ABSTRACT
Background: Meta-analytic results indicate that posttraumatic stress disorder (PTSD) is associated with hypoactivation of the medial prefrontal cortex (mPFC), hyperactivation of the amygdala, and volume reductions of the hippocampus. Effective psychotherapeutic treatments were hypothesized to normalize these neural patterns via upregulation of prefrontal structures, which in turn downregulate limbic regions.
Objective: To gain a sound understanding of the effects of successful psychotherapy on the brain, neural changes from pre- to post-treatment in PTSD patients will be aggregated.
Method: A systematic literature search identified 24 original studies employing structural or functional MRI measurements both before and after treatment of patients diagnosed with PTSD.
Results: In conjunction, the review returned little evidence of an activation increase in the mPFC/rostral anterior cingulate cortex (rACC) following successful treatment. Five out of 12 studies observed such an increase (especially during emotion processing tasks), albeit in partially non-overlapping brain regions. Conversely, neither the putative related activation decrease in the amygdala nor volumetric changes or altered activation during the resting state could be convincingly established.
Conclusion: Successful psychological treatments might potentially work via upregulation of the mPFC, which thus may be involved in symptom reduction. However, the role of the amygdala in recovery from PTSD remains unclear. There is currently no indication that the various PTSD treatment approaches employed by the reviewed studies differ regarding their action mechanisms, but further research on this topic is needed.
KEYWORDS: Review, PTSD, trauma, psychotherapy, exposure therapy, fMRI, neurobiology, amygdala, prefrontal cortex, hippocampus
HIGHLIGHTS
There is little evidence for an activation increase in mPFC/rACC following successful PTSD treatment.
Most studies detected no significant activation changes in amygdala, insula, or hippocampus.
There is no consistent evidence for post-treatment volume changes in any brain region.
Abstract
Antecedentes: Los resultados de metanálisis indican que el trastorno de estrés postraumático (TEPT) se asocia con la hipoactivación de la corteza prefrontal medial (CPFm), la hiperactivación de la amígdala y la reducción del volumen del hipocampo. Se hipotetizó que los tratamientos psicoterapéuticos eficaces normalizan estos patrones neuronales a través de la regulación aumentada de las estructuras prefrontales, que a su vez regulan a la baja las regiones límbicas.
Objetivo: Para obtener una comprensión sólida de los efectos de la psicoterapia exitosa en el cerebro, se agregarán los cambios neuronales de antes a después del tratamiento en pacientes con TEPT.
Método: Una búsqueda bibliográfica sistemática identificó 24 estudios originales que empleaban mediciones de resonancia magnética estructural o funcional antes y después del tratamiento de pacientes diagnosticados con TEPT.
Resultados: En conjunto, la revisión arrojó escasas pruebas de un aumento de la activación en las cortezas CPFm y cingulada anterior rostral (CCAr) tras el éxito del tratamiento. Cinco de 12 estudios observaron dicho aumento (especialmente durante las tareas de procesamiento de emociones), aunque en regiones cerebrales parcialmente no superpuestas. Por el contrario, no se pudo establecer de forma convincente ni la supuesta disminución de la activación relacionada en la amígdala ni los cambios volumétricos o la activación alterada durante el estado de reposo.
Conclusión: Los tratamientos psicológicos exitosos podrían funcionar potencialmente a través de la regulación aumentada de la CPFm, que por lo tanto puede estar involucrada en la reducción de los síntomas. Sin embargo, el papel de la amígdala en la recuperación del TEPT sigue sin estar claro. En la actualidad, no hay indicios de que los diversos enfoques de tratamiento del TEPT empleados por los estudios revisados difieran en cuanto a sus mecanismos de acción, pero es necesario seguir investigando sobre este tema.
PALABRAS CLAVE: Revisión, TEPT, trauma, psicoterapia, terapia de exposición, fMRI, neurobiología, amígdala, corteza prefrontal, hipocampo
Short abstract
背景:元分析结果表明, 创伤后应激障碍 (PTSD) 与内侧前额叶皮层 (mPFC) 激活不足, 杏仁核过度激活以及海马体积减小有关。有效的心理治疗方法假设通过前额叶结构的上调来使这些神经模式正常化, 从而反过来下调边缘区。
目的: 为了更好地了解成功的心理治疗对大脑的影响, 将汇总PTSD患者治疗前后的神经变化。
方法: 系统的文献检索确定了24项原始研究, 这些研究在对诊断为PTSD的患者进行治疗前后均采用结构或功能MRI测量。
结果: 结合起来, 本综述发现了很少成功治疗后mPFC/喙前扣带回皮层 (rACC) 激活增加的证据。 12个研究中有5个观察到了这种增加 (特别是在情绪处理任务期间), 尽管是在部分不重叠的脑区。相反, 既不能确信杏仁核假定的相关激活减少, 也不能确定体积变化或激活状态改变。
结论: 成功的心理治疗可能通过上调mPFC发挥作用, 这可能与症状减轻有关。然而, 杏仁核在PTSD恢复中的作用仍不清楚。目前尚无迹象表明所综述的研究采用的各种PTSD治疗方法在其作用机理方面存在差异, 但需要对此主题进行进一步研究。
关键词: 综述, PTSD, 创伤, 心理治疗, 暴露疗法, fMRI, 神经生物学, 杏仁核, 前额叶皮层, 海马
1. Introduction
Every year, posttraumatic stress disorder (PTSD) affects 1.1–2.9% of the general population in Western societies (Wittchen et al., 2011), causing severe distress as well as high societal costs (Habetha, Bleich, Weidenhammer, & Fegert, 2012). PTSD is defined as the exposure to a traumatic event (Criterion A) complemented by four groups of symptoms: persistent re-experiencing of the traumatic event (Criterion B), avoidance of trauma-associated stimuli (Criterion C), negative thoughts or feelings (Criterion D), and increased arousal (Criterion E) (American Psychiatric Association, 2013). Various evidence-based PTSD treatments show large treatment effects (Cusack et al., 2016; Watts et al., 2013) including e.g. Cognitive Behavioural Therapy (CBT), Prolonged Exposure Therapy (PE), and Eye Movement Desensitization and Reprocessing (EMDR). These approaches have in common that they are trauma-focused and exposure-based. However, drop-out rates (e.g. Imel, Laskab, Jakcupcakc, and Simpson (2014) report 18%) and non-response rates remain relatively high across different treatments (Schottenbauer, Glass, Arnkoff, Tendick, & Gray, 2008). A detailed understanding of how neural processing needs to be altered in order to achieve recovery may help identify mechanisms of change and thus inform the enhancement of treatments.
2. Neural correlates of PTSD
The standard neurobiological model of PTSD assumes insufficient top-down regulation, i.e. hyperactivation of limbic structures facilitated by hypoactivated prefrontal structures (Rauch, Shin, & Phelps, 2006).
In line with this view, the (ventro)medial prefrontal cortex (vmPFC), which subserves emotion regulation as well as the inhibition of acquired fear responses (Fitzgerald, Digangi, & Phan, 2018; Nicholson et al., 2017), has been shown to be hypoactivated in PTSD by various meta-analyses (Hayes, Hayes, & Mikedis, 2012; Patel, Spreng, Shin, & Girard, 2012) (Figure 2, left side). Structural alterations in grey (Wang et al., 2020) or white matter (Dennis et al., 2019) have not been convincingly established.
Figure 2.
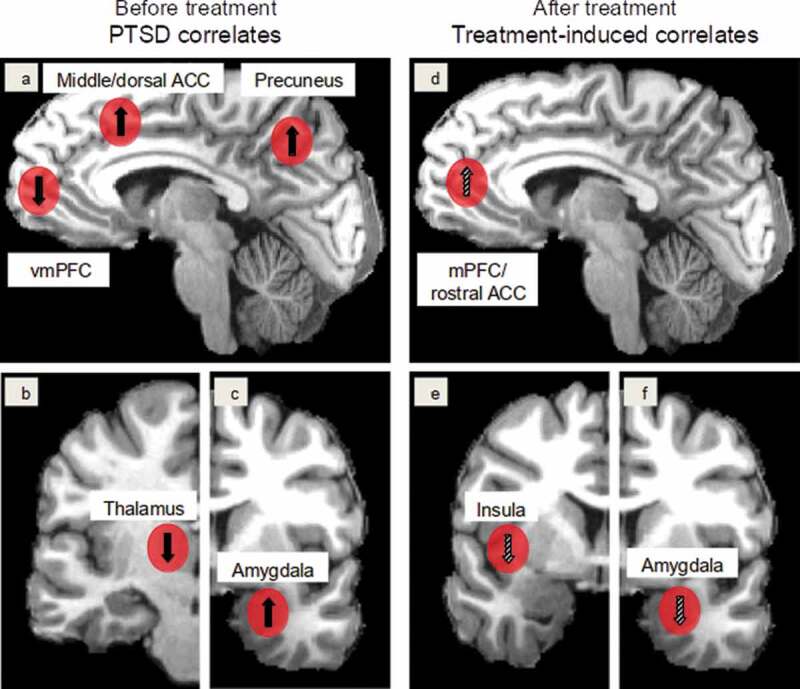
PTSD correlates found in meta-analyses (see Introduction) (left column, 2a,b,c) and treatment-induced activation changes discussed in this review (right column, 2d,e,f). Red spheres mark the neural regions. In the left column, upward and downward arrows indicate hyperactivation and hypoactivation of the regions before treatment, respectively, and in the right column upward and downward arrows indicate increases and decreases in activation changes following treatment, respectively. Limited and inconsistent findings are marked with hatched arrows. Abbreviations: ACC: anterior cingulate cortex, (v)mPFC: (ventro)medial prefrontal cortex
Convergently, hyperactivation of the amygdala has been reported by several meta-analyses (Patel et al., 2012; Hayes et al., 2012) (Figure 2, left side); however, potentially associated with trauma exposure rather than PTSD development (Patel et al., 2012). The amygdala plays a crucial role in diverse processes such as emotional responding, memory formation, fear conditioning, but also fear extinction (Fitzgerald et al., 2018; Koenigs & Grafman, 2009; Maren & Holmes, 2016). However, as recent studies suggested an involvement of the amygdala in extinction learning, the meaning of specific activation differences might be quite ambiguous unless their exact location can be determined (Zhang, Kim, & Tonegawa, 2020). Its morphology seems to be unaltered (Kühn & Gallinat, 2013; Logue et al., 2018), indicating that full normalization of neural processing might be achievable.
