Abstract
Tick-borne bacteria, Ehrlichia canis, Anaplasma platys, and Ehrlichia chaffeensis are significant pathogens of dogs worldwide, and coinfections of E. canis and A. platys are common in dogs on the Caribbean islands. We developed and evaluated the performance of a multiplex bead-based assay to detect antibodies to E. canis, A. platys, and E. chaffeensis peptides in dogs from Grenada, West Indies, where E. canis and A. platys infections are endemic. Peptides from outer membrane proteins of P30 of E. canis, OMP-1X of A. platys, and P28–19/P28–14 of E. chaffeensis were coupled to magnetic beads. The multiplex peptide assay detected antibodies in dogs experimentally infected with E. canis and E. chaffeensis, but not in an A. platys experimentally infected dog. In contrast, the multiplex assay and an in-house enzyme-linked immunosorbent assay (ELISA) detected A. platys antibodies in naturally infected Grenadian dogs. Following testing of 104 Grenadian canine samples, multiplex assay results had good agreement with commercially available ELISA and immunofluorescent assay for E. canis antibody-positive dogs (K values of 0.73 and 0.84), whereas A. platys multiplex results had poor agreement with these commercial assays (K values of −0.02 and 0.01). Prevalence of seropositive E. canis and A. platys Grenadian dogs detected by the multiplex and commercial antibody assays were similar to previous reports. Although the multiplex peptide assay performed well in detecting the seropositive status of dogs to E. canis and had good agreement with commercial assays, better antigen targets are necessary for the antibody detection of A. platys.
Keywords: Anaplasma, canine antibodies, Ehrlichia, multiplex bead assay, peptides
Tick-borne bacteria are significant pathogens of dogs in the United States and the Caribbean. Specifically in Grenada, West Indies, there is serologic and polymerase chain reaction (PCR) evidence that dogs are infected with Ehrlichia canis and Anaplasma platys.19 A study of 177 Grenadian dogs evaluated in 2004 and 2006 indicated that the seroprevalence of E. canis is 42–49% and 20% for A. platys. In the same study, 44% of dogs were detected as PCR-positives for E. canis and A. platys in 2006.19 With the concerns of cross-reactivity among antigens found in immunofluorescent assays (IFAs)12,15 and a commercially available enzyme-linked immunosorbent assay (ELISA),a,2 use of multiplex peptide bead-based technology was proposed as an alternative that may improve detection of infections and coinfections of these tick-borne agents. Advantages of this technology over traditional IFA and ELISA are the multiplex format (analysis of several analytes or antibody–antigen reactions at the same time) and high throughput capabilities (e.g., 96-well capacity), smaller sample volume, greater cost-effectiveness, and higher sensitivity.3,4,16
We developed a multiplex peptide bead assay to detect antibodies to peptides specific to immunodominant outer membrane proteins (OMPs) P30 of E. canis, OMP-1X of A. platys, and P28–19 and P28–14 of E. chaffeensis.8,13,14 A similar multiplex assay has been developed to detect serum antibodies to outer surface proteins of Borrelia burgdorferi in dogs.17 We also compared the agreement of the multiplex assay results with the results of a commercial ELISAa and IFA.b Because the ELISAa does not differentiate antibody responses to Ehrlichia chaffeensis from E. canis, we also included peptides from OMPs expressed in E. chaffeensis–infected macrophages (P28–19) and in infected tick cells (P28–14)5 to determine the cross-reactivity of E. canis and A. platys antibodies in serum to these peptides of E. chaffeensis. The OMP-1X gene for A. platys was chosen because it is reported as unique to this species and is considered not to cross-react with mouse anti–Anaplasma phagocytophilum serum.8 To validate the multiplex bead assay, we tested sera from 3 purpose-bred experimentally infected Beagles (carried out at Kansas State University)11 and from 104 dogs of Grenada, West Indies. The population of Grenadian dogs allowed us to test for E. canis– and A. platys–exposed dogs, because Rhipicephalus sanguineus, vector of E. canis and A. platys, is the only known tick to parasitize dogs in Grenada.19
For experimentally infected samples, sera from 3 purpose-bred Beagles obtained from a previous study11 were tested for antibodies to E. canis, A. platys, and E. chaffeensis before (control serum) and 14, 27, and 35 d post experimental infection. Days post-infection were selected based on peak total immunoglobulin (Ig)G ELISA values.11
For naturally infected samples, community-owned Grenadian dogs from St. George’s parish and outlying parishes were brought to the Veterinary Teaching Hospital at St. George’s University School of Veterinary Medicine for assessment and blood sampling prior to spay and neuter surgeries (September-December of 2014). Collection of blood samples from these dogs was approved by the Institutional Animal Care and Use Committee (IACUC, protocol 14002-R) at St. George’s University. The population consisted of 28 male dogs and 76 female dogs, 101 mixed-breed dogs, and 3 purebred dogs with a median age of 1.8 y (2 mo to 8 y).
