Abstract
Among sarcomas with a round cell morphology that lack rearrangement of the EWSR1 gene, rearrangements involving the CIC gene are the most common. In comparison with Ewing Sarcoma, CIC-rearranged sarcomas present at an older average age, arise almost exclusively in soft tissues, are clinically more aggressive, and are more likely to be resistant to the chemotherapy regimens used for Ewing sarcoma. CIC-rearranged sarcomas present more commonly in a deep location, and we suspect that superficial presentations may be underrecognized. In this case series, we report three of such cases. Overall, the morphology is similar to CIC-rearranged sarcomas of deeper locations. We hope to raise awareness among the dermatopathology community by expanding the differential of superficial tumors with round cell morphology.
Keywords: CIC, Round Cell Sarcoma, CIC-DUX4, CIC-DUX, CIC-Rearranged
1. Introduction
Among the category of round cell sarcomas that lack EWSR1 rearrangements, the most common recurrent genetic alteration is rearrangement of the CIC gene (19q13.2) with a resulting gene fusion.1 This translocation was described in karyotypes as early as 1996 and has been the subject of a number of case reports.2 In 2012–2013, there were three case series that provided significantly more information about this entity and helped to draw attention to it.3–5 In 2017, Antonescu et al. published an extensive case series of 115 patients with CIC-rearranged sarcomas, detailing their clinical and molecular features.1 CIC-rearranged sarcomas, in comparison with Ewing sarcoma, present at an older average age, arise almost exclusively in soft tissues, are clinically more aggressive, and are more likely to be resistant to the chemotherapy regimens used for Ewing sarcoma.
Most CIC-rearranged sarcomas present in deep tissues, but they can also present in a superficial location, such as the dermis and subcutaneous tissue.6 Herein we report three cases of superficial CIC-rearranged sarcomas in an effort to raise awareness among dermatopathologists by expanding the differential of superficial tumors with round cell morphology.
2. Case Reports
2.1. Case 1
A 55-year-old woman without any history of malignancy presented to a general surgeon for a mass on her left shoulder. The mass was first noticed by the patient eight months prior. It was painful and increasing in size. Physical examination revealed a large, “softball-sized” lobulated mass with necrotic surface and subsequently an incisional biopsy was performed.
