Abstract
Purpose
To determine the relationship between fine particulate matter (PM2.5) and ocular outcomes such as visual impairment and age-related eye disease.
Methods
Baseline data were used from the Canadian Longitudinal Study on Aging. The Comprehensive Cohort consisted of 30,097 adults ages 45 to 85 years. Annual mean PM2.5 levels (µg/m3) for each participant's postal code were estimated from satellite data. Ozone, sulfur dioxide, and nitrogen dioxide levels were also estimated. Binocular presenting visual acuity was measured using a visual acuity chart. Intraocular pressure (IOP) was measured in millimeters of mercury using the Reichart Ocular Response Analyzer. Participants were asked about a diagnosis of glaucoma, macular degeneration, or cataract. Logistic and linear regression models were used.
Results
The overall mean PM2.5 level was 6.5 µg/m3 (SD = 1.8). In the single pollutant models, increased PM2.5 levels (per interquartile range) were associated with visual impairment (odds ratio [OR] = 1.12; 95% confidence interval [CI], 1.02–1.24), glaucoma (OR = 1.14; 95% CI, 1.01–1.29), and visually impairing age-related macular degeneration (OR = 1.52; 95% CI, 1.10–2.09) after adjustment for sociodemographics and disease. PM2.5 had a borderline adjusted association with cataract (OR = 1.06; 95% CI, 0.99–1.14). In the multi-pollutant models, increased PM2.5 was associated with glaucoma and IOP only after adjustment for sociodemographics and disease (OR = 1.24; 95% CI, 1.05–1.46 and β = 0.24; 95% CI, 0.12–0.37).
Conclusions
Increased PM2.5 is associated with glaucoma and IOP. These associations should be confirmed using longitudinal data and potential mechanisms should be explored. If confirmed, this work may have relevance for revision of World Health Organization thresholds to protect human health.
Keywords: fine particulate matter, air pollution, glaucoma, eye disease, intraocular pressure, CLSA
Contaminated air increases the risk of disease and premature mortality. Prior research has identified consistent relationships between air pollution and lung cancer, stroke, hospital admission, and mortality.1–5 The effects of air pollution on the eye, which is directly exposed, are much less understood. It has been known for some time that smoking is harmful to vision, increasing the risks of cataract and age-related macular degeneration (AMD).6,7 However, the association between ambient air pollutants, such as fine particulate matter (PM2.5), and the risk of eye disease has not been widely studied. PM2.5, a consistent risk factor for mortality,1,3 is defined as having a mass median aerodynamic diameter of <2.5 µm. Common sources of PM2.5 include motor vehicles, smelters, power plants, industry, residential fireplaces and wood stoves, and forest fires.8 The World Health Organization has determined that long-term exposure to levels of PM2.5 >10 µg/m3 is associated with health hazards.9
Little epidemiological research exists examining the relationship between PM2.5 and eye disease. Relationships have been identified between PM2.5 and visual impairment,10 glaucoma,11,12 and AMD,13 but the results for cataract are unclear.14,15 To our knowledge, all of the existing studies have been done in east Asia or the United Kingdom with none being done in the United States or Canada. Some evidence indicates that these findings are biologically plausible.16,17 Using the baseline data from a large, population-based database called the Canadian Longitudinal Study on Aging (CLSA), we investigated the association between PM2.5 and four ocular outcomes: visual impairment, glaucoma, AMD, and cataract.
Methods
Study Population
Baseline data were used from the CLSA Comprehensive Cohort.18 We focused on the baseline data because not enough people have developed incident eye disease over the 3 years of follow-up completed thus far to be able to do a longitudinal analysis at this time. The CLSA Comprehensive Cohort consists of 30,097 people 45 to 85 years old who live near one of 11 data collection sites in seven Canadian provinces. The 11 CLSA data collection sites are located in Victoria, Vancouver, Surrey, Calgary, Winnipeg, Hamilton, Ottawa, Montreal, Sherbrooke, Halifax, and St. John's. Stratified random sampling was done using provincial healthcare registration databases and random digit dialing of landline telephones. When sampling from provincial healthcare databases, people who were temporary visa holders or had transitional health coverage (when the information was available) were excluded. Non-permanent residents and non-Canadian citizens were excluded from both sampling frames. Further inclusion criteria were that participants had to be community dwelling, be cognitively unimpaired at baseline, speak English or French, and provide written informed consent. Full-time members of the Canadian Armed Forces, individuals residing on a federal First Nations reserve or settlement, and individuals living in nursing homes were excluded.