The hippocampus, however, exhibits significant volume reductions associated with PTSD symptom severity (Kühn & Gallinat, 2013; Logue et al., 2018; Nelson & Tumpap, 2017). A recent mega-analysis also identified aberrations in its interhemispheric structural connectivity (Dennis et al., 2019). However, activation changes of the hippocampus are still contentiously debated; whereas no activation alterations were observed in two meta-analyses (Hayes et al., 2012; Sartory et al., 2013), a third one found hyperactivation in the right hippocampus (Patel et al., 2012). Take together, these results seem crucial as the hippocampus subserves context-encoding (such as during the traumatic event) as well as extinction memory recall and thus likely plays an important role in context differentiation (Rauch et al., 2006; Shin, Rauch, & Pitman, 2006).
Going beyond the standard neurobiological model of PTSD, hyperactivation in precuneus and hypoactivation in the thalamus have been observed in several meta-analyses (Hayes et al., 2012; Patel et al., 2012; Sartory et al., 2013) (Figure 2). Conversely, it remains unclear whether the insula shows altered activation as two meta-analyses reported hyperactivation/alteration of this structure (Patel et al., 2012; Stark et al., 2015), while this was not confirmed by two others (Hayes et al., 2012; Sartory et al., 2013).
3. Neural correlates of treatment effects
It has been suggested that top-down inhibition is generally a crucial component of psychological treatment approaches (Quidé, Witteveen, El-Hage, Veltman, & Olff, 2012), presumably acting via extinction learning, which involves activation of brain regions subserving threat appraisals such as the dorsal ACC, the anterior insula, and the amygdala (Suarez-Jimenez et al., 2020). For extinction learning to occur, recall of trauma memories seems necessary (Maeng & Milad, 2017), which would implicate brain structures subserving autobiographical memory during the recovery process. In turn, extinction recall is necessary to translate learning into stable therapeutic gains, which would require recruitment of the anterior hippocampus, amygdala regions, and medial prefrontal areas (Suarez-Jimenez et al., 2020).
In summary, the current literature provides good evidence that the main neurobiological correlates of PTSD are alterations of the mPFC, the amygdala, and the hippocampus as well as that PTSD treatment processes seem to be related to these regions as well as to the dorsal ACC and the anterior insula. The aim of the current systematic review is to investigate whether the available evidence supports these models and whether recovery from PTSD can indeed be understood as a normalization of neural activation patterns as observed in trauma-exposed healthy subjects. Specifically, we aim at i) gaining a better understanding of the neural alterations induced by psychological treatment, ii) identifying which brain regions exhibit such alterations consistently across studies, iii) noting which pre-treatment differences might predict treatment success, and iv) whether there are substantial differences between the different treatment forms.
4. Method
4.1. Study selection
Following PRISMA guidelines (Moher et al., 2015), a literature search was conducted with the scientific search engines Cochrane, PsycINFO, Pubmed, PubPsych, Scopus, and Web of Science using the search terms: (i) neuroimaging, brain imaging, fMRI, MRI, magnetic resonance imaging, BOLD, blood oxygen level dependent, VBM, voxel-based morphometry, voxelwise, voxel-wise, DTI, diffusion tensor imaging, white matter, cortical thickness, grey matter which were fully crossed with the search terms (ii) therapy, psychotherapy, therapeutics, therapeutic, treatment, intervention and again fully crossed with the terms (iii) posttraumatic stress disorder, posttraumatic stress syndrome, PTSD, PTSS, posttraumatic stress, traumatic stress, psychological trauma without searching for the following terms animals, non-human, rodent, mice, SPECT, single photon emission computerized tomography, PET, positron emission tomography, CT, and computer tomography. Peer-reviewed original articles published in English, German, and French from January 2005 up to the end of October 2019 were selected from the search results. An initial search omitting duplicates automatically and manually revealed 2627 peer-reviewed articles (see Figure 1). Title and abstract of all articles were screened by two scientists (AS and AM) for meeting the inclusion criteria: (i) pre- and post-measurement by MRI or functional MRI (fMRI) technique, (ii) psychological treatment between the two neuroimaging scans, and (iii) PTSD diagnosis by using Clinician-Administered PTSD Scale (CAPS-IV; Blake et al., 1995) at pre-treatment in order to have a comparable diagnostic. Theoretical work and reviews were only used for the search of additional articles. In total, 107 full-texts were assessed of which 29 articles met our criteria. In a subsequent consideration process, five studies were excluded: Roy et al. published two studies (Roy, Costanzo, Blair, & Rizzo, 2014; Roy et al., 2010) with the same paradigm, of which only the more recent and extensive was included. A study by Jung, Chang, and Kim (2016) with participants with partial PTSD with a very low baseline CAPS score of 29.4 was excluded as it was challenging to conclude on PTSD diagnostic status. Further, Shou et al. (2017), Yang et al. (2018a), and Yang et al. (2018b) were excluded because they examined patients with major depressive disorder (MDD) and PTSD, but gave little information on PTSD alone. In sum, 24 studies are included in this review.
Figure 1.
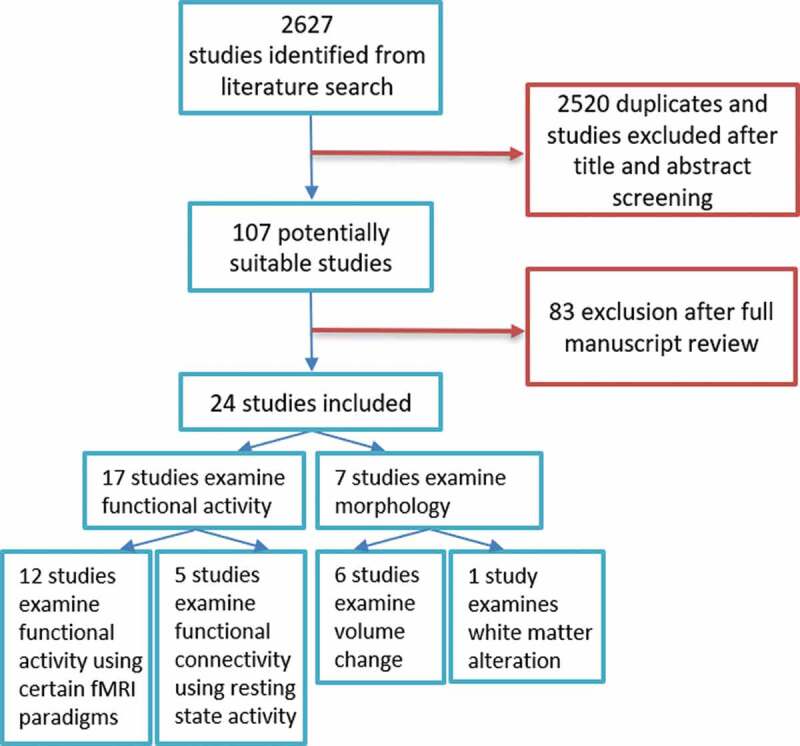
Stepwise inclusion of studies for the current analysis
4.2. Study analysis
From the studies meeting the above criteria, we extracted information concerning i) treatment (interaction) effects or pre-post-treatment comparisons, ii) correlation results between CAPS change and neural change, and iii) baseline predictors related to treatment outcome. Because of the focus on treatment-specific changes, group-differences (without treatment interaction) are not reported in this review. In addition, only results that remained significant after correction for multiple comparisons are considered.
Of the 24 identified papers, 17 studies analysed functional alterations after psychotherapeutic treatment (incl. five resting-state studies) and seven studies analysed structural alterations (see Figure 1). A total of 351 PTSD patients (range = 8–39 per study) and 447 controls (range = 8–70; no control group in three studies) were included. Out of the 24 papers, some were based on overlapping or identical samples (five of the studies co-authored by Van Rooij and Kennis, three by Helpman and Rubin, two each by King et al. and Fonzo et al.).
4.2.1. Subject characteristics
4.2.1.1. PTSD diagnosis
Along with the CAPS cut-off reported by Weathers et al. (1999), all studies included patients with an average total CAPS value ≥ 45 pre-treatment (cf. Tables 1, 2, and 3), 23 studies even reported average CAPS values ≥ 60. 21 studies reported post-treatment total CAPS scores of treated PTSD subjects (or information from which this could be calculated) with a mean = 37.9 (SD = 21.8, range = 16.29–80.67, as four outliers showed post-treatment scores > 50).
Table 1.