The biopsy specimen consisted of two fragments of friable red-brown tissue. Microscopic examination showed an ulcerated, multilobulated tumor with zonal necrosis, involving the entire thickness of the dermis and extending to all specimen margins (Fig. 1A). The tumor lobules were composed of small round cells with pale to vacuolated cytoplasm and slightly irregular nuclei, embedded in a myxoid stroma (Fig. 1B). Focally the tumor nodules consisted of larger cells with more striking pleomorphism. These cells exhibited abundant clear cytoplasm, round nuclei, and prominent nucleoli (Fig. 1C). Mitotic rate was brisk throughout the tumor.
Figure 1.
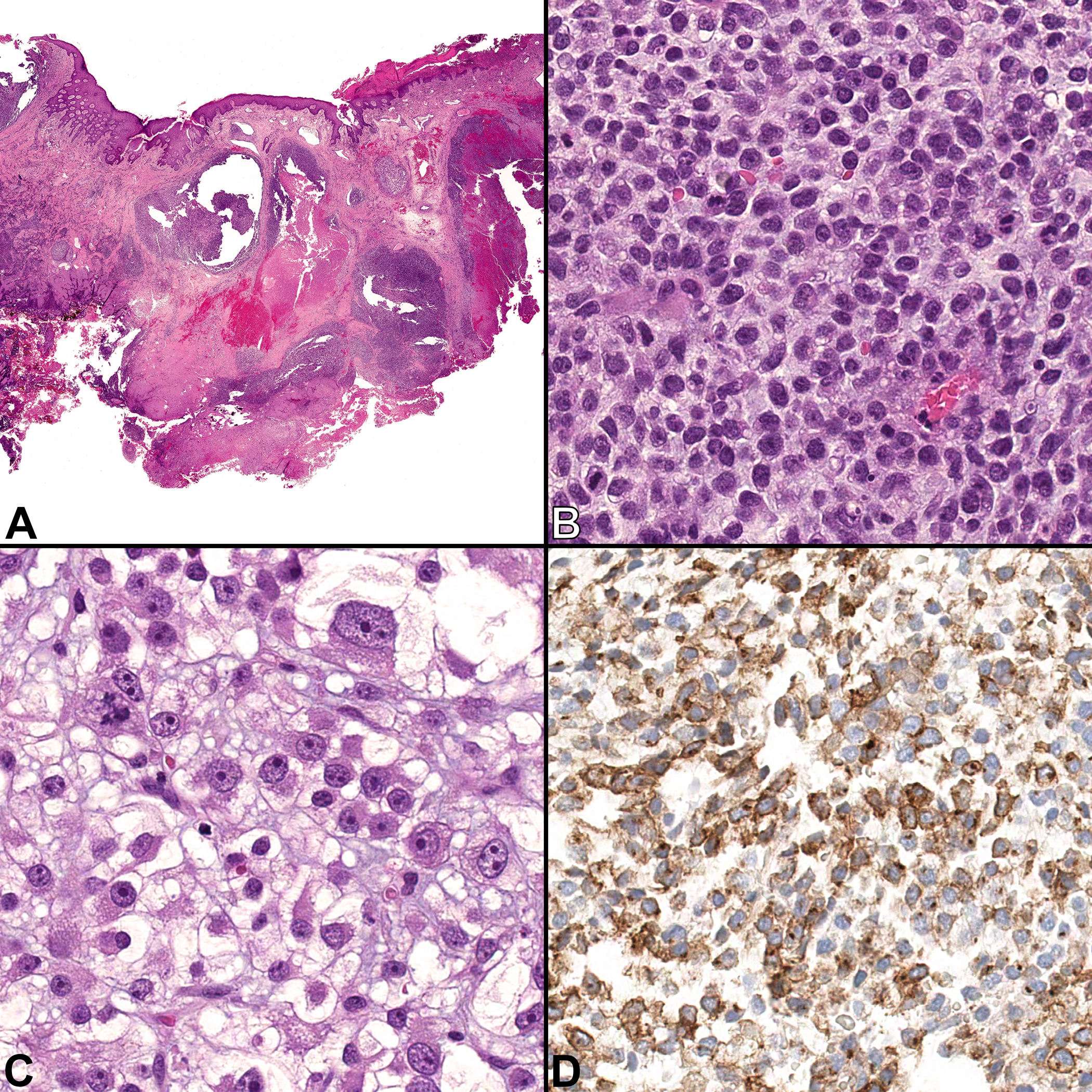
Case 1, incisional biopsy of a shoulder mass. A) A multilobulated tumor involving the entire thickness of the dermis. Ulceration and zonal necrosis can be seen in the right side of the image. [H&E, ×40] B) Most of the tumor lobules are composed of sheets of uniform small round blue cells with little vacuolated cytoplasm and slightly irregular nuclei, embedded in a myxoid stroma. Mitoses are frequent. [H&E, ×400] C) Rare tumor nodules consist of markedly larger cells with abundant clear cytoplasm, pleomorphic nuclei, and prominent nucleoli. [H&E, ×400] D) The tumor is positive for CD99 with membranous and paranuclear staining patterns. [CD99 immunohistochemistry, ×400]
By immunohistochemistry, the tumor demonstrated patchy staining for CD99 (membranous and paranuclear dot-like patterns, Fig. 1D) and diffuse staining for vimentin. Focal expression of cytokeratin cocktail (CK AE1/AE3 and Cam5.2) and MNF116 was noted in the pleomorphic component only. The tumor was negative for S100-protein, SOX10, CK5/6, p63, EMA, MyoD1, myogenin, HMB45, Melan-A, CD57, chromogranin, synaptophysin, CD45, CD20, CD3, smooth muscle actin, desmin, and ERG. Expression for INI-1 was retained.
Dual-color fluorescence in situ hybridization (FISH) using a break-apart probe to CIC (19q13) as previously described was performed on a 4-micrometer-thick section.7 A positive result was defined as >10% nuclei with split signals. Dual-color FISH using a break-apart probe to EWSR1 (22q12) was also performed as previously described.5 Rearrangement of CIC was detected, whereas EWSR1 rearrangement was absent. These findings, coupled with the histomorphologic features and immunophenotype, supported the diagnosis of cutaneous CIC-rearranged sarcoma.