Study Design
Data collection by CLSA staff consisted of a home visit and a visit to a data collection site.18 All CLSA staff collecting data underwent standardized training in order to collect data in a uniform way across all sites. Baseline assessments were done between December 2011 and July 2015. Written informed consent was obtained from all participants. Research Ethics Board approval was received in July 2010 from all affiliated sites. Ethics approval was received from the University of Ottawa for this analysis in May 2019.
Data Collection
Visual Impairment and Eye Disease
At the data collection sites, visual acuity was measured using an illuminated Early Treatment of Diabetic Retinopathy Study chart and its standard protocol.19 Scores were converted to the log of the minimum angle of resolution (logMAR). Visual acuity was evaluated at a distance of 2 meters using habitual distance correction (i.e., wearing normal corrective lenses for distance vision). Visual impairment (VI) was defined as presenting binocular acuity worse than 20/40 (0.301 logMAR), as is often used in North American research.20 Participants were asked to report if they have ever had a diagnosis of cataract, AMD, or glaucoma. To try to separate out those with late-stage AMD, we distinguished between those with a report of AMD without VI and those with a report of AMD with VI. Corneal-compensated intraocular pressure (IOP) was measured using the Reichart Ocular Response Analyzer (Reichart Technologies, Depew, NY, USA). The average IOP of the right and left eyes was used. If one eye had missing IOP data, then the IOP value of the other eye was used. If the person reported a diagnosis of glaucoma, we assumed that they were taking pressure-lowering eye drops. To estimate their pretreatment IOP, we imputed values by dividing their mean IOP by 0.7, which is the mean treatment effect of pressure-lowering eye drops.21 This approach has been used previously.22
Air Pollution
Air pollution measures were provided by the Canadian Urban Environmental Health Research Consortium (CANUE) and were merged into the CLSA data.23 Ground-level PM2.5 concentration levels were estimated by satellite by combining aerosol optical depth retrievals using the GEOS-Chem chemical transport model from the following National Aeronautics and Space Administration (NASA, Washington, DC, USA) instruments: Moderate Resolution Imaging Spectroradiometer, Multi-angle Imaging SpectroRadiometer, and Sea-viewing Wide Field-of-view Sensor. These measurements were subsequently calibrated to regional ground-based observations using geographically weighted regression. These 0.01° × 0.01° gridded surface datasets were used to assign values of annual mean concentration (µg/m3) of PM2.5 to the postal code of each CLSA participant.24–26
Hourly ground-level ozone (O3) concentrations were estimated with the Global Environmental Multi-Scale Modelling Air Quality and Chemistry model by Environment and Climate Change Canada (Gatineau, QC, Canada) staff. Estimates incorporate ground-level observation data. These datasets were used to assign values of annual mean concentration of O3 in parts per billion to the postal code of each CLSA participant.27–30
Ground-level sulfur dioxide (SO2) concentrations were estimated from the Ozone Monitoring Instrument satellite data using SO2 profiles from the Global Environmental Multiscale (GEM) model for air quality and chemistry over North America. These annual gridded datasets were aggregated to 3-year running averages and used by CANUE staff to assign values of annual mean concentration of SO2 in parts per billion to the postal code of each CLSA participant.26,31–33
Nitrogen dioxide (NO2) concentrations were estimated using data from national air pollution surveillance monitoring following methods reported in Hystad et al.34 Background and regional components were estimated with land use regression procedures using satellite-derived NO2 estimates and geographic variables, and local scale variation was modeled using deterministic gradients. The model included road length within 10 km, satellite NO2 estimates, area of industrial land use within 2 km, and summer rainfall.26,35
Demographic, Health, and Lifestyle Data
Demographic data including age, sex, ethnicity, education, and income were obtained during the in-home visit using an interviewer-administered questionnaire. In order to assess household income, participants were asked, “What is your best estimate of the total household income received by all household members, from all sources, before taxes and deductions, in the past 12 months?” Participants were asked if they had ever received a physician diagnosis of several comorbid conditions including diabetes and hypertension. Blood pressure was measured six times using the BpTru BPM200 blood pressure monitor (Medaval, Dublin, Ireland). The first reading was discarded, and the average of the subsequent five readings was used. Hypertension was defined if a participant reported a physician diagnosis of hypertension or if the average systolic blood pressure was 130 mmHg or higher or diastolic blood pressure was 80 mmHg or higher.36 Smoking status was classified as current, never, or former based on these interview questions: “Have you smoked at least 100 cigarettes in your life?” and “At the present time, do you smoke cigarettes daily, occasionally (at least once in last 30 days), or not at all (not in last 30 days)?” A current smoker was defined as a person who reported smoking at least 100 cigarettes and currently smokes daily or occasionally, whereas a former smoker was someone who reported smoking at least 100 cigarettes in life but had not smoked in the last 30 days.