All canine samples were tested for the presence of antibodies to E. canis and A. platys by commercial ELISAa during clinical examination. All canine sera were tested by IFA using a commercial kitb in which slides were coated with semipurified elementary bodies and morulae from cell culture–propagated E. canis and A. phagocytophilum. Positive and negative canine control serum was provided from the same company as the kit. Serum samples were tested as recommended by the manufacturer,b and were considered positive if they reacted at a dilution >1:80.
To develop the multiplex bead assay, peptides were synthesized using published sequences for the immunodominant OMP of E. canis P30 [GenBank accession ACC85904], A. platys OMP-1X,8E. chaffeensis P28–19,10 and E. chaffeensis P28–14.5 The peptides were modified by the addition of a 19-atom nonimmunogenic spacer consisting of N-terminal amine-polyethylene glycol acid (PEG 5).c The carboxyl terminus of PEG 5 was attached to the primary amine N-terminus of each peptide by an amide bondc (Table 1). Four carboxylated magnetic bead regionsd were identified and coupled to 20 μg/mL of NH2-PEG 5–modified peptides using a 2-step carbodiimide reactione to chemically couple the carboxylated bead regions to the amine N-terminus of each modified peptide (Table 1).
Table 1.
Peptide sequence, source, and bead region.
| Organism | OMP | Peptide sequence and spacer | Bead region |
|---|---|---|---|
| Ehrlichia chaffeensis | P28–19 | NH2-[PEG5]-VFGLKQNWDGSAISA | R15 |
| P28–14 | NH2-[PEG5]-VFGLKKDGDIAQSA | R34 | |
| Ehrlichia canis | P30 | NH2-[PEG5]-VFGLKEEWNGGTIPA | R48 |
| Anaplasma platys | OMP-IX | NH2-[PEG5]-AVQEKKPPEA | R53 |
Serum samples from the experimentally infected dogs were then tested against 4 distinct Ehrlichia/Anaplasma peptide-coupled beads to confirm coupling of these peptides to the beads. The multiplex assay was performed using a modification of an indirect immunoassay protocol as described4 using multiplex technology.f In brief, a working mixture of the 4 peptide coupled bead regions was prepared and diluted to a final concentration of 100 microspheres of each set per microliter in assay buffer.4 Fifty microliters of the microsphere mixture was placed in a 96-well plateg containing 50 μL of filter-sterilized assay buffer. Sera from all dogs were diluted 1:1,000 in assay buffer containing 0.1% blockerh to reduce nonspecific binding of antibody,18 and 50 μL of the diluted sample was added to the appropriate wells in duplicate. Background wells consisted of the microsphere mixture without diluted serum. Following incubation overnight at 4°C on a plate shaker, the beads were washed according to the published protocol using a magnetic separator.i Biotin-affinity purified goat anti-dog IgG H+Lj (50 μL of 2 μg/mL) was added to all of the wells, and the plate was incubated for 1 h at room temperature while on a plate shaker. Three washes using assay buffer were performed followed by 30-min incubation at room temperature with 50 μL/well of 4 μg/mL of phycoerythrin (PE)-labeled streptavidin.k The beads were then washed with assay buffer and resuspended in 100 μL of manufacturer’s drive fluidl and analyzed. The median fluorescent intensity (MFI) of each sample performed in duplicate was compared to the MFIs of the background wells (beads without serum). An average of duplicate samples was determined by analysis softwarem and recorded as the average MFI. Cutoffs for positive samples for each bead set were based on 2 standard deviations of the MFIs of 10 replicates of a negative control sample (serum from an uninfected dog). Background wells, negative control serum, and known positive control serum from experimentally infected dogs were assayed in each plate. During the initial testing of the multiplex assay, it was noted that seroreactivity to OMP-1X peptide by the A. platys–infected dog was below the MFI negative control cutoff (Table 2). The E. canis– and E. chaffeensis–infected canine serum reacted well with the E. canis P30 and E. chaffeensis P28–19 peptide-coated beads as expected (Table 2 with the highest MFI values). There was some cross-reactivity noted with the E. canis–infected serum to E. chaffeensis P28–19 and P28–14 coated beads and E. chaffeensis–infected serum with E. canis P30 coated beads; however, the MFIs were lower.