2.2. Case 2
A 50-year-old man presented with 10-year history of a slowly growing, painful mass on the left thigh that had begun enlarging more rapidly over the last year. Physical examination revealed a freely mobile subcutaneous mass that was approximately 3 cm in size. The patient was otherwise asymptomatic and free of other skin lesions. An initial excisional biopsy was performed that was followed by a wide local excision with a sentinel lymph node biopsy. Although identified as malignant, no definitive diagnosis was made on this initial resection. Approximately three months later, the patient noticed a second nodule adjacent to the scar of the first resection, which was resected. The patient subsequently developed bilateral lung masses and a right parietal scalp nodule, identified on PET scan, which were also resected.
The parietal scalp nodule was received as a 1.2 cm ellipse of skin with an unremarkable epidermal surface. Cross-sectioning revealed a single tan 1 cm nodule within the dermis and subcutaneous adipose tissue.
Microscopic examination confirmed the presence of a nodule within the dermis and subcutaneous adipose tissue (Fig. 2A) which comprised nests and sheets of epithelioid cells with a vague rhabdoid cytology that extended to lateral specimen borders and a background of fibrosis (Fig. 2B and 2C). The lesional cells were immunohistochemically positive for CD56, BCL-2, CD99 (Fig. 2D), and INI1; they were negative for MNF116, CK20, EMA, S100, desmin, CD34, myeloperoxidase, CD3, CD4, CD8, CD68, kappa and lambda light chains, beta-catenin, synaptophysin, chromogranin, and PD-L-1.
Figure 2.
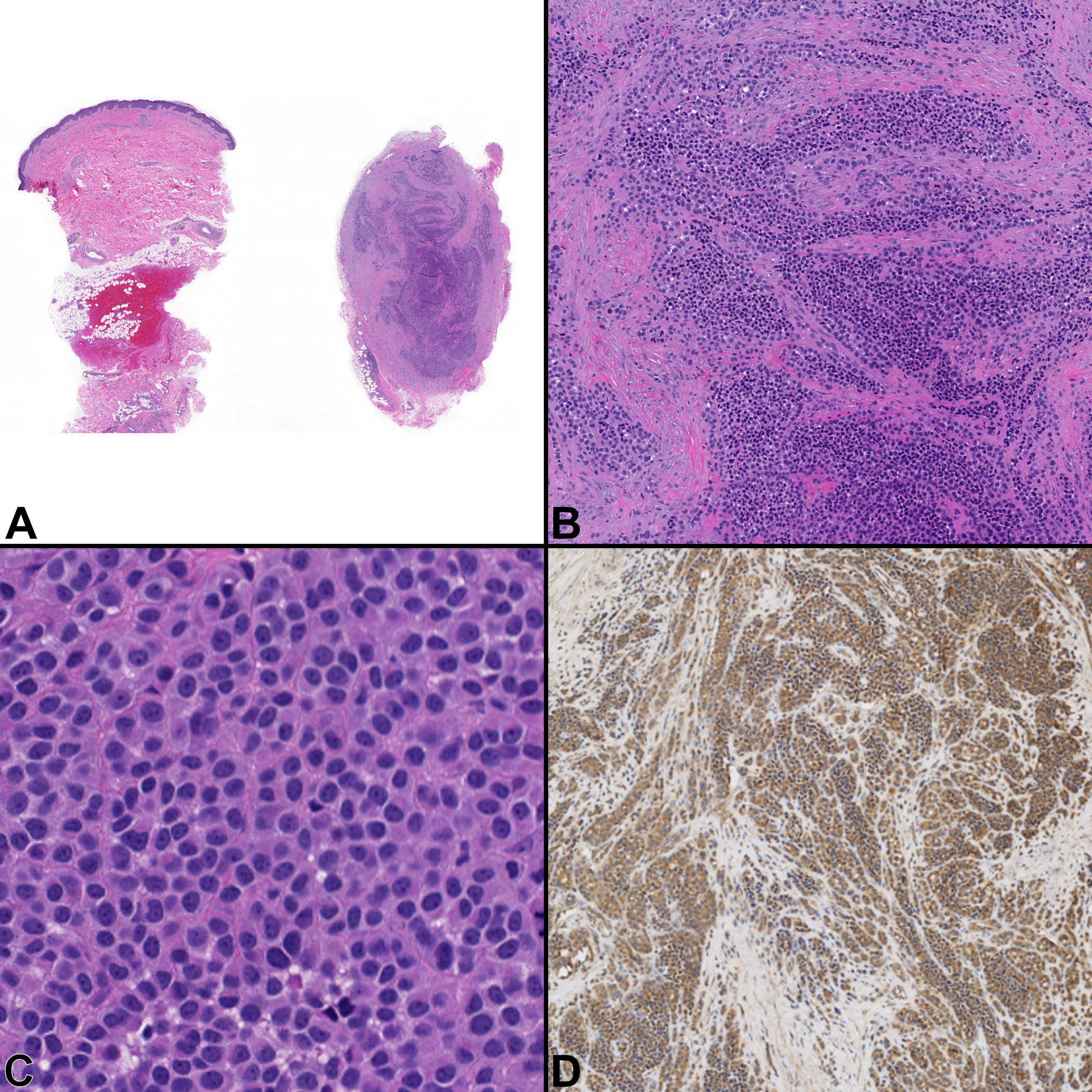