Statistical Analysis
Mean values of PM2.5 and their standard deviations were given by categorical demographic, health, lifestyle, and ocular variables. Pearson's correlation coefficient was calculated between PM2.5 and IOP. IOP values greater than 60 were excluded, as these were considered probable measurement errors. Relationships between PM2.5 and categorical ocular variables were tested by linear regression.
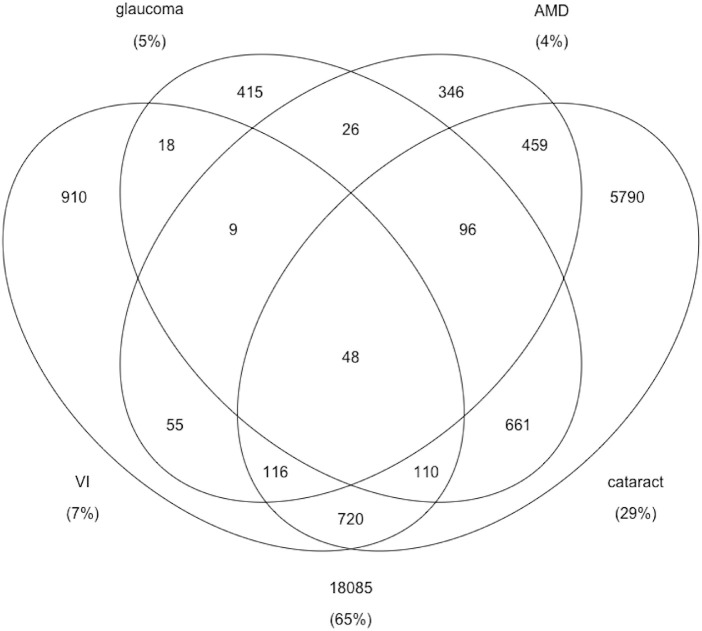
In separate multivariable analyses, logistic regression was used to determine the relationship between PM2.5 and VI, glaucoma, and cataract; multinomial regression was used for the three-category AMD variable; and linear regression was used for IOP. These regression models were first adjusted for potentially confounding variables such as age, sex, education, household income, ethnicity, diabetes, hypertension, smoking, and province. In a second phase of adjustment, models were also adjusted for other pollutants including O3, SO2, and NO2. The correlation between PM2.5 and the other pollutants was checked to guard against multicollinearity. Variance inflation factors were checked, as well. Sampling weights and strata variables were incorporated into all analyses using the SVY commands in Stata/SE 16 (StataCorp, College Station, TX, USA). The Venn diagram was produced in R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) with the limma 3.46.0 package.37 The Venn diagram was produced for those who had non-missing values for all four ocular outcomes and non-missing PM2.5 data (n = 27,864).
Results
Ninety-seven percent of CLSA participants (n = 29,147) had a non-missing value for PM2.5. The 950 people who were missing data on PM2.5 were almost all from British Columbia (78%). Those missing PM2.5 data were also older, more likely to be female, and more likely to have VI and cataract than those who had PM2.5 data.
Certain people lived in postal codes that had higher mean levels of PM2.5 (Table 1). These included older people, certain ethnic groups, people who had lower incomes, current smokers, and people with type 1 diabetes. PM2.5 levels also differed by province, with higher levels in Alberta, Ontario, and Quebec than in the other provinces. Also, people who reported AMD, glaucoma, or cataract lived in areas with higher mean PM2.5 levels (P < 0.05) (Table 2). There is some overlap among those affected by our four ocular outcomes (VI, AMD, glaucoma, and cataract). In the Venn diagram shown in the Figure, for example, 5790 have only cataract, 661 have cataract and glaucoma, 720 have cataract and VI, and 48 have all 4 outcomes. The average unimputed IOPs of those with and without glaucoma were 17.7 mmHg (SD = 5.2) and 15.8 mmHg (SD = 3.5), respectively. Pearson's correlation coefficient between PM2.5 and IOP was 0.01 (P = 0.20).
Table 1.
