Endowment (to ZZ), a Fellow Scholar Award from the American Society of Hematology (to ZZ), and a Career Development Award from the American Heart Association (to ZZ, 19CDA34660043), a startup fund from the Medical College of Wisconsin (to YC), a Scientist Development Grant from the American Heart Association (to YC, 17SDG33661117). The Medical College of Wisconsin Clinical Research Data Warehouse was supported by the NIH National Center for Advancing Translational Sciences (to Clinical & Translational Science Institute of Southeast Wisconsin, UL1TR001436).
Data availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
CRediT authorship contribution statement
Wen Dai: Conceptualization, Writing – original draft, Formal analysis, Writing – review & editing. Hayley Lund: Data curation, Writing – original draft, Writing – review & editing. Yiliang Chen: Writing – original draft, Formal analysis, Writing – review & editing. Jue Zhang: Formal analysis, Writing – review & editing. Kristen Osinski: Data curation, Writing – review & editing. Stephanie Zellner Jones: Data curation, Writing – review & editing. Lisa Baumann Kreuziger: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. José A. López: Conceptualization, Data curation, Writing – review & editing. Ivor J. Benjamin: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Roy L. Silverstein: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Ze Zheng: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors have declared that no relevant conflict of interest exists.
Acknowledgments
We are grateful to all medical professionals at the Froedtert & MCW medical center in Milwaukee, Wisconsin for their dedication to caring for the patients and generating the data included in this study. We thank Drs. Daisy Sahoo, Mary Sorci-Thomas, Shawn Jobe, and Renren Wen for helpful discussions.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Schroder J., et al. Prognosis and reclassification by YKL-40 in stable coronary artery disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perico L., et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fajgenbaum D.C., June C.H. Cytokine storm. New Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alipour A., et al. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:792–797. doi: 10.1161/atvbaha.107.159749. [DOI] [PubMed] [Google Scholar]
- 8.Wang H., Yuan Z., Pavel M.A., Hansen S.B. The role of high cholesterol in age-related COVID19 lethality. bioRxiv. 2020 doi: 10.1101/2020.05.09.086249. [DOI] [Google Scholar]
- 9.Klop B., Elte J.W., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg I.J. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 11.Hendren N.S., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American heart association COVID-19 cardiovascular disease registry. Circulation. 2021;143:135–144. doi: 10.1161/circulationaha.120.051936. [DOI] [PubMed] [Google Scholar]
- 12.Gregory J.M., et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526–532. doi: 10.2337/dc20-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward Z.J., et al. Projected US state-level prevalence of adult obesity and severe obesity. New Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 14.Third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Stone N.J., et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Sproston N.R., Ashworth J.J. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmielewski P.P., Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol (Praha) 2018;77:171–178. doi: 10.5603/FM.a2017.0101. [DOI] [PubMed] [Google Scholar]
- 18.Webb B.J., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., et al. Corticosteroid therapy is associated with improved outcome in critically Ill patients with COVID-19 with hyperinflammatory phenotype. Chest. 2021;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group R.C., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner E.J., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 Outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(481–491):e483. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultze A., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipsson H., Monson J.P., Koltowska-Haggstrom M., Mattsson A., Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91:3954–3961. doi: 10.1210/jc.2006-0524. [DOI] [PubMed] [Google Scholar]
- 24.Berglund L., et al. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–2989. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagdade J.D., Yee E., Albers J., Pykalisto O.J. Glucocorticoids and triglyceride transport: effects on triglyceride secretion rates, lipoprotein lipase, and plasma lipoproteins in the rat. Metabolism. 1976;25:533–542. doi: 10.1016/0026-0495(76)90007-x. [DOI] [PubMed] [Google Scholar]
- 26.Khovidhunkit W., et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Zhai T., Wu X., Zhang N., Huang X., Zhan Q. Inflammatory risk factors for hypertriglyceridemia in patients with severe influenza. J Int Med Res. 2020;48 doi: 10.1177/0300060520918058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robin A.P., et al. Lipoprotein lipase activity in surgical patients: influence of trauma and infection. Surgery. 1981;90:401–408. [PubMed] [Google Scholar]
- 29.Laufs U., Parhofer K.G., Ginsberg H.N., Hegele R.A. Clinical review on triglycerides. Eu. Heart J. 2020;41:99–109c. doi: 10.1093/eurheartj/ehz785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berryman D.E., Bensadoun A. Heparan sulfate proteoglycans are primarily responsible for the maintenance of enzyme activity, binding, and degradation of lipoprotein lipase in Chinese hamster ovary cells. J Biol Chem. 1995;270:24525–24531. doi: 10.1074/jbc.270.41.24525. [DOI] [PubMed] [Google Scholar]
- 31.Gopal S. Syndecans in inflammation at a glance. Front Immunol. 2020;11:227. doi: 10.3389/fimmu.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanford K.I., et al. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/jci38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K., et al. Possible involvement of Syndecan-1 in the state of COVID-19 related to endothelial injury. Thromb J. 2021;19:5. doi: 10.1186/s12959-021-00258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu B., Moser A., Shigenaga J.K., Grunfeld C., Feingold K.R. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–1741. doi: 10.1016/j.bbrc.2009.12.145. [DOI] [PubMed] [Google Scholar]
- 35.Sammalkorpi K., Valtonen V., Kerttula Y., Nikkilä E., Taskinen M.R. Changes in serum lipoprotein pattern induced by acute infections. Metab Clin Exp. 1988;37:859–865. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 36.Saja M.F., et al. Triglyceride-rich lipoproteins modulate the distribution and extravasation of Ly6C/Gr1(low) monocytes. Cell Rep. 2015;12:1802–1815. doi: 10.1016/j.celrep.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swantek J.L., Cobb M.H., Geppert T.D.Jun. N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/MCB.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 40.Chester Wasko M., Dasgupta A., Ilse Sears G., Fries J.F., Ward M.M. Prednisone use and risk of mortality in patients with rheumatoid arthritis: moderation by use of disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken) 2016;68:706–710. doi: 10.1002/acr.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truelove S.C., Watkinson G., Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J. 1962;2:1708–1711. doi: 10.1136/bmj.2.5321.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monton C., et al. Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. Eur Respir J. 1999;14:218–220. doi: 10.1034/j.1399-3003.1999.14a37.x. [DOI] [PubMed] [Google Scholar]
- 43.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J.W., et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:956–963. doi: 10.1016/j.cmi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Robinson P.C., Morand E. Divergent effects of acute versus chronic glucocorticoids in COVID-19. Lancet Rheumatol. 2021;3:e168–e170. doi: 10.1016/S2665-9913(21)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz-Price L.S., et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.