Case 2, Superficial thigh mass with metastasis to superficial scalp. A) Low power view of the specimen (fragmented into two pieces) with the mass present in the dermis and subcutaneous adipose tissue. [H&E, ×20] B) Tumor cells have a relatively monotonous, round cell morphology diffusely infiltrating surrounding tissues in a background of fibrosis. [H&E, ×100] C) A slight rhabdoid morphology can be appreciated at higher magnification. [H&E, ×400] D) They are diffusely positive for CD99. [×100]
Molecular analysis was performed at a reference laboratory. RT-PCR was negative for EWSR1/FLI1 and SS18/SSX fusion transcripts. Dual-color FISH using a break-apart probe to CIC (19q13) demonstrated homozygous deletion of the 3’ (telomeric) CIC (19q13.2) locus in 190 (95%) of the 200 interphase nuclei examined. Dual-color break-apart FISH for the BCOR (Xp11.4) locus was negative. By targeted next generation sequencing (NGS), the tumor was found to have loss of CDKN2A/B and CIC, was microsatellite stable, and had a low tumor mutation burden (4 muts/Mb). Based on the histomorphologic findings and the CIC alterations by FISH and NGS assays, a diagnosis of CIC-rearranged sarcoma was rendered.
2.3. Case 3
A 27-year-old woman presented to an orthopedic surgeon with a complaint of a recently identified palpable nodule in her left leg that was superficially located. A wide local excision with grossly negative margins was subsequently performed. Gross examination of the mass demonstrated a well-circumscribed mass centered in the subcutaneous adipose tissue that appeared to abut the dermis.
Microscopically, it was well-circumscribed and composed of round cells with a vesicular chromatin pattern and background hemangiopericytomatous vasculature (Fig. 3A–B). In between the cells, there was myxoid as well as collagenous stroma and occasional interspersed giant cells (Fig. 3C). There were ten mitotic figures per ten high-power fields. Immunohistochemical stains for S100-protein, EMA, and CD99 (Fig. 3D) were multifocally positive, whereas CD34, SMA, STAT6, MNF116, ERG, SOX10, and desmin were negative.
Figure 3.