provides information on the reviewed studies with functional paradigms including participant characteristics, type and duration of treatment, employed fMRI paradigm, as well as neuroimaging results with (i) findings of pre-post-treatment differences and group-by-time interaction effects, and (ii) findings of correlation analysis between CAPS score change (indicator of symptom improvement) and neural patterns. Only results and sample of subjects are mentioned who completed treatment and the second fMRI scan. Only significant results (p<0.05) are reported. The individual studies are grouped by the type of paradigm applied (mainly emotional vs. cognitively demanding)
|
Study |
Sample size (n) of (R=remitted) patients, number of male subjects, type of trauma event |
Sample size (n) and type of control subjects |
Pre-CAPS scores Mean (and SD) of total CAPS scores |
Post-CAPS scores Mean (and SD) of total scores |
Psychiatric comorbidities in patient group (in subsample size or in percent) |
Type & duration of treatment |
Type of paradigm within (f)MRI scans |
Neuronal alterations from pre- to post-treatment |
Correlation analysis between symptom improvement (reduction in CAPS scores) & neuronal changes ↑ means pos. correlation ↓ means neg. correlation |
|
(Social) emotional processing fMRI paradigms (mainly not cognitively demanding) | |||||||||
| Van Rooij et al. (2016) | R=21 (all male) war veterans |
H-Res=23 Non-R=22 |
R: 66.3 (12.6) Non-R: 74.4 (13.1) |
R: 24.3 (14.1) Non-R: 66.0 (15.3) |
R (Pre/Post): Mood disorder: 10/1 Anxiety disorder: 3/2 Non-R (Pre/Post): Mood: 14/6 Anxiety: 11/5 |
Trauma-focused CBT and/or EMDR R/Non-R (mean): 10.6/7.30 |
Emotional processing task (viewing & rating neutral, positive, negative, trauma-unrelated emotional pictures, only congruent are analyzed) |
ROI analyses (ROIs: amygdala, dACC, insula, hippocampus, vmPFC): R and H-Res show no change Non-R: ↓ amygdala Whole-brain analyses: not significant |
(↓ amygdala (r=-.34, p=.026), but can be explained by change in Non-R group only) |
| Felmingham et al. (2007) | 8 (3 males) assault or car accident |
No control group | 78.1 (20) | 28.9 (20.3) → All patients at least 30% reduction in total CAPS score |
Mood: 4 | Imaginal exposure & cognitive restructuring 8 once-weekly sessions |
Presentation of fearful & neutral facial expressions | Pre-post-comparison: Contrast fearful & neutral faces: ROI analyses (ROIs: amygdala, ACC): ↑ in bilateral rACC (left: p=.021, k=10, right: p=.036, k=36) Whole-brain analysis: ↓ right postcentral gyrus (k=172) ↓ right middle temporal gyrus (k=29) ↓ left superior temporal gyrus (k=41) ↑ left middle temporal gyrus (k=213) ↑ right inferior frontal gyrus (k=57) ↑ left parietotemporal gyrus (k=47) ↑ right hippocampus (p=.001, k=6) |
↑ right rACC activity (r=.84, p<.01) ↓ bilateral amygdala (r=-.85, p<.01) |
| King et al. (2016b) | 13 (all male) combat veterans |
Control group therapy=8 (PTSD patients, all male, combat veterans, PCGT incl. elements of affective PTSD treatment) |
72.29 (18.32) controls: 74.11 (15.34) |
Decrease of average 16 points (ES: d=0.92) controls: decrease of average 7 points (ES d=0.43) |
Mood: 93% Anxiety: 21% Substance: 21% |
Mindfulness-based exposure therapy (MBET) group therapy 16 weeks |
Emotional faces matching task (angry, fearful, neutral faces; instruction: choose target between two faces) |
Whole-brain & ROI analyses (ROIs: amygdala, mPFC/ACC): Group-by-time-interaction: In MBET patients (not in PCGT): Angry faces: ↑ left amygdala (F=10.65, k=16) right parahippocampus (F=21.43, k=25) ↑ right fusiform/lingual gyrus (F=20.8, k=36) right precuneus (F=18.11, k=202) PCC (F=10.76, k=15) Fearful faces: ↑ left medial frontal gyrus (BA10, F=17.19, k=16) Neutral faces: ↑ bilateral fusiform/lingual gyrus (right: F=20.80, k=71, left: F=11.28, k=30) left lingual gyrus (F=12.27, k=73) left caudate body (F=11.12, k=36) |
ROI analyses: in both treatment groups in response to angry faces: ↑ rACC/mPFC (BA32/BA10, k=42) ↑ left amygdala/peri-amygdala area (k=15) |
| Simmons et al. (2013) | R=9 (all male) combat veterans |
Non-R=15 | R: 86.7 (15.4) Non-R: 91.1 (13.4) |
R: 25.8 (16.5) Non-R: 75.1 (16.2) |
R/Non-R: MDD: 8/8 Anxiety: 9/15 Personality disorder: 1/4 |
PE 8-12 weeks à 90 min. |
Affective anticipation task (combat-related/ negative images vs. noncombat-related/positive images) |
Group-by-time interaction: During negative anticipation (after treatment): R: ↓ left ventral anterior insula During positive anticipation (after treatment): Non-R: ↑ left ventral anterior insula Functional Connectivity analysis (seed: left ventral anterior insula): R: ↑ connectivity with right cingulate/medial frontal gyrus right mid-posterior insula/middle frontal gyrus Non-R: Mild reduction in connectivity Regions differed between the groups: left cerebellum |
--- |
| Aupperle et al. (2013) | 14 n=11 meet full PTSD criteria, n=3 partial PTSD (all female) intimate partner violence |
No control group | 66.07 (16.78) | 16.29 (16.81) In 12 patients: symptom reduction ≥ 50% |
BDI-II mean (pre/post): 20.50/7.07 > sign. decline (t(13)=4.71, p <.001) |
Cognitive trauma therapy for battered women Weekly sessions à 90 min., mean sessions=11.57 (SD=1.60) |
Anticipation task (1) continuous performance task (with cues about stimuli) & (2) interspersed presentation of positive & negative affective images |
From pre-to-post-treatment: Image anticipation phase (negative-positive images): Whole-brain analyses: ↑ left PCC (BA29, cluster size=2176 mm3) ↑ left ACC, medial frontal gyrus (BA9 & 32, 1472 mm3) ↑ right cerebellum (2048 mm3) ↑ left cerebellum (1088 mm3) ↑ right superior/middle temporal gyrus (1664 mm3) ↓ right middle frontal gyrus (BA10, 960 mm3) ↓ right mid Insula (BA13, 832 mm3) ROI analyses (bilateral insula, amygdala, cingulate): No additional findings. Image presentation phase: Whole-brain analyses: ↑ right precuneus, inferior parietal (BA40, 1344 mm3) ↑ left precuneus, PCC (BA31, 896 mm3) ↑ right precuneus (BA31, 896 mm3) ↓ left dlPFC (BA9, 1280 mm3) ROI analyses additionally: ↓ right amygdala (448 mm3) |
Linear mixed effect analyses: Image anticipation phase: ↓ left anterior insula (BA 13, 1216 mm3) ↓ PCC (BA 29, 1472 mm3, ROI: BA 31, 448 mm3) ↓ precuneus (BA 7, 896 mm3) ROI analyses additionally: ↑ right posterior insula (384 mm3) Image presentation phase: ↑ bilateral precuneus (BA 18&19, left: 9344 mm3, right: 1664 mm3) ↑ right PCC (BA 31, 832 mm3) ↑ medial frontal gyrus (BA 6, 1920 mm3) ↑ precentral gyrus (BA 4, 1216 mm3) ↑ lingual gyrus (BA 18, 1216 mm3) ↑ cerebellum (2048 mm3) ↑ inferior temporal gyrus (448 mm3) ↓ right superior/ transverse temporal gyrus (BA: 41, 1344 mm3) ↓ cerebellum (3840 mm3) ↓ caudate body (896 mm3) ROI analyses additionally: ↑ left posterior insula (BA:13, 448 mm3) |
| Peres et al. (2011) | 12 partial PTSD (all male) policemen, gunfire attacks |
Wait list=12 (partial PTSD) H-Res=12 (policemen) |
Patients: 48 (3.62) Wait list: 43 (4.82) |
Patients: 19 (5.03) > at least 37% fewer PTSD symptoms Wait list: 46 (2.70) > no sign. change |
No comorbidities | Exposure and Cognitive Restructuring Therapy | Acoustic-cue paradigm: recall/retrieval cued by pleasant, neutral, & traumatic memories =symptom provocation task |
Pre-post-comparison: ROI analyses (ROIs: OFC, PFC, parietal lobes, ACC, amygdala, insula, thalamus, hippocampus): PTSD group during traumatic memory retrieval: ↓ left amygdala than before treatment (Pcorr<.001, k=92) ↑ mPFC than for wait list after treatment R and H-Res: ↓ left amygdala (Pcorr<.001, k=232) ↑ mPFC (Pcorr<.001, k=1852) Wait list at post scan – like pre-scan: ↑ left amygdala (Pcorr<.001, k=576) ↓ mPFC (Pcorr<.001, k=1623) Whole-brain analysis and other ROIs: no significant differences. |
Only in R: ↑ mPFC (r=.82, p=.02) ↓ left amygdala (r=-0.86, p=.04) |
| Roy et al. (2014) | 10 (mainly male) combat veterans (partly with TBI, from Roy et al. (2010) |
H-Res=18 combat-exposed |
VRET + PE: 84.1 (12.62) VRET: 80.44 (13.31) PE: 72.7 (13.01) |
VRET + PE: 80.67 (14.97) > no sign. change VRET: 64.5 (23.07) > sign. change PE: 75.9 (11.79) > no sign. change |
PE: BDI: 27.2 pre-treatment (from (Roy et al., 2010), n=8) |
VRET (n=4) or PE (n=6) 12-20 sessions à 90 min. |
Affective Stroop task (emotions: negative, positive, neutral) |
Whole-brain analyses: VRET + PE: Treatment group emotion-by-time interaction: Response to negative images: ↓ right amygdala ↑ vmPFC Response to neutral images: ↑ ACC (ROIs: amygdala, hippocampus, ACC (Roy et al., 2010) |
--- |
| Thomaes et al. (2012) | 16 (all female) child abuse-related complex PTSD |
H=22 (all female) and TAU treatment group |
EXP and TAU: 88.5 (13.9) EXP: 92.7 (9.5) TAU: 83.1 (17.5) |
EXP and TAU: 66.2 (22.0) > sign. reduction > no sign. diff. between both treatments EXP: 62.4 (27.4) TAU: 71.0 (12.9) |
Anxiety: 76% Mood: 62% Personality: 75% Dissociative symptoms (DES=22.1) |
2 treatments: EXP = 9 (Psycho-educational & cognitive behavioral stabilizing group therapy added to TAU) TAU = 7 (supportive care/pharmacotherapy) 20 weekly 2 hour session about 6 months |
Classical & affective Stroop task (trauma-relevant, general negative, & neutral words) |
Classical stroop task: Treatment type-by-time interaction: Post-treatment in EXP group (post<pre): ↓ dorsal ACC (right: p(SVC)=.008, left: p(SVC)=.032) ↓ left anterior insula (p(SVC)=.038) (ROI: dorsal ACC, anterior insula, superior frontal cortex) |
Emotional stroop task: Negative/trauma vs. neutral words: Positive correlation between CAPS improvement and ↓ dorsal ACC (p(SVC)<.05) |
| Helpman et al. (2016a) |
16 car accidents, sexual or physical assaults, witnessing serious injuries/ deaths |
H-Res=16 |
PTSD: 78.53 (16.31) |
Sign. reduction in CAPS in PTSD group (mean difference= 49.93, SE=3.49, p = 0.001) |
