Focal lesions of the midbrain may cause parkinsonism, dystonia, and limb tremor.1, 2
We describe clinical, neurophysiological and imaging features of a patient who developed hemidystonia and irregular rest, postural and kinetic upper limb tremor after a midbrain stroke, initially misdiagnosed as “Holmes tremor” (HT).
In March 2009, a 40‐year‐old man presented an abrupt onset of left hemiparesis, left‐sided miosis and eyelid ptosis, caused by a right paramedian midbrain infarct. Hemiparesis improved gradually over the next weeks. Five months later, he gradually developed severe impairment of finger dexterity during repetitive movements and prominent irregular postural and kinetic tremor of the left upper limb (inconstantly occurring also at rest), without any cogwheel rigidity or shuffling gait.
Brain magnetic resonance imaging (MRI) exhibited a right dorsal paramedian midbrain ischemic lesion, including the red nucleus. Dopamine transporter imaging revealed a complete loss of substantia nigra terminals on the right striatum (Fig. 1). Diffusion‐weighted MRI 3 T confirmed the disrupted microstructural integrity within the midbrain lesion, evidenced by lower fractional anisotropy and higher mean diffusivity.
FIG. 1.
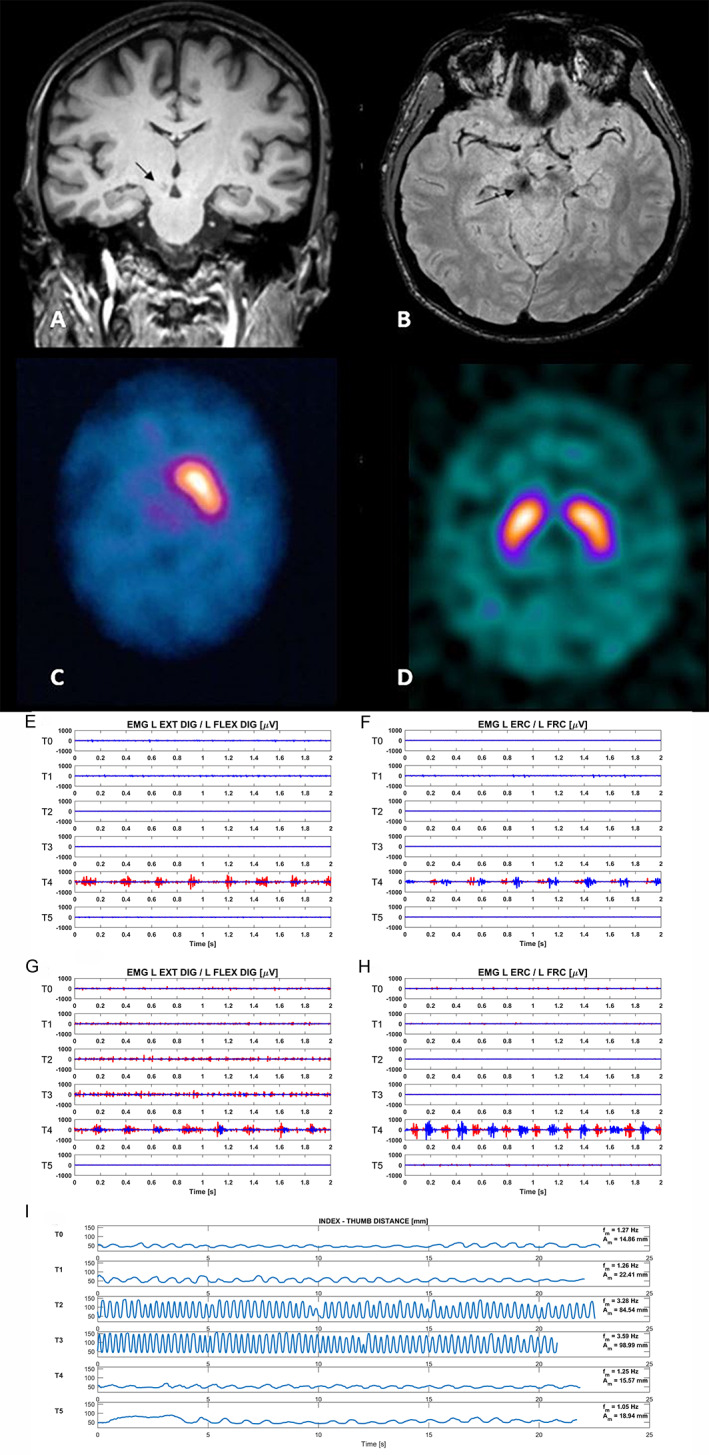
T1weighted brain MRI shows a hypointense lesion in right dorsal paramedian midbrain region (arrows; coronal view in A; axial view in B). 123Iodine‐FP‐CIT SPECT shows complete loss of uptake on the right striatum of the index case (C) and a normal uptake in an age‐matched healthy subject (D). Trapezius, deltoid, biceps and triceps brachii, extensor carp radialis (ECR)/flexor carpi radialis (FCR), extensor digitorum comunis (EDC)/flexor digitorum superficialis (FDS). Rest position: at T4, an irregular 3.7 Hz tremor with synchronous pattern with co‐contraction between EDC/FDS (antagonist) with clear predominance of extensors (E) and 3.7 Hz tremor with predominant alternating pattern between antagonist muscles (ERC/FRC) has been recorded (F). Finger‐to‐finger position: at T4, an 4.2 Hz irregular tremor with synchronous pattern of co‐contraction between EDC and FDS with mild predominance of extensors (G) and 4.2 Hz regular tremor with predominant alternating pattern between wrist antagonist muscles (ERC and FCR) (H). Kinematic data (frequency and amplitude) at different time points, during the finger tapping task performed with the left hand, best performance recorded from T2 to T3 along with an overall similar performance at T0, T4 and T5 (I). Legenda: red color: extensors muscles; blu color: flexors muscles.