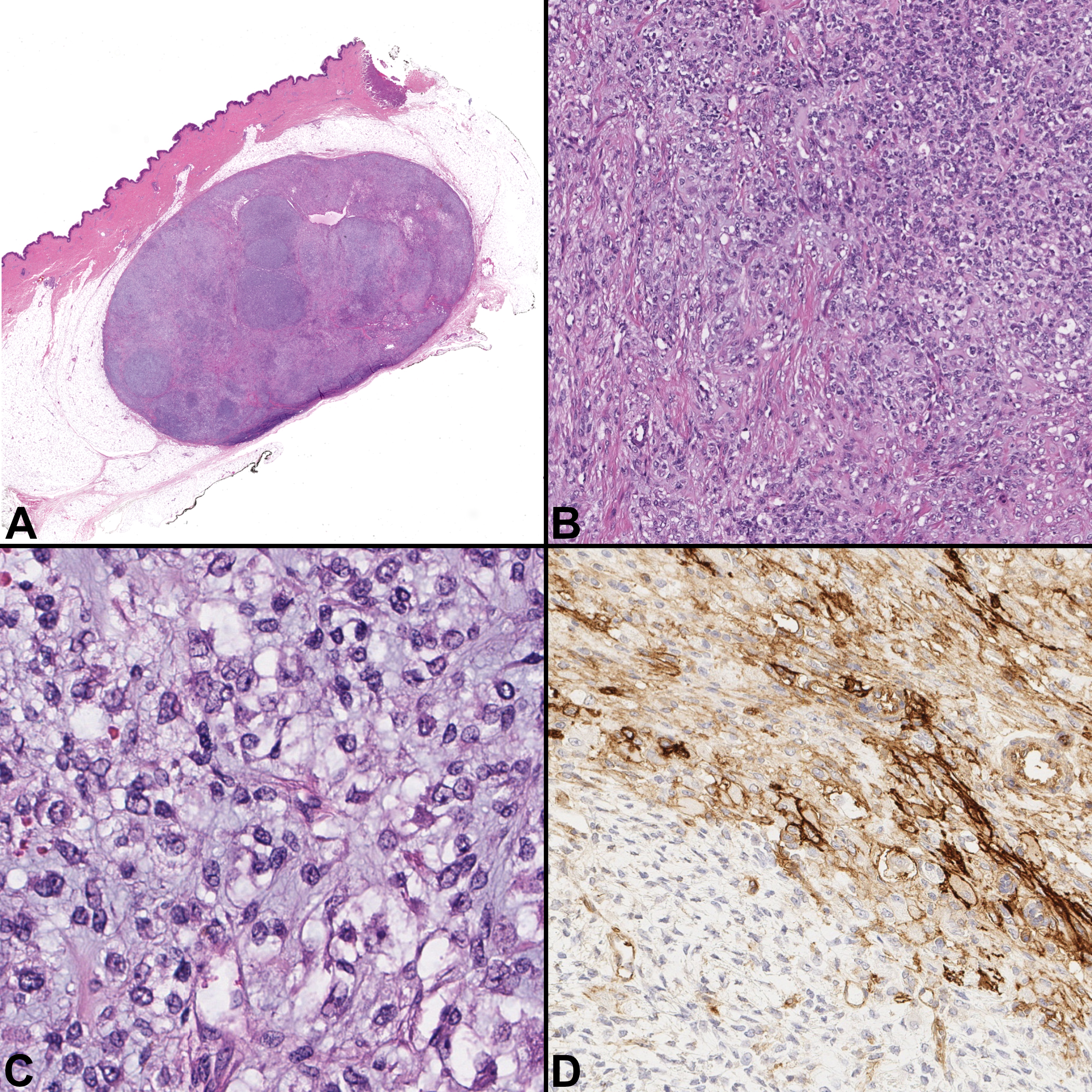
Case 3, Superficial leg mass. A) The nodule is well circumscribed and centered within the subcutaneous adipose tissue, but focally abuts the dermis. [H&E, ×20] B) Tumor cell appearance is variable from a round cell morphology to a plump, slightly spindled shape. [H&E, ×100] C) Some areas demonstrate a myxoid stroma [H&E, ×400] D) CD99 demonstrates patchy membranous positivity [CD99, ×200]
Fusion of the CIC and DUX4 genes was confirmed by FISH analysis as previously described by Antonescu et al (Fig. 4).1 Based on the morphologic features and the FISH result, a diagnosis of CIC-rearranged sarcoma was made. The patient had a follow-up PET scan which was negative, and the patient was free of disease at 36 months after excision.
Figure 4.