Table 2.
Multiplex results (median fluorescent intensity) for experimentally infected dogs.*
|
Ehrlichia chaffeensis
|
Ehrlichia canis
|
Anaplasma platys
|
||
|---|---|---|---|---|
| Serum samples | P28–19, R15 bead | P28–14, R34 bead | P30, R43 bead | OMP-IX, R58 bead |
| Negative control samples prior to infection | 185 ± 73 (>258) | 179 ± 51 (>230) | 181 ± 66 (>247) | 156 ± 22 (>178) |
| E. chaffeensis collected 35 dpi | 1,550 | 195 | 383 | 78 |
| E. canis collected 14 dpi | 600 | 287 | 1,440 | 257 |
| A. platys collected 27 dpi | 174 | 162 | 159 | 146 |
Mean ± 2 standard deviations. Cutoff in parentheses, dpi = days postinfection
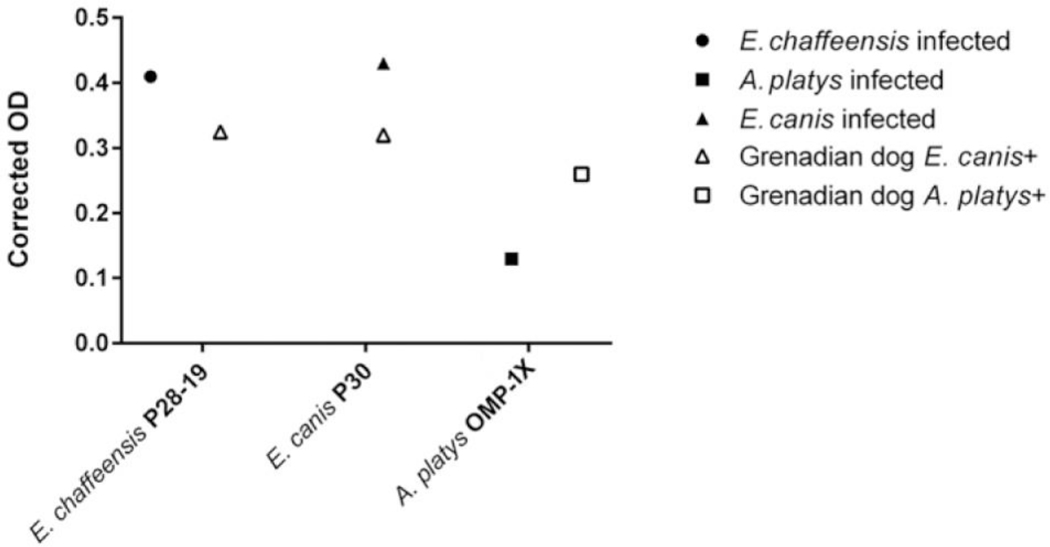
Because the A. platys experimentally infected dog did not produce a MFI reading above the negative cutoff, an in-house ELISA was developed to determine if the serum immunoreactivity to A. platys OMP-1X and Ehrlichia P28/P30 peptides bound to an ELISA plate was in any way different than when tested against the peptide-coated beads. In this experiment, sera were compared from 3 experimentally infected dogs and 2 Grenadian dogs that were known to be E. canis or A. platys antibody-positive by ELISAa and IFAb as well as PCR-positive for 16S rRNA of E. canis or A. platys (data not shown). The in-house ELISA performed similarly to the peptide bead assay for E. canis P30 and E. chaffeensis P28–19, however, the serum from a known A. platys–seropositive Grenadian dog reacted readily with A. platys OMP-1X peptide-coated wells with a higher OD than the serum from the A. platys experimentally infected dog (Fig. 1). This same Grenadian canine sample was above the MFI cutoff for A. platys in the multiplex assay performed later. The A. platys strain used for experimental infection is from a naturally infected dog from Florida.11 This observation suggest that the OMP-1X peptide is suitable for detecting canine infections with A. platys at more prevalent geographic locations such as Grenada in the Caribbean, but requires further optimization to detect wider geographical strains.