Average Levels of PM2.5 by Demographic, Smoking, or Health (N = 29,147)
| Variable* | Fine Particulate Matter (µg/m3) Mean (SD) |
|---|---|
| Age group (y) | |
| 45–54 (n = 7405) | 6.4 (1.4) |
| 55–64 (n = 9575) | 6.5 (1.9) |
| 65–74 (n = 7107) | 6.6 (2.1) |
| 75–85 (n = 5060) | 6.7 (2.1) |
| Sex | |
| Female (n = 14,830) | 6.5 (1.8) |
| Male (n = 14,317) | 6.5 (1.8) |
| Ethnic or racial background | |
| White (n = 27,467) | 6.5 (1.8) |
| Black (n = 255) | 6.9 (1.8) |
| Asian (n = 663) | 6.5 (1.5) |
| Aboriginal (n = 347) | 6.3 (1.7) |
| Other (n = 415) | 6.8 (1.7) |
| Education | |
| More than bachelor's degree (n = 6223) | 6.5 (1.7) |
| Bachelor's degree (n = 6862) | 6.6 (1.7) |
| Less than bachelor's degree (n = 16,010) | 6.5 (1.8) |
| Annual household income | |
| ≥$150,000 (n = 10,003) | 6.4 (1.6) |
| $50,000–$100,000 (n = 9564) | 6.5 (1.8) |
| $20,000–$50,000 (n = 6167) | 6.7 (1.9) |
| <$20,000 (n = 1525) | 7.0 (2.0) |
| Refused/don't know (n = 1888) | 6.6 (1.9) |
| Smoking | |
| Never (n = 13,812) | 6.5 (1.7) |
| Former (n = 12,723) | 6.5 (1.8) |
| Current (n = 2511) | 6.8 (1.8) |
| Diabetes | |
| None (n = 23,909) | 6.5 (1.8) |
| Type 1 (n = 170) | 6.7 (1.6) |
| Type 2 (n = 2694) | 6.5 (1.9) |
| Suspect/neither type (n = 2032) | 6.3 (1.7) |
| Hypertension | |
| No | 6.5 (1.7) |
| Yes | 6.5 (1.8) |
| Province | |
| Alberta (n = 2949) | 7.8 (1.9) |
| British Columbia (n = 5509) | 5.8 (1.3) |
| Manitoba (n = 3110) | 6.1 (1.1) |
| Newfoundland and Labrador (n = 2124) | 5.7 (1.8) |
| Nova Scotia (n = 3043) | 5.0 (1.2) |
| Ontario (n = 6355) | 7.0 (1.8) |
| Quebec (n = 6057) | 7.2 (1.6) |
*The following variables have missing data: education (n = 52), smoking (n = 101), diabetes (n = 342), and hypertension (n = 1).
Table 2.
Average Levels of PM2.5 by Visual Impairment or Eye Disease (N = 29,147)
| Variable* | Fine Particulate Matter (µg/m3) Mean (SD) | P |
|---|---|---|
| Visual impairment | ||
| No (n = 26,655) | 6.5 (1.8) | |
| Yes (n = 2,067) | 6.5 (2.0) | 0.780 |
| Glaucoma | ||
| No (n = 27,525) | 6.5 (1.8) | |
| Yes (n = 1,467) | 6.7 (2.0) | <0.001 |
| Age-related macular degeneration | ||
| No (n = 27,715) | 6.5 (1.8) | |
| Yes but not visually impaired (n = 948) | 6.6 (1.9) | 0.014 |
| Yes and visually impaired (n = 237) | 6.8 (2.2) | 0.011 |
| Cataract | ||
| No (n = 20,264) | 6.5 (1.7) | |
| Yes (n = 8,286) | 6.6 (2.0) | <0.001 |
Note that 425 were missing data on visual impairment, 155 were missing data on glaucoma, 247 were missing data on AMD, and 597 were missing data on cataract.
Figure.
Venn diagram to illustrate the overlap in the number of people affected by ocular outcomes.
In single pollutant regression models, those who lived in areas with higher PM2.5 levels (per interquartile range [2.9 µg/m3]) were more likely to have visual impairment (odds ratio [OR] = 1.12; 95% confidence interval [CI], 1.01–1.24) after adjusting for age, sex, ethnicity, education, household income, smoking, diabetes, hypertension, and province (Table 3). In addition, those who lived in areas with higher PM2.5 levels were more likely to have glaucoma (OR = 1.14; 95% CI, 1.01–1.29) and visually impairing AMD (OR = 1.51; 95% CI, 1.10– 2.08). There was a borderline association between PM2.5 and cataract after adjustment, although the odds ratio was quite small (OR = 1.06; 95% CI, 0.99–1.14). There was no relationship between PM2.5 and IOP (β = –0.00; 95% CI, –0.10–0.09) in the single-pollutant model.