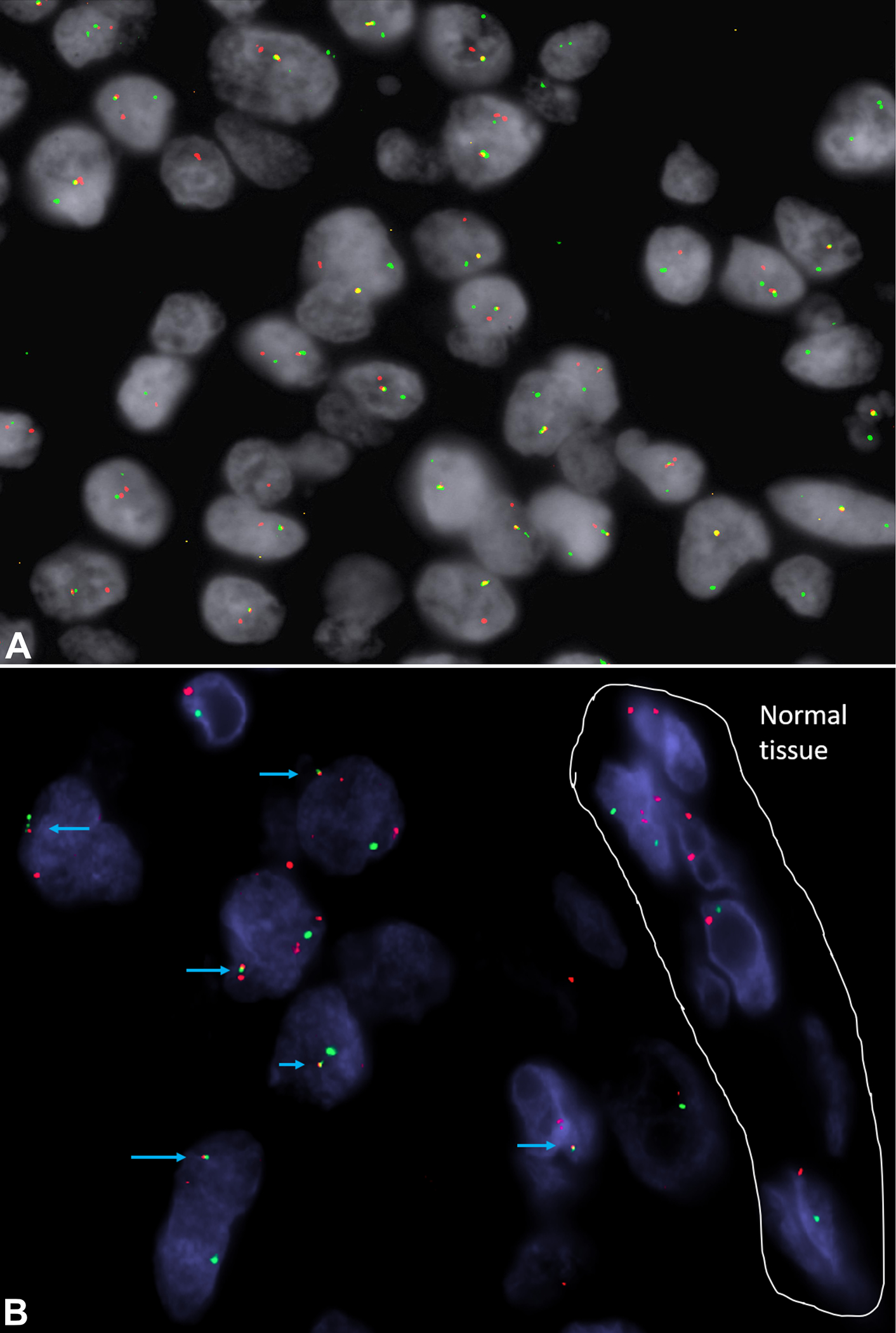
CIC and DUX4 dual color dual fusion fluorescence in situ hybridization (FISH) from case 3. Blue arrows show the fusion signals between the green-telomeric portion of CIC gene and the red centromeric of DUX4. A partial deletion of telomeric CIC is also seen.
Discussion
As the name implies, CIC-rearranged sarcomas are defined by rearrangements of the CIC gene, which is found at 19q13.2. CIC is a high mobility group protein that is important for transcription, replication, recombination, and DNA repair processes. Themost common fusion partner by far is the retrogene DUX4, found on the long arm of chromosome 4 (4q35.2). The fusion results in the disruption of transcriptional repression leading to, among other changes, an upregulation of PEA3 transcription factors (e.g. ETV1/4/5).8 In addition to forming fusions with DUX4 on chromosome 4, CIC also forms fusions with a nearly identical gene (DUX4L) present on the long arm of chromosome 10 (10q26), and rarely other genes such as FOXO4, NUTM1, and NUTM2A.9–12
Like many translocation-associated sarcomas, a relative cellular monotony can be appreciated in CIC-rearranged sarcomas. However, when compared with Ewing sarcoma, the prototypical round cell sarcoma, CIC-rearranged sarcomas demonstrate a slightly increased nuclear size, increased nuclear pleomorphism, increased mitotic rate, and more frequent tumor necrosis. They often have a vaguely nodular morphology, whereas foci of myxoid stroma and spindle cell morphology with fascicle formation are present in some cases.