Exclusion criteria: diagnosis of psychosis, substance/ alcohol dependence within the past six months or abuse within past two months; HAM-D-17 score>24 |
PE 10 weeks |
Fear conditioning and extinction on day 1, extinction recall on day 2 |
Pre-post-comparison during extinction recall: ROI analyses (ROIs: amygdala, hippocampus, insula, subcallosal cortex, mPFC, OFC, ACC, thalamus, vmPFC): PTSD group: ↓ right rACC (t(15)=3.79, k=23, p=.021) H-Res: ↑ left vmPFC (t(15)=3.76, k=61, p=.019) Pre- to post-treatment changes during recall and CAPS changes among PTSD group: ↓ right subgenual ACC (cluster size=84, p=0.017) ↓ left hippocampal (cluster size = 45 p = 0.011) ↓ parahippocampal region > were significantly associated with percent decrease in CAPS score See Supplement for further analyses. |
No sign. correlation between CAPS and rACC change. |
|
Cognitively demanding fMRI tasks (mainly with emotional stimuli) | |||||||||
| Fonzo et al. (2017b) | 25 PTSD patients to treatment (23 female) mainly sexual/ physical assault, injury, combat, natural disaster |
Wait list=26 (PTSD patients) |
66.33 (15.17) Wait list: 71.37 (14.99) |
29.60 (21.26) Wait list: 64.23 (21.77) |
Mood: n=23 (64%) | PE 9-12 sessions à 90 min. |
(1) Emotional reactivity task: identify color of presented tinted fearful/neutral faces (2) Emotional conflict task: fearful & happy faces with (in-)congruent emotion words; identify emotion (3) Gender conflict task (as control task): same facial stimuli as in (2); identify gender (4) Reappraisal task: (a) experience emotional response while viewing negative & positive pictures, (b) try to reduce emotional distress while viewing negative pictures |
Voxel-wise analyses (with post hoc): Time-by-treatment arm effects: Reappraisal task (contrast of reducing emotional response to just looking at negative pictures): Treatment group: ↑ left lateral frontopolar cortex (middle frontal gyrus, BA 10) (No change in wait list group) ↑ left lateral frontopolar context-dependent connectivity (by PPI) with vmPFC (spanning olfactory cortex, ACC, mid-orbital gyrus)/ventral striatum No sign. time-by-treatment arm interaction effects in other regions of whole-brain or ROI analyses (ROIs: bilateral amygdala, anterior insula); no sign. effects in emotional reactivity or conflict task. No additional effects of remission status. |
Generalized linear model: Treatment group: ↑ left lateral frontopolar cortex with improvements in CAPS hyperarousal symptoms (Wald X2=7.71, p=.005) |
| Farrow et al. (2005) | 13 (9 males) assault, traffic, or industrial accident |
No control group | 54 | 20 | No comorbidities | Modified CBT: with a forgiveness component in average 7.3 (± 2.4) sessions |
Empathy judgments & social reasoning (reading & judging scenarios: 1) social reasoning as baseline, 2) empathic judgment, 3) forgivability, 4) intention task) |
Post-minus-pre-treatment: Empathic vs. social reasoning judgments: ↑ left middle temporal gyrus (BA 21, k=24) Forgivability vs. social reasoning judgments: ↑ PCC/precuneus (BA31/7, k=19) ↑ left middle frontal gyrus (BA 8/9, k=14) |
--- |
| Van Rooij et al. (2015a) | R=22 (all male) war veterans |
Non-R=17 H-Res=22 (all male) |
R: 71.7 (15.2) Non-R: 70.3 (11.3) |
R: 28.1 (17.8) Non-R: 66.1 (16.2) |
R (Pre/Post): Mood: 11/3 Anxiety: 4/2 Somatic: 1/0 Non-R (Pre/Post): Mood: 9/2 Anxiety: 8/5 Somatic: 1/1 |
Trauma-focused CBT and/or EMDR R/Non-R (mean): 8.8/9.8 |
Inhibition task stop-signal anticipation task withholding response (inhibition) & cues for anticipation (contextual cue processing) |
All patients vs. H-Res: No group-by-time interactions in whole-brain or ROI analyses (ROIs: amygdala, dACC, insula, hippocampus, vmPFC, left motor cortex, rIFG, right striatum). |
--- |
Abbreviations: ACC=anterior cingulate cortex, BA=Brodmann’s area, CAPS=Clinician-Administered PTSD Scale for DSM-IV, CBT=Cognitive Behavioral Therapy, dACC=dorsal ACC, dlPFC=dorsolateral prefrontal cortex, EMDR=Eye Movement Desensitization and Reprocessing, EXP=experimental treatment, FC=functional connectivity, H=healthy, trauma-unexposed subjects (without traumatic event in the past), H-Res=healthy, resilient controls (healthy, trauma-exposed subjects; no fulfilling of PTSD despite exposure to a traumatic event), k=cluster size, Non-R=non-responder (with persistent PTSD diagnosis after therapy; ‘non-remitted’), MBET=Mindfulness-based exposure therapy, OFC=orbitofrontal cortex, mPFC=medial prefrontal cortex, PCC=posterior cingulate cortex, PCGT=Present-Centered Group Therapy, PE=Prolonged exposure therapy, R=responder, rACC=rostral ACC, ROI=region of interest, SD=standard deviation, TAU=treatment as usual, vmPFC=ventromedial prefrontal cortex, VRET=Virtual reality exposure therapy, ↑=significant increase (of activation) in mentioned region, ↓=decrease in mentioned region.
Table 2.
provides details on the individual studies employing resting-state connectivity analyses. Only results and sample of subjects are mentioned who completed treatment and the second (f)MRI scan. Only significant results (p<0.05) are reported
|
Resting-state analyses | ||||||||
| Study | Sample size (n) of (R=remitted) patients, number of male subjects, and type of trauma event | Sample size (n) and type of control subjects |
Pre-CAPS scores Mean (and SD) of total CAPS scores |
Post-CAPS scores Mean (and SD) of total scores |
Type & duration of treatment | Type of paradigm within (f)MRI scans | Neuronal alterations from pre- to post-treatment |
Correlation analysis between symptom improvement (reduction in CAPS scores) & neuronal changes ↑ means pos. correlation ↓ means neg. correlation |
| King et al. (2016a) | 12 (all male) combat veterans |
Control group therapy=8 (PTSD patients, all male, combat veterans, PCGT) |
72.29 (18.32) Control group: 74.11 (15.34) |
56.71 (22) | Mindfulness-based exposure therapy group therapy 16 weeks à 120 min. |
Seed-based analysis | Whole-brain analyses: Group-by-time-interaction: MBET group („spreading interaction“): ↑ Connectivity of PCC seed with: bilateral dlPFC (left: k=26, right: k=30) dorsal ACC (k=26) ROI/seed analyses: Post>pre-contrast MBET group: ↑ Connectivity of PCC seed with: bilateral dlPFC (left: k=212, right: k=119) dorsal ACC (k=78) ↑ Connectivity of left amygala seed with: left hippocampus (k=136) dorsal ACC (k=51) Post>pre control/PCGT group: ↑ Connectivity of PCC seed with: Bilateral precuneus (left: k=46, right: k=49) left cuneus (k=105) superior parietal lobule (k=39) |
MBET group: ↑ PCC-dlPFC connectivity with avoidant and hyperarousal symptoms (not with intrusive symptoms) |
| Fonzo et al. (2017b) | 25 (treatment group, 23 female) |
Wait list=26 (PTSD patients) |
66.33 (15.17) Wait list: 71.37 (14.99) |
29.60 (21.26) Wait list: 64.23 (21.77) |
PE 12 weeks à 90 min. |
Seed-based analysis | No sign. treatment-by-time interaction effect between frontopolar cortex and vmPFC/ventral striatum (during reappraisal) | --- |
| Zhu et al. (2018) | PTSD=24 (7 male) car accidents, sexual/physical assaults, witnessing serious injuries/deaths |
H-Res=26 | 82.0 (15.2) | 31.2 (22.8) | PE 10 sessions |
Seed-based analysis | Group-by-time interaction (seeds: BLA, CMA, hippocampus): Difference between PTSD patients and H-Res: BLA connectivity: with OFC (z=3.71, p(FWE)=.01), with vmPFC (z=3.42, p=.008), and with thalamus (right: z=3.97, p=.003, left: z=2.99, p=.033) CMA connectivity: with OFC (z=3.76, p=.009) hippocampus connectivity: with vmPFC (z=3.84, p=.003) Post-hoc analysis (direction of effect of time): in patients (not in H-Res): ↑ FC in BLA-OFC (p=.001, t=3.77), ↑ FC in CMA-OFC (p=.002, t=3.49), ↑ FC in hippocampus-vmPFC (p=.004, t=3.20) Post-hoc analysis (direction of effect of group): in patients at pre-treatment (not at post-treatment or in H-Res): ↓ FC in BLA-OFC (p=0.0054, t=2.91) ↓ FC in BLA-thalamus (p=0.0069, t=2.82) ↓ FC in CMA-OFC (p =0.0003, t=3.99) ↓ FC in hippocampus-vmPFC (p=0.0045, t=2.94) |
Uncorrected correlation findings in the Supplement. |
| Santarnecchit et al. (2019) | TF-CBT group=14 EMDR group=17 (19 male) natural disaster, witnessing serious injuries/deaths |
TF-CBT: 45.7 EMDR: 57.6 |
>no statistically significant differences between EMDR/TF-CBT | Trauma-focused CBT or EMDR CBT:10±2 weeks EMDR:4±2 weeks |
Seed-based analysis | Similar positive CAPS change across TF-CBT and EMDR: ↓ FC between left visual cortex (i.e., cuneus) and left temporal pole/middle temporal gyrus (F(1,29)=4.76, p<.0031) ↑ FC between bilateral superior frontal gyrus and right temporal pole structures (F(1,29)=4.13, p<.015) |
--- | |
| Kennis et al. (2016) | R=17 (all male) combat veterans |
H-Res=22 Non-R=22 (all male) |
R: 65.00 (12.45) Non-R: 72.95 (14.39) |
R: 21.29 (14.11) Non-R: 61.36 (17.14) |
Trauma-focused CBT and/or EMDR R/Non-R (mean): 9.18/9.50 |
Graph-based network analysis |
Treatment effects: No significant group or group-by-time interaction effect Post-hoc analysis of R vs. Non-R: Group-by-time interaction effect: R: ↑ pallidum degree or clustering coefficient |
No sign. correlation |
Abbreviations: ACC=anterior cingulate cortex, BLA=basolateral amygdala, CAPS=Clinician-Administered PTSD Scale for DSM-IV, CBT=Cognitive Behavioral Therapy, CMA=centromedial amygdala, dlPFC=dorsolateral prefrontal cortex, EMDR=Eye Movement Desensitization and Reprocessing, FC=functional connectivity, H=healthy, trauma-unexposed subjects (without traumatic event in the past), H-Res=healthy, resilient controls (healthy, trauma-exposed subjects), k=cluster size, Non-R=non-responder, MBET=Mindfulness-based exposure therapy, OFC=orbitofrontal cortex, mPFC=medial prefrontal cortex, PCC=posterior cingulate cortex, PCGT=Present-Centered Group Therapy, PE=Prolonged exposure therapy, R=responder, ROI=region of interest, SD=standard deviation, vmPFC=ventromedial prefrontal cortex, ↑=significant increase (of resting state connectivity) in mentioned region, ↓=decrease in mentioned region.