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) data of serum obtained from 3 experimentally infected dogs (closed symbols) and 2 naturally infected Grenadian dogs (open symbols) reacting with peptide-coated wells. Corrected optical densities (ODs; mean of 3 replicates) represent the OD after negative control serum was subtracted
The intra-assay precision or repeatability of the multiplex assay was determined by assaying 5 Grenadian canine samples that covered a dynamic range (very low: <250 MFI; low to moderate: 251–999 MFI; moderate to high: 1,000–2,500; very high: >2,500 MFI) in triplicate on the same plate. Inter-assay precision was determined by testing serum from 4 dogs covering the dynamic range in duplicate on 2 different plates 1 mo apart. The coefficient of variation (CV) calculation of the intra-assay was 5–8% for the 4 bead sets. The inter-assay precision was 7–14% for all Ehrlichia peptide-coated beads and 18% for A. platys peptide-coated beads. According to published norms, acceptable intra-assay and inter-assay CVs are <10% and <20%, respectively.1
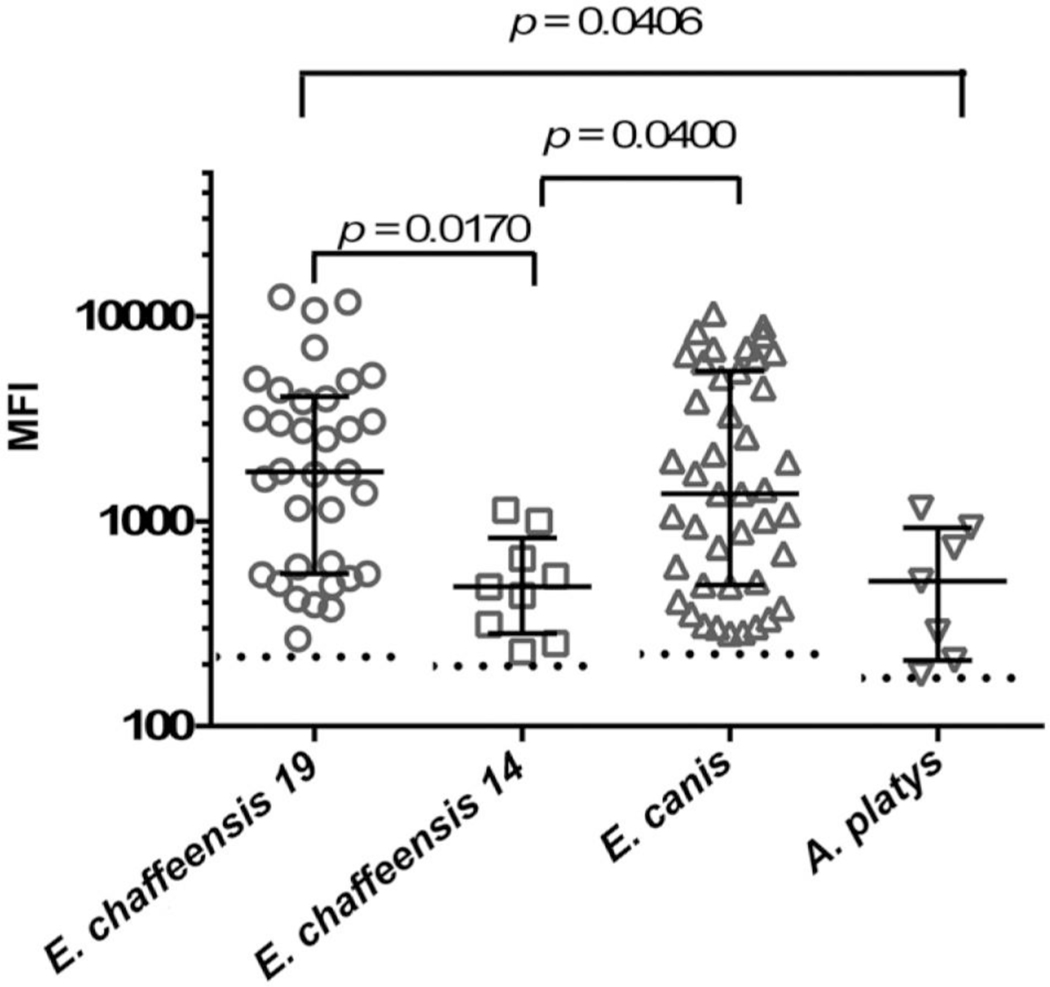
Following these preliminary assay optimizations, the multiplex bead assay was used to test 104 Grenadian canine serum samples. The average age of the dogs was ~2 y. Three dogs were <6 mo of age, with 1 testing antibody positive for E. canis (likely because of maternal antibody), whereas the other 2 dogs tested antibody negative against the E. canis and A. platys peptides. There were 60 antibody-negative dogs and 44 positive samples (Fig. 2). Analysis of variance and Dunn multiple comparison testn detected significant differences between the MFIs of the canine sera against each peptide-coated bead (p = 0.0019). The MFIs for E. chaffeensis P28–19 and E. canis P30 were similar (p > 0.99), whereas the MFIs for E. chaffeensis P28–14 and A. platys OMP-1X were significantly lower than E. chaffeensis P28–19. E. chaffeensis P28–14 MFI was significantly lower than the results for E. canis P30 (Fig. 2). Although the median MFI (511) results for A. platys–positive dogs was lower than E. canis P30 (1363), there was no significant difference (p = 0.0854). Seven samples from Grenadian dogs reacted with the A. platys peptide with MFIs above the cutoff. Six of these dogs also reacted to E. canis, indicating co-exposure, whereas only 1 dog reacted to A. platys peptide alone. All serum that reacted with E. chaffeensis P28–19 peptide also reacted with P30 of E. canis. However, 9 canine sera reacted with the E. canis peptide, but did not recognize the E. chaffeensis P28–19 peptide. Of the 9 canine sera that reacted with E. chaffeensis P28–14, 4 samples also reacted with E. chaffeensis P28–19 and/or E. canis P30 peptides. Sera from 4 dogs reacted with all bead sets with low to moderate range MFIs.
Figure 2.
Multiplex bead assay results from testing 44 Grenadian dog serum samples that had median fluorescent intensities (MFIs) above the cutoffs defined in Table 2 (dotted lines). Each symbol represents the MFI from a dog serum sample reacting to a peptide-coated bead. The lower and upper lines represent the 25th and 75th percentiles of the interquartile range. The middle line represents the median of the data. Significant differences between data sets are listed above each column of data set (Dunn multiple comparisons test, p < 0.05)
The multiplex peptide bead assay results of all positive and negative Grenadian canine sera for E. canis and A. platys were compared to the ELISAa and IFAb results (Table 3). Based on the analyte used for antibody detection, considerably more dogs were detected as antibody-positive for E. canis in the multiplex peptide bead assay than either commercial assay (35.5% for multiplex bead assay vs. 20% for commercial ELISA and 19% for IFA). The ELISA and IFAb assays detected more A. platys–positive dogs (5% for ELISA and 9% IFAb vs. 1% for multiplex assay), and more dogs were antibody-positive for both E. canis and A. platys than the peptide assay (12.5% for ELISA and 18% for IFAb vs. 5.7% for multiplex assay). The proportion of dogs that were antibody negative for E. canis, E. chaffeensis, and A. platys were similar for all 3 methods (62.5% for ELISA, 53.8% for IFA,b and 57.6% for multiplex assay), with the IFA having fewer negatives as expected.