CD99 is positive in the majority of cases, but it has a much greater variation in staining pattern than in Ewing Sarcoma, which almost universally shows a strong and diffuse membranous staining.1 In cases in which CIC is fused with DUX4, immunohistochemistry for DUX4 was found to be a sensitive and specific marker.13 A case series by Hung et al. evaluated the use of ETV4 and WT1 immunohistochemistry in 40 CIC-rearranged sarcomas and potential histologic mimics and found that they were both very sensitive and specific markers.14
The differential diagnosis for a superficial tumor with a relatively monotonous round cell morphology can potentially be extensive. Also, given that CIC-rearranged sarcomas can occur in a relatively broad age range, this will also influence the breadth of differential diagnosis. Merkel cell carcinoma most often occurs in the elderly but can been seen in a wide age range. It is generally centered in the dermis of sun-damaged skin and has a variety of growth patterns, including nested and solid, with a relatively monotonous cytology. Nuclei are typically stippled to vesicular and mitotic figures are abundant. Expression of CK20 (in a dot-like paranuclear pattern) and neuroendocrine markers such as synaptophysin and chromogranin is typical. Positivity for the Merkel cell polyomavirus is present in the majority of cases.15 Melanoma can arise in the background of a preexisting melanocytic nevus or de novo, can occur in any age, and can mimic the morphology of CIC-rearranged sarcomas, particularly the small cell variant. This variant has a more monomorphic appearance than usual and can have similar appearance to CIC-rearranged sarcomas. The presence of melanin, a background melanocytic nevus, and epidermal involvement would prompt one to consider this diagnosis and a panel of melanocytic markers, such as Melan-A, S100, and SOX-10 are expected to be positive in most cases. The small cell variant of anaplastic large cell lymphoma can occur in the skin in which the predominating cells are monotonous and small. Nevertheless, scattered large atypical cells are still.16 ALK, in a subset, and CD30 immunostains are positive and will help distinguish it from a CIC-rearranged sarcoma. Myoepithelioma can arise in a superficial location and can take on a variety of appearances, but has monotonous, slightly vesicular nuclei with small nucleoli and can mimick the appearance of CIC-rearranged sarcomas. They are usually positive for S100 protein, SOX10, and pan-cytokeratin, and about 50% of cases demonstrate rearrangements of EWSR1. Lastly, leukemia cutis of any type can mimic the round cell morphology of CIC-rearranged sarcomas, but is often relatively easy to separate based on clinical history and appropriate immunostains. CIC-rearranged sarcomas should reliably lack expression of CD45.
Precise and efficient diagnosis of translocation-associated sarcomas has often relied on sensitive and relatively inexpensive fluorescent in situ hybridization (FISH). This is because the FISH assay can be designed to detect multiple different gene fusion sites or can be designed to detect the break apart of a single important gene when there are potentially many partner genes. Nevertheless, two case series published in 2017 have shown that FISH has a relatively low sensitivity in detecting CIC-rearranged sarcomas, missing approximately 15% of cases.17,18 Interestingly there were five cases in the aforementioned series by Hung et al. that were positive for both ETV4 and WT1 by immunohistochemistry, but were negative for CIC break apart by FISH, which raises the possibility of FISH cryptic CIC-rearranged sarcomas.14 RNA sequencing has been shown to have higher sensitivity than FISH for detection of CIC rearrangement, but false negatives may arise with data processing algorithms.17,18
In summary, we report three cases of CIC-rearranged sarcomas that presented in a superficial location. As seen in case 2, both the primary tumor and metastases can be seen superficially. Morphologically, these three cases fall within the spectrum of what has been described in the literature in other sites. Not much attention has been drawn to this aspect of CIC-rearranged sarcomas in the published literature and we suspect that may be an under-recognized presentation of this sarcoma. We reviewed the histology and molecular pathology to help familiarize dermatopathologists with this recently described entity. We hope that increased awareness of the potential for CIC-sarcoma to arise in a superficial location will aid other colleagues in identifying similar cases.