Table 3.
gives information on the reviewed studies analyzing morphological alterations following treatment. Only results and sample of subjects are mentioned who completed treatment and the second MRI scan. Only significant results (p<0.05) are reported
|
Morphological analyses | |||||||||
| Study | Sample size (n) of (R=remitted) patients, number of male subjects, and type of trauma event | Sample size (n) and type of control subjects |
Pre-CAPS scores Mean (and SD) of total CAPS scores |
Post-CAPS scores Mean (and SD) of total scores |
Psychotropic medication in patient group (in subsample size or in percent) | Type & duration of treatment | Type of paradigm within (f)MRI scans | Neuronal alterations from pre- to post-treatment |
Correlation analysis between symptom improvement (reduction in CAPS scores) & neuronal changes ↑ means pos. correlation ↓ means neg. correlation |
| Levy-Gigi et al. (2013) | 39 (9 male) mainly environmental disaster, accident, violent crime |
H-Res=31 (11 male) |
62.4 (12.8) | 40.4 (20.3) | Benzodiazepine: 16 (< 4 weeks) no antidepressants |
CBT 12 weekly 1.5-hour sessions |
Volume change | Group-by-time interaction (Tukey’s HSD): PTSD patients (not in controls): ↑ hippocampal volumes at the second assessment relative to the first (p<.05) |
↑ total hippocampal volume (r=.52, p<.005) |
| Van Rooij et al. (2015b) | R=22 (all male) veterans |
Non-R=22 H-Res=23 H=25 |
R: 66.5 (12.3) Non-R: 76.3 (12.5) |
R: 25.0 (14.2) Non-R: 66.4 (15.2) |
R (Pre/Post): SSRI/SARI: 4/4 Benzodiazepine: 6/6 Antipsychotics/other: 3/1 Non-R (Pre/Post): SSRI/SARI: 9/13 Benzodiazepine: 4/2 Antipsychotics/other: 2/3 |
Trauma-focused CBT and/or EMDR mean session R: 9.2 (SD:6.5) Non-R: 10.0 (4.6) |
Volume change | No sign. time-by-group interaction effect. | No correlations with change in hippocampal volume. |
| Rubin et al., (2016) | R=23 (22% male) mainly accident, sexual/physical assault, natural disaster |
Non-R=17 H-Res=36 |
R: 81.3 (16.4) Non-R: 81.0 (14.5) |
R: 23.0 (20.4) Non-R: 68.0 (10.8) |
No medication usagee four weeks prior to participation | PE 10 weeks |
Volume change | No sign. time-by-group interaction effect. | |
| Laugharne et al. (2016) | EMDR=10 PE=10 (6 males) mainly adult sexual assault, witnessing death/injury |
(both treatment groups) | EMDR: 87.60 (20.92) PE: 80.70 (18.50) |
EMDR: 28.40 (27.85) PE: 22.30 (19.17) |
PE/EMDR: No psychotropic medication: 4/3 Antidepressant: 6/6 Benzodiazepine: 1/0. Antipsychotics: 0/3 |
EMDR or PE Twice-weekly for 12 sessions |
Volume change | Time-by-side-by-treatment interaction: after EMDR: ↑ left amygdala volume (p<.04) |
|
| Helpman et al. (2016b) | R=11 (2 males) mainly accidents, sexual/physical assault, witnessing death/injury |
H-Res=25 Non-R=14 |
R + Non-R: Total: 80.63 (15.58) |
R: <20 | Exclusion criteria: use of any psychotropic medication 4 weeks prior to participation (6 weeks for fluoxetine) | PE 10 weeks |
Volume change | Time-by-remission status-interaction effect: R (vs. Non-R): ↓ volume reduction & cortical thinning in left rACC |
|
| Bossini et al. (2017) | 19 (10 male) mainly assault/robbery, sudden death of a family member, terrorist attack |
H=19 | 75.8 (21.8) | 19.3 (15.5) | No medication | EMDR 12 sessions over 3 months |
Volume change | Group-by-time interaction for grey matter volume: Patients after treatment (vs. H): ↑ left parahippocampal gyrus (F(1,35)=11.237; p=.001, k=246), ↓ left thalamus (F(1,35)=9.432; p=.002, k=168) |
|
| Kennis et al. (2015) | R=16 (all male) veterans |
Non-R=23 H-Res=22 |
R: 63.25 (10.55) Non-R: 73.00 (14.37) |
R: 22.56 (14.63) Non-R: 58.91 (15.75) |
R (Pre/Post): SSRI/SARI: 4/3 Benzodiazepine: 5/3 Antipsychotics/other: 2/0 Non-R (Pre/Post): SSRI/SARI: 5/7 Benzodiazepine: 4/1 Antipsychotics/other: 2/4 |
Trauma-focused CBT with exposure and/or EMDR R/Non-R (mean): 9.33/9.35 |
White matter alteration using DTI (FA) | Tract-Based Analyses: Group-by-time interaction: Non-R: ↑ FA of left dorsal cingulum (p=.026) compared to R and H-Res → FA increase over treatment in Non-R Whole-brain analyses: Group-by-time interaction: R: ↓ FA H-Res: ↑ FA in two clusters: left posterior corona radiate (k=218, p=.004) superior longitudinal fasciculus (k=16, p=.049) ROIs: dorsal and hippocampal cingulum bundle, stria terminalis, fornix |
Voxel-wise analysis: posterior corona radiata (change in FA correlated with change in CAPS, Pearson’s r=.451, p=.004) |
Abbreviations: ACC=anterior cingulate cortex, CAPS=Clinician-Administered PTSD Scale for DSM-IV, CBT=Cognitive Behavioral Therapy, DTI=diffusion tensor imaging, EMDR=Eye Movement Desensitization and Reprocessing, FA=fractional anisotropy, H=healthy, trauma-unexposed subjects (without traumatic event in the past), H-Res=healthy, resilient controls (healthy, trauma-exposed subjects), k=cluster size, Non-R=non-responder, mPFC=medial prefrontal cortex, PE=Prolonged exposure therapy, R=responder, rACC=rostral ACC, ROI=region of interest, SD=standard deviation, ↑=significant increase (of morphological alteration) in mentioned region, ↓=decrease in mentioned region.
4.2.1.2. Different trauma types
One half of the studies (n = 12) included patients with mixed trauma events such as (sexual) assault, violent crime, traffic accident, environmental disaster, and injury/illness. Ten studies exclusively included combat veterans or police officers who witnessed gunfire attacks and two studies included patients exposed to interpersonal violence (intimate partner violence or child abuse).
4.2.1.3. Comorbidity of MDD
There were varying degrees of comorbidity, with the majority of patients reporting MDD in five studies, half of the patients diagnosed with MDD in nine studies and six studies analysed patients with no or few depressive symptoms.
4.2.1.4. Psychotropic Medication
Patients took no or very little medication in ten studies, approximately half of the patients were medicated in seven studies, and almost all patients in three studies (20 out of 24 studies allowed to draw conclusions on the subject number with regard to MDD and psychotropic medication; cf. Supplement Table A.1).
4.2.2. Treatment approach and fMRI tasks
4.2.2.1. Form of psychotherapy
PE and CBT-based interventions including exposure elements or EMDR were employed in the majority of treatment approaches (cf. Tables 1–3, and Supplement Table A.2).
4.2.2.2. FMRI paradigms
Emotionally valenced tasks without high cognitive demands predominate in nine out of 12 functional MRI studies (cf. Table 1).
5. Results
5.1. Treatment effects
5.1.1. Altered brain activation patterns following treatment
In total, 12 out of 24 studies used behavioural paradigms, out of which 10 analysed activation differences in specified regions without whole-brain correction (cf. Table 1 and Supplement Table A.3). However, activations outside of the a priori determined regions of interest (ROIs) were also reported by some studies but should be considered exploratory (cf. Table 1).
5.1.1.1. Cortical regions
Of the 12 studies investigating activation changes in mPFC, rACC, or orbitofrontal cortex, five studies reported an increased activation of the mPFC/rACC region following successful treatment (Aupperle et al., 2013; Felmingham et al., 2007; King et al., 2016b; Peres et al., 2011; Roy et al., 2014) (Figure 2, right side). Conversely, Helpman et al. (2016a) observed decreased activation in the right rACC, while the remaining six studies did not reported any change in these regions (Farrow et al., 2005; Fonzo et al., 2017b; Simmons et al., 2013; Thomaes et al., 2012; Van Rooij et al., 2015a; Van Rooij et al., 2016). However, all reported activation changes were found in studies employing a ROI approach and thus were not whole-brain corrected. The exact positions of the peak coordinates only partially overlapped across studies (see Supplement Table A.4).
Two studies did not employ any control group, limiting their ability to attribute the observed changes to the intervention (Aupperle et al., 2013; Felmingham et al., 2007). The type of task needs to be taken into account when interpreting these findings, as activation increases in mPFC/rACC was shown after emotion processing tasks, whereas the activation decrease was observed during extinction recall. All of these six studies evaluated diverging exposure-based treatment approaches (see Table 1).
Of the 12 studies (with a ROI approach in three studies), only one study reported a significant activation decrease in dorsal ACC by treatment (Thomaes et al., 2012).
Regarding the lateral PFC, divergent treatment effects were reported. While two studies indicated significant activation increases in the left middle frontal gyrus using rather cognitively demanding fMRI tasks (Farrow et al., 2005; Fonzo et al., 2017b), a study by Aupperle et al. (2013) indicated an activation decrease in the right middle frontal gyrus during the anticipation of negative images.
Activation changes in the insula were investigated by 12 studies, of which two reported a significant decrease in the left (ventral) anterior insula after successful intervention (Simmons et al., 2013; Thomaes et al., 2012) and one study observed a decrease in the right middle insula (Aupperle et al., 2013). The remaining studies (of which five defined the insula as ROI) returned no further significant results, although all findings employed comparable tasks (cf. Table 1). However, these results could be driven by high degrees of comorbid MDD (see Table 1 and Supplement Table A.1).
Finally, some individual results across diverging functional paradigms were reported, such as activation changes in dorsolateral PFC, in parietal, posterior and temporal cortical regions as well as the cerebellum, but still await replication (see Table 1 for details).
With regard to the different symptom clusters such as re-experiencing, avoidance, or hyperarousal, only three of the 12 studies analysed associations with neural outcomes, two of which reported significant associations (between left lateral frontopolar reappraisal activation and CAPS hyperarousal symptoms in Fonzo et al. (2017b) and between left inferior parietal lobe activation and re-experiencing symptoms before treatment in Van Rooij et al. (2015a)) (cf. Supplement).
5.1.1.2. Subcortical regions
Of the 12 studies investigating activation changes in the amygdalae, three studies reported an activation decrease (right-lateralized during exposure to negative stimuli (Aupperle et al., 2013; Roy et al., 2014) and left-lateralized during traumatic memory retrieval (Peres et al., 2011). One study reported a left-lateralized increase in response to angry faces (King et al., 2016b). The remaining eight studies (of which 5 chose an ROI approach) returned null results. Comparing the peak coordinates, the amygdala findings overlap across studies, including the correlations between symptom improvement and amygdala change (cf. 3.2), but again partly in different hemispheres (cf. Supplement Table A.4). The choice of the control group might have impacted these findings as activation decreases were found in comparison to healthy, trauma-exposed controls (Peres et al., 2011; Roy et al., 2014), or no controls (Aupperle et al., 2013), whereas activation increases were found in comparison to other PTSD patient groups (King et al., 2016b; Peres et al., 2011). The activation decreases were observed after CBT (Aupperle et al., 2013; Peres et al., 2011) and PE/Virtual reality exposure therapy (Roy et al., 2014)), activation increase after group Mindfulness-based exposure therapy (MBET) (King et al., 2016b), thus, different treatment forms could affect the amygdala differentially.
However, as the remaining five studies did not find any evidence of altered processing in the amygdalae it seems unlikely that such neural changes are central to the recovery from PTSD.