Table 3.
Grenadian dog sample results by enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), and multiplex bead assay for presence of antibodies to Ehrlichia canis and Anaplasma platys.*
| Results | No. of dogs tested for ELISA | No. of dogs tested for IFA | No. of dogs tested for peptide assay |
|---|---|---|---|
| E. canis+/A. platys− | 21 (20) | 20(19.2) | 37(35.5) |
| E. canis+/A. platys+ | 13(12.5) | 19(18.2) | 6(5.7) |
| E. canis−/A. platys+ | 5(5) | 9 (8.6) | 1 (0.96) |
| E. canis−/A. platys− | 65 (62.5) | 56 (53.8) | 60 (57.6) |
| Total | 104(100) | 104(100) | 104(100) |
A total of 104 dogs were tested. Numbers in parentheses are percentages
Kappa statisticso (Table 4) indicated very good to good agreement between the ELISA,a IFA,b and the multiplex peptide bead assay when naturally exposed E. canis antibody-positive serum from Grenadian dogs were compared. In contrast, the agreement among the 3 assays for A. platys antibody detection was poor (Table 5).
Table 4.
Kappa values for Ehrlichia assay agreement*
| Ehrlichia sp. ELISA | E. canis P30 peptide assay | |
|---|---|---|
| IFA | 0.85 (very good)) | 0.84 (very good) |
| ELISA | Nota applicable | 0.73 (good) |
Kappa values for Ehrlichia assay agreement
Table 5.
Kappa values for Anaplasma assay agreement*
| Anaplasma sp. ELISA | A. platys OMP-1X peptide assay | |
|---|---|---|
| IFA | 0.5 (moderate) | 0.01 (poor) |
| ELISA | Nota applicable | −0.02 (poor) |
Kappa values for Anaplasma assay agreement
To test the implementation of the canine Ehrlichia/Anaplasma assay developed in our study and to compare the performance with a commercial ELISAa that uses E. canis and A. phagocytophilum peptides, we selected serum samples from Grenadian dogs because this population is likely exposed to only 1 known tick species, R. sanguineus, which carries both E. canis and A. platys. Because we detected E. canis–positive serum samples that also reacted with E. chaffeensis peptides, this likely represents cross-reactions with E. canis antibodies because the tick vector, Amblyomma americanum, has not been found on the island.19 It is also possible that the antibodies cross-reacted with the peptides for the E. canis P30, E. chaffeensis P28–9 and P28–14 because several amino acids are common within the peptide sequences of the pathogens. Nonetheless, the possibility of the presence of E. chaffeensis on this island cannot be ruled out and requires further investigation.
The multiplex bead assay had good agreement with the ELISAa and IFAb when antibody reactivity to E. canis was compared. The agreement findings for the multiplex peptide bead assay and the ELISA is expected because the commercial assay uses peptides from the P30 and P30–1 immunodominant proteins of E. canis and the multiplex bead assay had a similar peptide to P30 of E. canis. In addition, the prevalence of Grenadian dogs seropositive for E. canis but not A. platys by the multiplex assay was 36%, which is very similar to an island-wide survey in 2013 that reported 34% positives for E. canis in dogs.9 Dogs coexposed to both pathogens were 6% for the multiplex assay, 13% for the commercial ELISA, and 18% for the IFA.