Table 3.
Results from Five Regression Models Showing the Relationships Between PM2.5 and Ocular Outcomes Adjusting for Demographics, Health, and Smoking
| Outcomes | PM2.5* Odds Ratio or β† | 95% CI | P |
|---|---|---|---|
| Visual impairment | 1.12 | 1.02–1.24 | 0.030 |
| Glaucoma | 1.14 | 1.01–1.29 | 0.035 |
| Intraocular pressure | –0.00 | –0.10–0.09 | 0.958 |
| AMD | |||
| No | 1.00 | Reference | — |
| Yes but not visually impaired | 1.00 | 0.86–1.15 | 0.959 |
| Yes and visually impaired | 1.51 | 1.10–2.08 | 0.011 |
| Cataract | 1.06 | 0.99–1.14 | 0.080 |
Measures of association are per interquartile range of PM2.5 (2.9 µg/m3).
Adjusted for age, sex, ethnicity, education, household income, smoking, diabetes, hypertension, and province.
PM2.5 was somewhat correlated with other pollutants such as O3 (r = –0.08), SO2 (r = –0.12, and NO2 (r = 0.20) (P < 0.001). When these three other pollutants were entered into the model, PM2.5 became more associated with glaucoma (OR = 1.24; 95% CI, 1.05–1.46) (Table 4). Higher levels of both PM2.5 and O3 were associated with IOP with the other three pollutants in the model (β = 0.24; 95% CI, 0.12–0.37 and β = 0.39; 95% CI, 0.28–0.50, respectively). Higher PM2.5 levels only had a borderline association with visually impairing AMD (OR = 1.41; 95% CI, 0.96–2.08; P = 0.08). Higher O3 levels were inversely associated with cataract (OR = 0.92; 95% CI, 0.85–0.99), and NO2 was inversely associated with VI (OR = 0.86; 95% CI, 0.74–0.99; P = 0.042).
Table 4.
Results from Five Regression Models Showing the Relationships Between PM2.5 and Ocular Outcomes Adjusting for Demographics, Health, Smoking, and Other Forms of Air Pollution
| Outcomes | PM2.5* Odds Ratio or β† | 95% CI | P |
|---|---|---|---|
| Visual impairment | 1.09 | 0.97–1.24 | 0.157 |
| Glaucoma | 1.24 | 1.05–1.46 | 0.010 |
| Intraocular pressure | 0.24 | 0.12–0.37 | <0.001 |
| AMD | |||
| No | 1.00 | Reference | — |
| Yes, but not visually impaired | 0.99 | 0.82–1.20 | 0.905 |
| Yes, and visually impaired | 1.41 | 0.96–2.08 | 0.079 |
| Cataract | 0.98 | 0.90–1.07 | 0.716 |
Measures of association are per interquartile range of PM2.5 (2.9 µg/m3).
Adjusted for age, sex, ethnicity, education, household income, smoking, diabetes, hypertension, province, ozone, sulfur dioxide, and nitric oxide.
Given that we calculated imputed IOP for people with glaucoma to account for presumed treatment effects, we did a sensitivity analysis excluding people with glaucoma to further investigate the relationships between air pollution and IOP. The results from the sensitivity analysis were consistent with our main results. Specifically, in the single pollutant model, PM2.5 was not associated with IOP (β = –0.07; 95% CI, –0.15–0.02). In the multi-pollutant model, PM2.5 and O3 were associated with IOP (β = 0.15; 95% CI, 0.04–0.26 and β = 0.40; 95% CI, 0.29–0.50, respectively).
In the multi-pollutant models, the variance inflation factors, which can indicate problems with collinearity, were less than 2.5 except for O3, which had a variance inflation factor of 2.6. When we ran the models without O3 in a sensitivity analysis, PM2.5 remained statistically significantly associated with glaucoma (OR = 1.21; 95% CI, 1.04–1.42), but the relationship with IOP was attenuated and had borderline significance (β = 0.11; 95% CI, –0.01–0.23; P = 0.067).