References
- 1.Antonescu CR, Owosho AA, Zhang L, et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. The American journal of surgical pathology. 2017;41(7):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richkind KE, Romansky SG, Finklestein J t. t (4; 19)(q35; q13. 1): a recurrent change in primitive mesenchymal tumors? Cancer genetics and cytogenetics. 1996;87(1):71–74. [DOI] [PubMed] [Google Scholar]
- 3.Italiano A, Sung YS, Zhang L, et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes, Chromosomes and Cancer. 2012;51(3):207–218. doi: 10.1002/gcc.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham C, Chilton-MacNeill S, Zielenska M, Somers GR. The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Human Pathology. 2012;43(2):180–189. doi: 10.1016/j.humpath.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 5.Choi E-YK, Thomas DG, McHugh JB, et al. Undifferentiated small round cell sarcoma with t (4; 19)(q35; q13. 1) CIC-DUX4 fusion: a novel highly aggressive soft tissue tumor with distinctive histopathology. The American journal of surgical pathology. 2013;37(9):1379–1386. [DOI] [PubMed] [Google Scholar]
- 6.Lehane F, Tsikleas G, Bettington A, Limarporn K, Wilkinson L, Lehane K. “Cyst” on the forearm of a 28-year-old female: Case report of a CIC-rearranged sarcoma. Journal of cutaneous pathology. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Smith SC, Buehler D, Choi E-YK, et al. CIC-DUX sarcomas demonstrate frequent MYC amplification and ETS-family transcription factor expression. Modern Pathology. 2015;28(1):57. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura-Saito M, Yamazaki Y, Kaneko K, et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t (4; 19)(q35; q13) translocation. Human molecular genetics. 2006;15(13):2125–2137. [DOI] [PubMed] [Google Scholar]
- 9.Sugita S, Arai Y, Tonooka A, et al. A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: a genetically distinct variant of Ewing-like sarcoma. The American journal of surgical pathology. 2014;38(11):1571–1576. [DOI] [PubMed] [Google Scholar]
- 10.Solomon DA, Brohl AS, Khan J, Miettinen M. Clinicopathologic features of a second patient with Ewing-like sarcoma harboring CIC-FOXO4 gene fusion. The American journal of surgical pathology. 2014;38(12):1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita S, Arai Y, Aoyama T, et al. NUTM2A-CIC fusion small round cell sarcoma: a genetically distinct variant of CIC-rearranged sarcoma. Human Pathology. 2017;65:225–230. doi: 10.1016/j.humpath.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 12.Le Loarer F, Pissaloux D, Watson S, et al. Clinicopathologic Features of CIC-NUTM1 Sarcomas, a New Molecular Variant of the Family of CIC-Fused Sarcomas. doi:info:doi/ 10.1097/PAS.0000000000001187 [DOI] [PubMed] [Google Scholar]
- 13.Siegele B, Roberts J, Black JO, Rudzinski E, Vargas SO, Galambos C. DUX4 immunohistochemistry is a highly sensitive and specific marker for CIC-DUX4 fusion-positive round cell tumor. The American journal of surgical pathology. 2017;41(3):423–429. [DOI] [PubMed] [Google Scholar]
- 14.Hung YP, Fletcher CD, Hornick JL. Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Modern Pathology. 2016;29(11):1324. [DOI] [PubMed] [Google Scholar]
- 15.Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nature Reviews Clinical Oncology. 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol. 1993;17(9):859–868. doi: 10.1097/00000478-199309000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida A, Arai Y, Kobayashi E, et al. CIC break-apart fluorescence in-situ hybridization misses a subset of CIC–DUX 4 sarcomas: a clinicopathological and molecular study. Histopathology. 2017;71(3):461–469. [DOI] [PubMed] [Google Scholar]
- 18.Kao Y-C, Sung Y-S, Chen C-L, et al. ETV transcriptional upregulation is more reliable than RNA sequencing algorithms and FISH in diagnosing round cell sarcomas with CIC gene rearrangements. Genes, Chromosomes and Cancer. 2017;56(6):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]