Because the P28/P30 OMP from these gene clusters are conserved between E. chaffeensis and E. canis, cross-reactive antibodies to peptides of E. canis P30 is likely. The majority of E. canis reactive sera also reacted well with E. chaffeensis P28–19 coated beads, with 4 samples reacting with all peptides. These findings suggest the possible conservation of antibody-binding epitopes among the various peptides. Ehrlichia chaffeensis P28 OMP consists of a multigene locus of 22 arranged genes that encode for immunodominant 28-kDa OMP and form porin-like structures on the membrane of the organism.7 The P28 OMP from gene 14 is also from the same multigene locus and is known to be highly expressed in infected tick cells and both P28–14 and P28–19 are paralogs of P30–10 in E. canis.5 P28–19 peptides of E. chaffeensis are highly expressed in infected canine macrophages.5 In our study, very few serum samples had antibodies that reacted with the peptide of E. chaffeensis P28 OMP-14, and the MFIs were low to moderate in range. The biologic significance of E. chaffeensis P28–14 is not completely known; however, prior studies in mice infected with bacteria grown in tick cells indicate that the immune response is less effective in clearing the organism grown in tick cells compared to the organism grown in canine macrophages.6
The agreement for A. platys seroreactive samples between the commercial assays and multiplex assay was poor. Similarly, A. platys beads were not very strong in detecting A. platys sera from a dog experimentally infected with a Florida isolate of the pathogen.11 We hypothesized this was a consequence of the lack of specificity of the assay, although the low prevalence of A. platys infection may also have contributed. In our study, A. platys seropositive samples were determined by the multiplex peptide assay and the peptide ELISAa to have a very low prevalence (1% and 5%, respectively) in this canine population. The IFAb assay also detected a low prevalence (9%) but it was relatively higher than the other assays and more consistent with the island-wide survey of 9% prevalence reported in 2013.9 The prevalence of A. platys reported in 2013 and in our study, regardless of the antibody assay, is less than the 20.5% reported in 2006,19 suggesting a change in the A. platys infection rate on the island over a 9-y period. Because more dogs reacted in the IFA than the other 2 peptide assays, it is likely that whole cell antigen provides more antigenic epitopes for the antibodies than the peptide assays. Several Grenadian canine samples had higher MFI values compared to negative control serum. This result suggests that the sera from the naturally infected Grenadian dogs may have more reactive antibodies specific to the peptide selected in our bead assay. According to the experimental infection study, the A. platys–infected dog was seroreactive to A. phagocytophilum in an in-house ELISA,11 and seroreactive by the IFA using whole cell antigen from the pathogen, and in the ELISA.a Because several of the Grenadian canine samples reacted with whole cell antigens in the IFA and the peptides in the ELISAa (reported to have a high sensitivity and specificity for E. canis and A. phagocytophilum),2 the low antibody reactivity of the Grenadian canine samples to the OMP-1X peptide of A. platys may be a consequence of the lack of immunogenic epitopes in this peptide. The ELISAa uses a different synthetic peptide from another major P44 OMP gene product of A. phagocytophilum than the multiplex assay developed in our study. Although A. phagocytophilum has been reported to have cross-reacting epitopes to A. platys, these epitopes may not be similar to various A. platys strains existing in nature, such as those in the Grenada island dogs. Even though the A. platys gene sequence was first identified to be identical in 2 naturally infected dogs from Venezuela and in 1 naturally infected dog from Taiwan, the report did not describe the testing of the OMP-1X peptide-coated ELISA plate against serum from infected Venezuelan dogs.8 Dogs inhabiting Venezuela are likely exposed to similar strains as dogs in Grenada compared to dogs from Taiwan. Therefore, it is unclear whether the A. platys strain found in Grenadian dogs is different than the strain against which the OMP-1X peptide sequence was originally defined and tested. Future studies are required to determine the differences in the sequences of OMP-1X in A. platys–infected Grenadian dogs. Further optimization of the multiplex assay is necessary before diagnostic sensitivity and specificity of the assay can be determined.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Windward Islands Research and Education Foundation (WINDREF SRGI-14005) located in Grenada, West Indies and Kansas State Veterinary Diagnostic Laboratory.
Sources and manufacturers
Snap 4DX, IDEXX Laboratories, Westbrook, ME.