Similarly, there is no convincing evidence that successful treatment is associated with altered processing in the hippocampi. Of the 12 studies (of which five chose the hippocampi as ROI), only one study reported a significant right-hemispheric activation increase (Felmingham et al., 2007), but the small cluster of six voxel and the lack of a control groups limit the interpretability of this result.
Across the studies examining brain activation, no clear association between therapy effects and comorbid anxiety disorders (which could potentially explain the non-convergence of results) emerged (details in Table 1 and Supplement 3.1.1). Despite the varying degree of post-treatment CAPS scores, no pattern can be detected with respect to specific neural changes.
No associations emerged between specific characteristics of the samples, such as age, gender, or ethnicity, and the aforementioned treatment-induced cortical and subcortical activation changes (cf. Supplement 3.4).
5.1.2. Treatment effects in resting-state analyses
Three out of five studies investigating treatment-associated changes in resting-state activation reported significant alterations. As various seed regions were employed by these studies, results (see Table 2 and the Supplement) were not directly comparable and still await replication.
5.1.3. Treatment effects in morphological analyses
Four of six studies investigating changes in grey matter volume reported significant group-by-time interactions, albeit in five different brain regions. Significant volume increases post-treatment were each reported by one study in the bilateral hippocampus (Levy-Gigi et al., 2013), the left parahippocampal gyrus (Bossini et al., 2017), and the left amygdala (Laugharne et al., 2016). Further, volume decreases were reported for the left thalamus (Bossini et al., 2017) and the left rACC (Helpman et al., 2016b) (see Table 3, Supplement A.5). In the only study analysing treatment-associated changes in white matter integrity, only non-responders showed a significant increase in fractional anisotropy in the left dorsal cingulum over time (Kennis et al., 2015). Neither differences in medication status nor comorbidity are likely to fully account for these inconsistencies (see Table 3 and Supplement A.1). Hence, there is currently no converging evidence that successful intervention is associated with significant morphological changes.
5.2. Correlation analyses between clinical improvement and brain changes
Eight studies using functional paradigms analysed correlations between changes in symptom severity and brain activation. Increased rACC/mPFC activation correlated with clinical improvement in three of these eight studies (Felmingham et al., 2007; King et al., 2016b; Peres et al., 2011). Regarding the amygdala, two studies reported a negative correlation with symptom improvement (bilaterally in Felmingham et al., 2007; left-hemispheric in Peres et al., 2011), one study observed a positive correlation (King et al., 2016b), while one study could not confirm this relationship for remitted subjects (Van Rooij et al., 2016). Concerning morphology, one study found a positive correlation between symptom improvement and total hippocampal volume (Levy-Gigi et al., 2013) which could, however, not be replicated by another study (Van Rooij et al., 2015b).
In summary, the evidence for changes in brain activation or morphology associated with symptom improvement is currently weak (see Table 1 and Supplement for individual findings).
5.3. Baseline predictors related to treatment outcome
Four studies with functional paradigms additionally analysed the predictive value of baseline brain activation for treatment outcome, of which three studies found pre-treatment activation of the dorsal ACC as a significant predictor (Aupperle et al., 2013; Fonzo et al., 2017a; Van Rooij et al., 2016) and two studies found amygdala and insula activation as significant predictors (Fonzo et al., 2017a; Van Rooij et al., 2016). These results appear consistent, as the fourth study analysed a different ROI, the inferior parietal lobe (and found that activation of this structure, too, had predictive value (Van Rooij et al., 2015a)). (See Supplement with Table A.5; including individual findings of two resting-state and two morphological studies.)
6. Discussion
Given its comparatively high prevalence, surprisingly few studies have investigated the neural correlates of successful PTSD treatment to date. The identified 24 studies only converge to a certain degree, with altered activation patterns in the prefrontal cortex associated with successful intervention receiving the most, but overall weak, support. In light of the central role of the amygdala in PTSD, the findings regarding this structure appear more inconsistent than expected. Further, there is currently no convincing evidence that therapeutic gains are accompanied by morphological changes.
6.1. Discussion of functional treatment-induced findings
An increase in mPFC/rACC activation was the most frequently observed treatment-associated alteration. However, the evidence for such alterations needs to be considered weak at this point as seven out of 12 studies did not observe any such changes and the studies which reported these alterations only overlap partially with regards to the exact brain regions involved. Since previous meta-analyses indicate that a hypoactive mPFC is an important neural correlate of PTSD, the current evidence would be in line with the hypothesis that successful treatment can compensate for PTSD-related neural aberrations. Psychotherapy has been suggested to strengthen especially the mPFC, enabling this structure to better contribute to the extinction of conditioned fear and the volitionally downregulation of negative emotions (Koenigs & Grafman, 2009). It is assumed that this is achieved via inhibition of the amygdala, i.e. by top-down regulation (Etkin, Egner, & Kalisch, 2011; Marek, Sun, & Sah, 2019).
However, there is also surprisingly little evidence for parallel changes in the amygdala. Five out of nine studies investigating the role of this brain structure reported no treatment-induced change. Only three studies found the predicted decrease in activation, the extent of which was correlated with clinical improvement. Conversely, one study reported a treatment-induced increase in activation. Additionally, two (out of three) studies found that higher pre-treatment amygdala activation predicted a better treatment outcome. Considering these highly inconsistent findings, the role of the amygdala (and its putative increased downregulation by the mPFC) in PTSD recovery remains unclear.
Some of the reported discrepancies could potentially be explained by different subregions of the amygdala being involved. The peak coordinates in the three studies reporting activation decreases after successful intervention seem to lie mainly in the basolateral amygdala (BLA). In two recent non-treatment studies, PTSD patients showed hyperactivation in the BLA to trauma-related words (Neumeister et al., 2018a) and fearful faces during unconscious face processing (Neumeister et al., 2018b). While the BLA has direct and indirect (via the thalamus) projections to the mPFC (Kelly & Stefanacci, 2009), these potentially show reduced connectivity in PTSD patients (Aghajani et al., 2016).
Concerning other brain regions, there is no robust evidence of therapy-induced changes as of now. Three out of four studies investigating brain activation in the hippocampi returned null results. Concerning dorsal ACC, no robust conclusions can be drawn, as only one out of 12 studies found a change in activation by treatment and three studies found baseline activation as a predictor of treatment outcome. Similarly, there is little evidence for significant pre-post-changes in insula activation as this was only reported by three out of eight studies. Two (out of three) studies found baseline insular activation to be a significant predictor for treatment outcome.
6.2. Discussion of treatment-induced morphological findings
Another main finding was that treatment-induced changes were more frequently observed on the level of brain activation rather than brain structure. Significant structural changes by treatment were found in five different structures, but each was only reported once and not confirmed by another study. No pattern of comorbidities or medications can be seen as an explanatory factor for these differences. The latter seems relevant as Quidé et al. (2012) and Thomaes et al. (2014) discussed that pharmacotherapy may especially have an effect on brain morphology, while psychotherapy may affect in particular the brain activation.
As meta-analyses consistently documented hippocampal volume reduction in PTSD patients, a volume increase following successful treatment might have been expected. However, only one out of six studies reported a significant volume increase (Levy-Gigi et al., 2013). Similar to the amygdala subregions, it seems more likely that only certain subregions are associated with PTSD changes. This was supported by Suarez-Jimenez et al. (2019) reporting that specifically the pre-treatment volume of the anterior hippocampus, which seems to be related to extinction recall (Suarez-Jimenez et al., 2020), predicted clinical improvement. Concerning the thalamus, only one out of six studies found a volume decrease following treatment.
In summary, these findings are still too heterogeneous and scarce to allow for robust conclusions regarding the psychotherapeutic effect on brain morphology. Future studies should focus on the hippocampal and amygdala subregions, comprehensively reporting coordinates.
6.3. Discussion of treatment forms
Significant pre-post-activation-differences in the mPFC/rACC and the amygdala were observed following different treatment forms: Imaginal Exposure and Cognitive Restructuring Therapy, Exposure and Cognitive Restructuring Therapy, PE and Virtual reality exposure therapy, group MBET, and individual and group CBT. Since all of these are trauma-focused and contain elements of exposure (see Supplement Table A.2), the observed brain alterations might be best conceptualized as neural correlates of extinction learning (Ball, Knapp, Paulus, & Stein, 2016; Graham & Milad, 2011). In order to help elucidate which specific changes in symptomatology drive the observed neural changes, future studies should report cluster-level symptom severity scores such as re-experiencing, avoidance, or hyperarousal. This might then also facilitate conclusions regarding which treatment or treatment component is associated with which brain activation changes. Similarly, other aspects of the sample composition should be explored regarding their effects on neural alterations such as trauma type, time since traumatic event, age, and gender. However, no diverging patterns of neural change associated with the different forms of psychotherapy can be identified based on the current state of evidence.
6.4. Comparison with the existing body of literature and new findings
Earlier reviews on therapy-associated alterations reported decreased activation of the amygdala (Malejko, Abler, Plener, & Straub, 2017; Thomaes et al., 2014), as well as decreased activation of the insula and increased activation of the dorsal ACC and hippocampus (Malejko et al., 2017) following successful treatment. However, including all null findings published to date brings us to the conclusion that the robustness of these observations is very limited. Further research is needed to substantiate or falsify the previous conclusions. Similarly, this review does not support the assumption of activation changes in the dorsolateral PFC since this was only found in one out of 12 studies (Aupperle et al., 2013).
However, there is agreement between the present review and previous reviews (Colvonen et al., 2017; Malejko et al., 2017) that pre-treatment dorsal ACC, amygdala, and insula activation might be potential predictors for treatment outcome (see Supplement).
The current review further extends the existing body of literature by carefully analysing the existing evidence for mPFC activation by treatment. The inconsistent findings on treatment-induced amygdala change challenge the standard PTSD model assuming that amygdala plays a key-role for therapeutic success. Thus, this review highlights the need for further research in PTSD to either verify or improve the standard model of PTSD.
6.5. Limitations and directions for future research
The results reviewed here potentially reflect heterogeneities regarding the inclusion of subjects who experienced different traumatic events, received different forms of treatment and medication, and exhibited comorbid disorders to a different degree. We opted to not conduct a meta-analytical review due to limited overlap regarding the employed methods and behavioural paradigms. Different fMRI paradigms trigger activation in different brain areas and can thus also only detect therapy-associated changes in these regions. For example, treatment-specific increases in mPFC/rACC activation were reported by studies which used emotion processing paradigms, while two studies using cognitively demanding tasks reported increases in the left middle frontal gyrus. Thus, the current review describes the state of the empirical evidence, but cannot directly account for the impact of diverging methodological and technical procedures. Future studies should focus on replicating previous studies with regards to the employed paradigms to allow for such an aggregation of data.