Discussion
In single-pollutant models, higher values of PM2.5 were associated with an increased odds of visual impairment, visually impairing AMD, and glaucoma. In multi-pollutant models, higher values of PM2.5 were associated with glaucoma and IOP. The relationship between PM2.5 and glaucoma is biologically plausible. Emerging evidence from mouse models suggests that PM2.5 exposure may contribute to glaucoma and ocular hypertension.16 A study by Li et al.16 in mice found that exposure of the ocular surface and trabecular meshwork to PM2.5 resulted in increases in IOP and upregulation of the nucleotide-binding domain, leucine-rich containing family, pyrin domain-containing-3 (NLRP3) inflammasome; caspase-1; IL-1β; and gasdermin D protein levels in outflow tissues. To understand the entry of PM2.5 into the eye, the investigators used fluorescent PM2.5 tracers and found that 10- to 500-nm particles passed through the cornea into the anterior chamber and were mainly deposited in the outflow tissue with most particles remaining in the ciliary body. Li et al.16 also conducted experiments on human trabecular meshwork cells in vitro and found that exposure to PM2.5 resulted in decreased human trabecular meshwork cell viability, increased oxidative stress, and activation of pyroptosis via the NLRP3 inflammasome pathway. Further evidence that oxidative stress is involved in the biological pathway is that Nwanaji-Enwerem et al.38 found that long-term ambient black carbon exposure, which is a subcomponent of PM2.5, was associated with IOP but only in those with a high oxidative stress allelic risk score, indicating that certain subgroups of people may be more susceptible than the average.
Our results are in agreement with a few studies. In the United Kingdom, Chua et al.12 reported that people living in areas with higher PM2.5 were more likely to report glaucoma, although that study's odds ratio per interquartile increase was much smaller than ours (OR = 1.06; 95% CI, 1.01–1.12). They also reported that higher PM2.5 levels were associated with thinner macular ganglion cell–inner plexiform layers. In a Taiwan nested case-control study, Sun et al.11 also reported an association between PM2.5 and a diagnosis of glaucoma using data from a health insurance administrative database (OR = 1.19; 95% CI, 1.05–1.36). Using Korean health insurance data, Min and Min39 found an association between particulate matter < 10 µg and childhood glaucoma using a longitudinal design (hazard ratio = 1.22; 95% CI, 1.15–1.28). None of these studies adjusted for other pollutants, which in our research resulted in stronger associations between PM2.5 and glaucoma.
We also found that higher levels of PM2.5 were associated with visually impairing AMD in a single pollutant model. However, this relationship was attenuated and had only borderline statistical significance in the multi-pollutant model, particularly after entering SO2. PM2.5 was not associated with all AMD in either single or multi-pollutant models. We assume the reason for this is that PM2.5 does not affect the early AMD process, which does not affect visual acuity, but rather only the late AMD neovascularization process, which does. A cross-sectional study by Riggs et al.17 supports the idea that PM2.5 induces endothelial dysfunction. They found that levels of PM2.5 were positively associated with proangiogenesis molecules such as angiopoietin-1, vascular endothelial growth factor, platelet-derived growth factor-BB, erythropoietin, and matrix metalloproteinase-9. More research on this topic is needed. In agreement with our findings is that Chua et al.13 reported that higher levels of PM2.5 were related to self-reported visually impairing AMD, thinner photoreceptor synaptic regions, thicker photoreceptor inner segment layer, and thinner retinal pigment epithelium. The authors did not adjust their model for other air pollutants, so we can only compare our single pollutant model to their results.
Strengths of this research include the use of a large, population-based dataset with information on multiple pollutants and data on other confounders such as age, sex, ethnicity, education, household income, smoking, and diabetes. To our knowledge, we are the first North American team to examine this question; however, our research does have some limitations. Glaucoma was based on self-report of a diagnosis from an eye care professional. However, IOP, an endophenotype for glaucoma, was measured, and the results with glaucoma and IOP were consistent in that both were related to PM2.5. Second, our data are cross-sectional, so we cannot establish the temporality of the exposure to air pollution and the presence of glaucoma. Although the CLSA is longitudinal, at this time there has only been one wave of 3-year follow-up data released, and there is insufficient power to conduct a longitudinal analysis. Finally, the air pollution data are based on the postal code of the residence of the participant, so if a person does not spend much time at their residence or they recently moved to that residence, there would be misclassification of exposure. This misclassification, though, would likely be nondifferential by glaucoma status, which would result in an underestimation of the true effect.