Ehrlichia canis and A. phagocytophilum MIF canine IgG antibody kit, Fuller Laboratories, Fullerton, CA.
Pierce Biotechnology, Rockford, IL
Magplex beads, Luminex, Austin, TX.
xMap antibody coupling kit, Luminex, Austin, TX.
MagPix instrument, Luminex, Austin, TX.
Greiner black plate (65509x), Greiner Bio-One North America, Monroe, NC.
EMD Millipore ChemiBLOCKER, Merck, Darmstadt, Germany.
Magnetic plate separator, Luminex, Austin, TX.
Biotin-affinity purified goat anti-dog IgG H+L, Kirkegaard and Perry Laboratories, Gaithersburg, MD.
Phycoerythrin (PE)-labeled streptavidin, Life Technologies, Grand Island, NY.
Magpix drive fluid, Luminex, Austin, TX.
xPonent 4.2 software for MagPix, Luminex, Austin, TX.
GraphPad Prism 6.05, GraphPad Software, La Jolla, CA.
Analyse-it (Method Evaluation Edition version 4), Analyse-it Software, Leeds, United Kingdom.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interests regarding the research, authorship, or publication of this article.
References
- 1.Angeloni SC. Optimization of immunoassays. In: xMAP® Cookbook: A Collection of Methods and Protocols for Developing Multiplex Assays with xMAP Technology. 3rd ed.Luminex Corp., 2016:55–63. [Google Scholar]
- 2.Chandrashekar R. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res 2010;71:1443–1450. [DOI] [PubMed] [Google Scholar]
- 3.Christopher-Hennings J. Opportunities for bead-based multiplex assays in veterinary diagnostic laboratories. J Vet Diagn Invest 2013;25:671–691. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar SAH, Hoffmeyer MR Microsphere-based multiplex immunoassays: development and applications using Luminex xMAP Technology. In: Wild D, ed. The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques. 4th ed.Amsterdam: Elsevier, 2013:168–169. [Google Scholar]
- 5.Ganta RR. Differential clearance and immune responses to tick cell-derived versus macrophage culture-derived Ehrlichia chaffeensis in mice. Infect Immun 2007;75:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganta RR. Molecular characterization of Ehrlichia interactions with tick cells and macrophages. Front Biosci (Landmark Ed) 2009;14:3259–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumagai Y. Expression and porin activity of P28 and OMP-1F during intracellular Ehrlichia chaffeensis development. J Bacteriol 2008;190:3597–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai TH. Cloning of the major outer membrane protein expression locus in Anaplasma platys and seroreactivity of a species-specific antigen. J Bacteriol 2011;193:2924–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanza-Perea M. Intraoperative bleeding in dogs from Grenada seroreactive to Anaplasma platys and Ehrlichia canis. J Vet Intern Med 2014;28:1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li JS. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J Immunol 2001;166:1855–1862. [DOI] [PubMed] [Google Scholar]
- 11.Nair AD. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum. PLoS One 2016;11(2):e0148239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor TP. Comparison of an indirect immunofluorescence assay, western blot analysis, and a commercially available ELISA for detection of Ehrlichia canis antibodies in canine sera. Am J Vet Res 2006;67:206–210. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi N. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol 1998;36:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi N. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun 1998;66:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suksawat J. Seroprevalence of Ehrlichia canis, Ehrlichia equi, and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J Vet Intern Med 2000;14:50–55. [DOI] [PubMed] [Google Scholar]
- 16.Wagner B, Freer H. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol 2009;127:242–248. [DOI] [PubMed] [Google Scholar]
- 17.Wagner B. A fluorescent bead-based multiplex assay for the simultaneous detection of antibodies to B. burgdorferi outer surface proteins in canine serum. Vet Immunol Immunopathol 2011;140:190–198. [DOI] [PubMed] [Google Scholar]
- 18.Waterboer T. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods 2006;309: 200–204. [DOI] [PubMed] [Google Scholar]
- 19.Yabsley MJ. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet Parasitol 2008;151:279–285. [DOI] [PubMed] [Google Scholar]