In many cases, it remains unclear whether the observed results are indeed disorder-specific. For example, as hypoactivation in the rACC (Diener et al., 2012) as well as treatment-induced activation increase in the rACC (Sankar et al., 2018) were also reported in MDD, the fact that four of the five studies which reported an activation increase in the mPFC/rACC used samples with high comorbidity of MDD might be relevant. Future studies should attempt to disambiguate which symptom changes are associated with which neural alterations, for example by masking with or covarying for changes in comorbid symptoms. In addition, future studies should explore associations with differential symptom profiles.
Thirdly, many studies likely suffered from limited statistical power due to small sample sizes ranging from 8 to 39 with a mean value of 18.25 across all studies. As some findings in mPFC/rACC and amygdala are based on less than 15 subjects (Felmingham et al., 2007; King et al., 2016b; Roy et al., 2014), the robustness of these results could be adversely impacted. Further, several studies used overlapping samples (cf. 2.2), which might render their results not fully independent.
Lastly, many studies did not include a control group. This means that the observed neural alterations could, in theory, also be an effect of the repeated scans, low retest reliability of the employed paradigms (Dichter, Sikich, Song, Voyvodic, & Bodfish, 2012) or other non treatment-related factors. Correlations with symptom severity or change scores as well as responder/non-responder analyses ameliorate these concerns to a certain degree. But future studies should aim to control for such effects, albeit costly.
7. Conclusion
Our systematic review identified limited evidence for a treatment-induced increase in mPFC/rACC activation. This would align well with the concept of therapy-induced normalization of brain processes, as previous meta-analyses found hypoactivation of the mPFC as a neural correlate of PTSD. However, one would then also expect to observe associated decreases in amygdala activation, which was not consistently the case. The putative increase in prefrontal activation seemed unrelated to the treatment approach; however, only trauma-focused and exposure-based approaches have been studied so far. The available data appear too inconsistent for valid conclusions regarding the role of the insula, or the hippocampus in therapeutic gains in PTSD. Further, there is little convergence regarding treatment-specific changes in brain morphology or resting-state activation.
Supplementary Material
Funding Statement
This work was funded by the German Research Foundation (DFG) grant to J. K. Daniels (former DA 1222/4-1, now WA 1539/8-2); the EU Rosalind-Franklin Fellowship Program to J. K. Daniels; and the German National Merit Foundation grant to A. Sierk. This work was supported by the EU Rosalind-Franklin Fellowship; Deutsche Forschungsgemeinschaft [DA 1222/4-1 & WA 1539/8-2]; Studienstiftung des Deutschen Volkes.
Data availability statement
As this article is a review of published work, there is no data to be archived.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics statement
As this article is a review of published work, there was no separate ethical review or informed consent procedure.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Aghajani, M., Veer, I. M., Van Hoof, M.-J., Rombouts, S. A. R. B., Van der Wee, N. J., & Vermeiren, R. R. J. M. (2016). Abnormal functional architecture of amygdala-centered networks in adolescent posttraumatic stress disorder. Human Brain Mapping, 37(3), 1120–22. doi: 10.1002/hbm.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). doi: 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Aupperle, R. L., Allard, C. B., Simmons, A. N., Flagan, T., Thorp, S. R., Norman, S. B., … Stein, M. B. (2013). Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Research: Neuroimaging, 214(1), 48–55. doi: 10.1016/j.pscychresns.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Ball, T. M., Knapp, S. E., Paulus, M. P., & Stein, M. B. (2016). Brain activation during fear extinction predicts exposure success. Depression and Anxiety, 34(3), 257–266. doi: 10.1002/da.22583 [DOI] [PubMed] [Google Scholar]
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. doi: 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Bossini, L., Santarnecchi, E., Casolaro, I., Koukouna, D., Caterini, C., Cecchini, F., … Fagiolini, A. (2017). Morphovolumetric changes after EMDR treatment in drug-naïve PTSD patients. Rivista Di Psichiatria, 52(1), 24–31. doi: 10.1708/2631.27051 [DOI] [PubMed] [Google Scholar]
- Colvonen, P. J., Glassman, L. H., Crocker, L. D., Buttner, M. M., Orff, H., Schiehser, D. M., … Afari, N. (2017). Pretreatment biomarkers predicting PTSD psychotherapy outcomes: A systematic review. Neuroscience and Biobehavioral Reviews, 75, 140–156. doi: 10.1016/j.neubiorev.2017.01.027 [DOI] [PubMed] [Google Scholar]
- Cusack, K., Jonas, D. E., Forneris, C. A., Wines, C., Sonis, J., Middleton, J. C., … Gaynes, B. N. (2016). Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review, 43, 128–141. doi: 10.1016/j.cpr.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Dennis, E. L., Disner, S. G., Fani, N., Salminen, L. E., Logue, M., Clarke, E. K., … Morey, R. A. (2019). Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: Results from the PGC-ENIGMA PTSD consortium. Molecular Psychiatry. doi: 10.1038/s41380-019-0631-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter, G. S., Sikich, L., Song, A., Voyvodic, J., & Bodfish, J. W. (2012). Functional neuroimaging of treatment effects in psychiatry: Methodological challenges and recommendations. International Journal of Neuroscience, 122(9), 483–493. doi: 10.3109/00207454.2012.678446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener, C., Kuehner, C., Brusniak, W., Ubl, B., Wessa, M., & Flor, H. (2012). A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage, 61(3), 677–685. doi: 10.1016/j.neuroimage.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow, T. F. D., Hunter, M. D., Wilkinson, I. D., Gouneea, C., Fawbert, D., Smith, R., … Woodruff, P. W. R. (2005). Quantifiable change in functional brain response to empathic and forgivability judgments with resolution of posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 140(1), 45–53. doi: 10.1016/j.pscychresns.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Felmingham, K., Kemp, A., Williams, L., Das, P., Hughes, G., Peduto, A., & Bryant, R. (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science, 18(2), 127–129. doi: 10.1111/j.1467-9280.2007.01860.x [DOI] [PubMed] [Google Scholar]
- Fitzgerald, J. M., Digangi, J. A., & Phan, K. L. (2018). Functional neuroanatomy of emotion and its regulation in PTSD. Harvard Review of Psychiatry, 26(3), 116–128. doi: 10.1097/HRP.0000000000000185.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo, G. A., Goodkind, M. S., Oathes, D. J., Zaiko, Y. V., Harvey, M., Peng, K. K., … Etkin, A. (2017a). PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. American Journal of Psychiatry, 174(12), 1163–1174. doi: 10.1176/appi.ajp.2017.16091072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo, G. A., Goodkind, M. S., Oathes, D. J., Zaiko, Y. V., Harvey, M., Peng, K. K., … Etkin, A. (2017b). Selective effects of psychotherapy on frontopolar cortical function in PTSD. American Journal of Psychiatry, 174(12), 1175–1184. doi: 10.1176/appi.ajp.2017.16091073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. M., & Milad, M. R. (2011). The study of fear extinction: Implications for anxiety disorders. The American Journal of Psychiatry, 168(12), 1255–1265. doi: 10.1176/appi.ajp.2011.11040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habetha, S., Bleich, S., Weidenhammer, J., & Fegert, J. M. (2012). A prevalence-based approach to societal costs occurring in consequence of child abuse and neglect. Child and Adolescent Psychiatry and Mental Health, 6(1), 1–10. doi: 10.1186/1753-2000-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, J. P., Hayes, S. M., & Mikedis, A. M. (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 1–13. doi: 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman, L., Marin, M.-F., Papini, S., Zhu, X., Sullivan, G. M., Schneier, F., … Neria, Y. (2016a). Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. NeuroImage: Clinical, 12, 715–723. doi: 10.1016/j.nicl.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman, L., Papini, S., Chhetry, B. T., Shvil, E., Rubin, M., Sullivan, G. M., … Neria, Y. (2016b). PTSD remission after prolonged exposure treatment is associated with anterior cingulate cortex thinning and volume reduction. Depression and Anxiety, 33(5), 384–391. doi: 10.1002/da.22471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imel, Z. E., Laskab, K., Jakcupcakc, M., & Simpson, T. L. (2014). Meta-analysis of dropout in treatment for PTSD. Journal of Consulting and Clinical Psychology, 81(3), 394–404. doi: 10.1037/a0031474.Meta-analysis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, W. H., Chang, K. J., & Kim, N. H. (2016). Disrupted topological organization in the whole-brain functional network of trauma-exposed firefighters: A preliminary study. Psychiatry Research: Neuroimaging, 250, 15–23. doi: 10.1016/j.pscychresns.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Kelly, R., & Stefanacci, L. (2009). Amygdala: Structure and circuitry in primates. Encyclopedia of Neuroscience (Pp, 341–345(Elsevier). doi: 10.1016/B978-008045046-9.00148-0 [DOI] [Google Scholar]
- Kennis, M., Van Rooij, S. J. H., Tromp, D. P. M., Fox, A. S., Rademaker, A. R., Kahn, R. S., … Geuze, E. (2015). Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology, 40(10), 2434–2442. doi: 10.1038/npp.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis, M., Van Rooij, S. J. H., Van den Heuvel, M. P., Kahn, R. S., & Geuze, E. (2016). Functional network topology associated with posttraumatic stress disorder in veterans. NeuroImage: Clinical, 10, 302–309. doi: 10.1016/j.nicl.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A. P., Block, S. R., Sripada, R. K., Rauch, S. A., Porter, K. E., Favorite, T. K., … Liberzon, I. (2016a). Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety, 33(4), 289–299. doi: 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- King, A. P., Block, S. R., Sripada, R. K., Rauch, S. A. M., Porter, K. E., Favorite, T. K., … Liberzon, I. (2016b). A pilot study of mindfulness-based exposure therapy in OEF/OIF combat veterans with PTSD: Altered medial frontal cortex and amygdala responses in social-emotional processing. Frontiers in Psychiatry, 7(SEP), 1–13. doi: 10.3389/fpsyt.2016.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs, M., & Grafman, J. (2009). Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. The Neuroscientist, 15(5), 540–548. doi: 10.1177/1073858409333072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S., & Gallinat, J. (2013). Gray matter correlates of posttraumatic stress disorder: A quantitative meta-analysis. Biological Psychiatry, 73(1), 70–74. doi: 10.1016/j.biopsych.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Laugharne, J., Kullack, C., Lee, C. W., McGuire, T., Brockman, S., Drummond, P. D., & Starkstein, S. (2016). Amygdala volumetric change following psychotherapy for posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences, 28(4), 312–318. doi: 10.1176/appi.neuropsych.16010006 [DOI] [PubMed] [Google Scholar]