To conclude, increased PM2.5 was associated with glaucoma and IOP. These associations should be confirmed using longitudinal data, and potential mechanisms should be explored. The mean long-term exposure levels of PM2.5 in this study were below the World Health Organization threshold of 10 µg/m3 and the Canadian threshold of 8.8 µg/m3. Therefore, if confirmed, this work may have relevance for revised thresholds to protect human health.40
Acknowledgments
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA) and has been conducted using the CLSA Comprehensive Dataset version 4.0 and Follow-up 1 Comprehensive Dataset version 1.0 under Application Number 190212. The CLSA is led by Parminder Raina, PhD; Christina Wolfson, PhD; and Susan Kirkland, PhD.
Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference LSA 94473 and the Canada Foundation for Innovation. The research by E.E.F. was funded by an operating grant from the CIHR (ACD-170303). The funders had no role in the design, analysis, or the interpretation of results. The opinions expressed in this manuscript are the authors’ own and do not reflect the views of the CLSA. Calculated air pollution metrics indexed to DMTI Spatial Inc. postal codes were provided by the Canadian Urban Environmental Health Research Consortium.
Disclosure: A. Grant, None; G. Leung, None; M.-J. Aubin, None; M.-J. Kergoat, None; G. Li, None; E.E. Freeman, None
References
- 1.Di Q, Dai L, Wang Y, et al.. Association of short-term exposure to air pollution with mortality in older adults. JAMA .2017; 318(24): 2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y, Wang Y, Di Q, et al.. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. BMJ .2019; 367: l6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Chen R, Sera F, et al.. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med .2019; 381(8): 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hvidtfeldt UA, Severi G, Andersen ZJ, et al.. Long-term low-level ambient air pollution exposure and risk of lung cancer - a pooled analysis of 7 European cohorts. Environ Int .2021; 146: 106249. [DOI] [PubMed] [Google Scholar]
- 5.Wellenius GA, Burger MR, Coull BA, et al.. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med .2012; 172(3): 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong R, Zhou B, Sun Q, Gu H, Tang N, Wang B.. Smoking and the risk of age-related macular degeneration: a meta-analysis. Ann Epidemiol .2008; 18(8): 647–656. [DOI] [PubMed] [Google Scholar]
- 7.Ye J, He J, Wang C, et al.. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci .2012; 53(7): 3885–3895. [DOI] [PubMed] [Google Scholar]
- 8.Ontario Ministry of the Environment, Conservation and Parks. Fine particulate matter. Available at: http://www.airqualityontario.com/science/pollutants/particulates.php. Accessed March 25, 2021.
- 9.World Health Organization. Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005. Available at: www.who.int/airpollution/publications/aqg2005/en/. Accessed March 25, 2021. [PubMed]
- 10.Yang BY, Guo Y, Zou Z, et al.. Exposure to ambient air pollution and visual impairment in children: a nationwide cross-sectional study in China. J Hazard Mater .2021; 407: 124750. [DOI] [PubMed] [Google Scholar]
- 11.Sun HY, Luo CW, Chiang YW, et al.. Association between PM2.5 exposure level and primary open-angle glaucoma in Taiwanese adults: a nested case-control study. Int J Environ Res Public Health .2021; 18(4): 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua SYL, Khawaja AP, Morgan J, et al.. The relationship between ambient atmospheric fine particulate matter (PM2.5) and glaucoma in a large community cohort. Invest Ophthalmol Vis Sci .2019; 60(14): 4915–4923. [DOI] [PubMed] [Google Scholar]
- 13.Chua SYL, Warwick A, Peto T, et al.. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank [published online ahead of print January 25, 2021]. Br J Ophthalmol , 10.1136/bjophthalmol-2020-316218. [DOI] [PubMed] [Google Scholar]
- 14.Shin J, Lee H, Kim H.. Association between exposure to ambient air pollution and age-related cataract: a nationwide population-based retrospective cohort study. Int J Environ Res Public Health .2020; 17(24): 9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YH, Park SJ, Paik HJ, Kim MK, Wee WR, Kim DH.. Unexpected potential protective associations between outdoor air pollution and cataracts. Environ Sci Pollut Res Int .2018; 25(11): 10636–10643. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Xing C, Zhou J, et al.. Airborne particulate matter (PM2.5) triggers ocular hypertension and glaucoma through pyroptosis. Part Fibre Toxicol .2021; 18(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riggs DW, Zafar N, Krishnasamy S, et al.. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ Res .2020; 180: 108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raina P, Wolfson C, Kirkland S, et al.. Cohort profile: the Canadian Longitudinal Study on Aging (CLSA). Int J Epidemiol .2019; 48(6): 1752–1753j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I.. New visual acuity charts for clinical research. Am J Ophthalmol .1982; 94(1): 91–96. [PubMed] [Google Scholar]