- Levy-Gigi, E., Szabó, C., Kelemen, O., & Kéri, S. (2013). Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biological Psychiatry, 74(11), 793–800. doi: 10.1016/j.biopsych.2013.05.017 [DOI] [PubMed] [Google Scholar]
- Logue, M. W., Van Rooij, S. J. H., Dennis, E. L., Davis, S. L., Hayes, J. P., Stevens, J. S., … Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. doi: 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng, L. Y., & Milad, M. R. (2017). Post-traumatic stress disorder: The relationship between the fear response and chronic stress. Chronic Stress, 1, 247054701771329. doi: 10.1177/2470547017713297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko, K., Abler, B., Plener, P. L., & Straub, J. (2017). Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: A systematic literature review. Frontiers in Psychiatry, 8. doi: 10.3389/fpsyt.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek, R., Sun, Y., & Sah, P. (2019). Neural circuits for a top-down control of fear and extinction. Psychopharmacology, 236(1), 313–320. doi: 10.1007/s00213-018-5033-2 [DOI] [PubMed] [Google Scholar]
- Maren, S., & Holmes, A. (2016). Stress and fear extinction. Neuropsychopharmacology, 41(1), 58–79. doi: 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., & Stewart, L. A.. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4(1), 1–9. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. D., & Tumpap, A. M. (2017). Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: A meta-analytic study. CNS Spectrums, 22(4), 363–372. doi: 10.1017/S1092852916000833 [DOI] [PubMed] [Google Scholar]
- Neumeister, P., Feldker, K., Heitmann, C. Y., Buff, C., Brinkmann, L., Bruchmann, M., & Straube, T. (2018b). Specific amygdala response to masked fearful faces in post-traumatic stress relative to other anxiety disorders. Psychological Medicine, 48(7), 1209–1217. doi: 10.1017/S0033291717002513 [DOI] [PubMed] [Google Scholar]
- Neumeister, P., Gathmann, B., Hofmann, D., Feldker, K., Heitmann, C. Y., Brinkmann, L., & Straube, T. (2018a). Neural correlates of trauma-related single word processing in posttraumatic stress disorder. Biological Psychology, 138, 172–178. doi: 10.1016/j.biopsycho.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A., Rabellino, D., Densmore, M., Frewen, P. A., Paret, C., Kluetsch, R., … Lanius, R. A. (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Human Brain Mapping, 38(1), 541–560. doi: 10.1002/hbm.23402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R., Spreng, R. N., Shin, L. M., & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36(9), 2130–2142. doi: 10.1016/j.neubiorev.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Peres, J. F. P., Foerster, B., Santana, L. G., Fereira, M. D., Nasello, A. G., Savoia, M., … Lederman, H. (2011). Police officers under attack: Resilience implications of an fMRI study. Journal of Psychiatric Research, 45(6), 727–734. doi: 10.1016/j.jpsychires.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Quidé, Y., Witteveen, A. B., El-Hage, W., Veltman, D. J., & Olff, M. (2012). Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: A systematic review. Neuroscience and Biobehavioral Reviews, 36(1), 626–644. doi: 10.1016/j.neubiorev.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Rauch, S. L., Shin, L. M., & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biological Psychiatry, 60(4), 376–382. doi: 10.1016/j.biopsych.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Roy, M. J., Costanzo, M. E., Blair, J. R., & Rizzo, A. A. (2014). Compelling evidence that exposure therapy for PTSD normalizes brain function. Annual Review of Cybertherapy and Telemedicine, 12, 61‐65. Retrieved from https://www.cochranelibrary.com/central/doi: 10.1002/central/CN-01753741/full [DOI] [PubMed] [Google Scholar]
- Roy, M. J., Francis, J., Friedlander, J., Banks-Williams, L., Lande, R. G., Taylor, P., … Rothbaum, B. (2010). Improvement in cerebral function with treatment of posttraumatic stress disordera. Annals of the New York Academy of Sciences, 1208(1), 142–149. doi: 10.1111/j.1749-6632.2010.05689.x [DOI] [PubMed] [Google Scholar]
- Rubin, M., Shvil, E., Papini, S., Chhetry, B. T., Helpman, L., Markowitz, J. C., … Neria, Y. (2016). Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Research: Neuroimaging, 252, 36–39. doi: 10.1016/j.pscychresns.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar, A., Melin, A., Lorenzetti, V., Horton, P., Costafreda, S. G., & Fu, C. H. Y. (2018). A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Research: Neuroimaging, 279, 31–39. doi: 10.1016/j.pscychresns.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Santarnecchi, E., Bossini, L., Vatti, G., Fagiolini, A., La Porta, P., Di Lorenzo, G., … Rossi, A. (2019). Psychological and brain connectivity changes following trauma-focused CBT and EMDR treatment in single-episode PTSD patients. Frontiers in Psychology, 10, 129. doi: 10.3389/fpsyg.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartory, G., Cwik, J., Knuppertz, H., Schürholt, B., Lebens, M., Seitz, R. J., & Schulze, R. (2013). In search of the trauma memory: A meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress Disorder (PTSD). PLoS ONE, 8(3), e58150. doi: 10.1371/journal.pone.0058150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenbauer, M. A., Glass, C. R., Arnkoff, D. B., Tendick, V., & Gray, S. H. (2008). Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry: Interpersonal and Biological Processes, 71(2), 134–168. doi: 10.1521/psyc.2008.71.2.134 [DOI] [PubMed] [Google Scholar]
- Shin, L. M., Rauch, S. L., & Pitman, R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences, 1071(1), 67–79. doi: 10.1196/annals.1364.007 [DOI] [PubMed] [Google Scholar]
- Shou, H., Yang, Z., Satterthwaite, T. D., Cook, P. A., Bruce, S. E., Shinohara, R. T., Rosenberg, B., & Sheline, Y. I. (2017). Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage: Clinical, 14, 464–470. doi: 10.1016/j.nicl.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, A. N., Norman, S. B., Spadoni, A. D., & Strigo, I. A. (2013). Neurosubstrates of remission following prolonged exposure therapy in veterans with posttraumatic stress disorder. Psychotherapy and Psychosomatics, 82(6), 382–389. doi: 10.1159/000348867 [DOI] [PubMed] [Google Scholar]
- Stark, E. A., Parsons, C. E., Van Hartevelt, T. J., Charquero-Ballester, M., McManners, H., Ehlers, A., … Kringelbach, M. L. (2015). Post-traumatic stress influences the brain even in the absence of symptoms: A systematic, quantitative meta-analysis of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 56, 207–221. doi: 10.1016/j.neubiorev.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Suarez-Jimenez, B., Albajes-Eizagirre, A., Lazarov, A., Zhu, X., Harrison, B. J., Radua, J., … Fullana, M. A. (2020). Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: A meta-analysis of functional magnetic resonance imaging studies. Psychological Medicine, 50(9), 1442–1451. doi: 10.1017/S0033291719001387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Jimenez, B., Zhu, X., Lazarov, A., Mann, J. J., Schneier, F., Gerber, A., … Markowitz, J. C. (2019). Anterior hippocampal volume predicts affect-focused psychotherapy outcome. Psychological Medicine, 1–7. doi: 10.1017/S0033291719000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes, K., Dorrepaal, E., Draijer, N., De Ruiter, M. B., Elzinga, B. M., Van Balkom, A. J., … Veltman, D. J. (2012). Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychological Medicine, 42(11), 2337‐2349. doi: 10.1017/S0033291712000499 [DOI] [PubMed] [Google Scholar]
- Thomaes, K., Dorrepaal, E., Draijer, N., Jansma, E. P., Veltman, D. J., & Van Balkom, A. J. (2014). Can pharmacological and psychological treatment change brain structure and function in PTSD? A systematic review. Journal of Psychiatric Research, 50(1), 1–15. doi: 10.1016/j.jpsychires.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Van Rooij, S. J. H., Geuze, E., Kennis, M., Rademaker, A. R., & Vink, M. (2015a). Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology, 40(3), 667–675. doi: 10.1038/npp.2014.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij, S. J. H., Kennis, M., Sjouwerman, R., Van Den Heuvel, M. P., Kahn, R. S., & Geuze, E. (2015b). Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychological Medicine, 45(13), 2737–2746. doi: 10.1017/S0033291715000707 [DOI] [PubMed] [Google Scholar]
- Van Rooij, S. J. H., Kennis, M., Vink, M., & Geuze, E. (2016). Predicting treatment outcome in PTSD: A longitudinal functional MRI study on trauma-unrelated emotional processing. Neuropsychopharmacology, 41(4), 1156–1165. doi: 10.1038/npp.2015.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Xie, H., Chen, T., Cotton, A. S., Salminen, L. E., Logue, M. W., Clarke-Rubright, E. K., Wall, J., Dennis, E. L., O’Leary, B. M., Abdallah, C. G., Andrew, E., Baugh, L. A., Bomyea, J., Bruce, S. E., Bryant, R., Choi, K., Daniels, J. K., Davenport, N. D., … Liberzon, I. (2020). Cortical volume abnormalities in posttraumatic stress disorder: An ENIGMA-psychiatric genomics consortium PTSD workgroup mega-analysis. Molecular Psychiatry. doi: 10.1038/s41380-020-00967–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, B. V., Schnurr, P. P., Mayo, L., Young-Xu, Y., Weeks, W. B., & Friedman, M. J. (2013). Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 74(6), e541–e550. doi: 10.4088/JCP.12r08225 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W., Ruscio, A. M., & Keane, T. M. (1999). Psychometric properties of nine scoring rules for the clinician- administered posttraumatic stress disorder scale. Psychological Assessment, 11(2), 124–133. doi: 10.1037/1040-3590.11.2.124 [DOI] [Google Scholar]
- Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jönsson, B., … Steinhausen, H. C. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679. doi: 10.1016/j.euroneuro.2011.07.018 [DOI] [PubMed] [Google Scholar]
- Yang, Z., Gu, S., Honnorat, N., Linn, K. A., Shinohara, R. T., Aselcioglu, I., … Sheline, Y. I. (2018a). Network changes associated with transdiagnostic depressive symptom improvement following cognitive behavioral therapy in MDD and PTSD. Molecular Psychiatry, 23(12), 2314–2323. doi: 10.1038/s41380-018-0201-7 [DOI] [PubMed] [Google Scholar]
- Yang, Z., Oathes, D. J., Linn, K. A., Bruce, S. E., Satterthwaite, T. D., Cook, P. A., … Sheline, Y. I. (2018b). Cognitive behavioral therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(4), 311–319. doi: 10.1016/j.bpsc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Kim, J., & Tonegawa, S. (2020). Amygdala reward neurons form and store fear extinction memory. Neuron, 105(6), 1077–1093.e7. doi: 10.1016/j.neuron.2019.12.025 [DOI] [PubMed] [Google Scholar]
- Zhu, X., Suarez-Jimenez, B., Lazarov, A., Helpman, L., Papini, S., Lowell, A., … Neria, Y. (2018). Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depression and Anxiety, 35(10), 974–984. doi: 10.1002/da.22816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this article is a review of published work, there is no data to be archived.