- 20.Aljied R, Aubin MJ, Buhrmann R, Sabeti S, Freeman EE.. Prevalence and determinants of visual impairment in Canada: cross-sectional data from the Canadian Longitudinal Study on Aging. Can J Ophthalmol .2018; 53(3): 291–297. [DOI] [PubMed] [Google Scholar]
- 21.van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH.. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology .2005; 112(7): 1177–1185. [DOI] [PubMed] [Google Scholar]
- 22.Khawaja AP, Cooke Bailey JN, Wareham NJ, et al.. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet .2018; 50(6): 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brook JR, Setton EM, Seed E, Shooshtari M, Doiron D, CANUE – The Canadian Urban Environmental Health Research Consortium. The Canadian Urban Environmental Health Research Consortium - a protocol for building a national environmental exposure data platform for integrated analyses of urban form and health. BMC Public Health .2018; 18(1): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Donkelaar A, Martin RV, Spurr RJ, Burnett RT.. High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environ Sci Technol .2015; 49(17): 10482–10491. [DOI] [PubMed] [Google Scholar]
- 25.Boys BL, Martin RV, van Donkelaar A, et al.. Fifteen-year global time series of satellite-derived fine particulate matter. Environ Sci Technol .2014; 48(19): 11109–11118. [DOI] [PubMed] [Google Scholar]
- 26.DMTI Spatial. CanMap Postal Code Suite v2015.3 [computer program]. Richmond Hill, ON: DMTI Spatial; 2015. [Google Scholar]
- 27.Robichaud A, Ménard R. Multi-year objective analyses of warm season ground-level ozone and PM2.5 over North America using real-time observations and Canadian operational air quality models. Atmos Chem Phys .2014; 14(4): 1769–1800. [Google Scholar]
- 28.Robichaud A, Ménard R, Zaïtseva Y, Anselmo D. Multi-pollutant surface objective analyses and mapping of air quality health index over North America. Air Qual Atmos Health .2016; 9(7): 743–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Environment and Climate Change Canada. Data files: CHRONOS_Ground-Level_O3_NA_2002.nc to CHRONOS_Ground-Level_O3_NA_2009.nc inclusive. Toronto, ON: Air Quality Research Division; 2017. [Google Scholar]
- 30.Environment and Climate Change Canada. Data files: GEMMACH_Ground-Level_O3_NA_2010.nc to GEMMACH_Ground-Level_O3_NA_2015.nc inclusive. Toronto, ON: Air Quality Research Division; 2017. [Google Scholar]
- 31.McLinden CA, Fioletov V, Boersma KF, et al.. Improved satellite retrievals of NO2 and SO2 over the Canadian oil sands and comparisons with surface measurements. Atmos Chem Phys .2014; 14: 3637–3656. [Google Scholar]
- 32.Kharol SK, McLinden CA, Sioris CE, et al.. OMI satellite observations of decadal changes in ground-level sulfur dioxide over North America. Atmos Chem Phys .2017; 17: 5921–5929. [Google Scholar]
- 33.Environment and Climate Change Canada. Data files: OMI_Ground-Level_SO2_NA_2005.nc to OMI_Ground-Level_SO2_NA_2015.nc inclusive. Toronto, ON: Air Quality Research Division; 2017. [Google Scholar]
- 34.Hystad P, Setton E, Cervantes A, et al.. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect .2011; 119(8): 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weichenthal S, Pinault LL, Burnett RT.. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep .2017; 7(1): 16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, et al.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension .2018; 71(6): 1269–1324. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie M, Phipson B, Wu D, et al.. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research .2015; 43(7): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwanaji-Enwerem JC, Wang W, Nwanaji-Enwerem O, et al.. Association of Long-term Ambient Black Carbon Exposure and Oxidative Stress Allelic Variants With Intraocular Pressure in Older Men. JAMA Ophthalmol .2019; 137(2): 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min KB, Min JY.. Association of Ambient Particulate Matter Exposure with the Incidence of Glaucoma in Childhood. Am J Ophthalmol .2020; 211: 176–182. [DOI] [PubMed] [Google Scholar]
- 40.Nazarenko Y, Pal D, Ariya PA.. Air quality standards for the concentration of particulate matter 2.5, global descriptive analysis. Bull World Health Organ .2021; 99(2): 125–137D. [DOI] [PMC free article] [PubMed] [Google Scholar]