Within 1 year, treatment with levodopa was started yielding an excellent response, as tremor and impaired finger dexterity completely recovered. After a few weeks, he developed end‐of‐dose left‐sided tonic postural hemidystonia that greatly improved after associating rotigotine (up to 10 mg/day) and entacapone.
To investigate the clinical and neurophysiological pattern of response to levodopa, we performed a suprathreshold levodopa challenge by administering 100 mg immediate‐release plus 100 mg sustained‐release formulation of levodopa after 12‐hour overnight withdrawal. The test was recorded in a movement analysis laboratory with an optoelectronic system (SmartD,Bts). An upper limbs surface PolyEMG and a total body kinematic study according to LAMB protocol3 was carried out in a timeline from T0 to T5 (T1, 45‐minutes; T2, 90‐minutes; T3, 120‐minutes; T4, 150‐minutes; T5, 240‐minutes), aiming to disentangle OFF‐related features (T0‐T5) from peak‐dose ON signs (T2‐T3) and biphasic motor features at early‐ON (T1) and at end‐of‐dose (T4) periods.
In the OFF‐state (T0 and T5), the main features were left‐sided upper limb dystonic posturing with right‐hand mirroring (without tremor) and some ankle and toes dystonia during gait. Forty‐five minutes after levodopa (T1), a mild left upper limb rest and postural tremor (not re‐emergent) was observed. After 150 minutes (T4) a rest (3.7 Hz) and postural (4.2 Hz) tremor was recorded at wrist and fingers along with a kinetic tremor during finger‐to‐nose task associated with dystonic posturing, reflecting an end‐of‐dose phenomenon. No tremor was recorded in the full‐ON state between 50 and 150 minutes as well as in the full‐OFF state, which started after 180 minutes (Video 1). Impaired dexterity during finger‐tapping task improved mainly between 90 and 120‐minutes and showed no substantial difference between T5 and T4 (Fig. 1).
Video 1.
Segment 1. T0 (OFF state, after 12‐hours overnight withdrawal of levodopa): severe impairment of finger dexterity during left‐hand finger‐tapping task with mild right‐hand mirror movements, reflecting dystonia and parkinsonism; left upper limb and left toes dystonic posturing on walking (no shuffling gait); no rest, postural (the arms outstretching posture without re‐emergent tremor) and kinetic tremor; no cerebellar sign during finger‐to‐nose task. Segment 2. T1 (45 minutes after 100 mg immediate‐release plus 100 mg sustained‐release formulation of levodopa): left upper limb rest and postural tremor (during contralateral task) associated with mild dystonic posturing. Segment 3. T2 (full‐ON state, after 90 minutes): right/left‐hand finger‐tapping task well performed. After 120 minutes (T3), the patient was still in the full‐ON state with similar clinical features (video not shown). Segment 4. T4 (after 150 minutes): note the rest, postural (during contralateral task) and kinetic tremor associated with mild left‐hand posturing. Segment 5. T5 (OFF state, 240 minutes): similarly to T0, note the coexistence of dystonia and parkinsonism during left‐hand finger‐tapping task with clear right‐hand mirroring, without significant tremor.
Clinical and neurophysiological data suggest a diphasic pattern of an unusual combination of rest, postural and action left upper limb tremor associated with dystonic postures (ie, dystonic tremor) and coexistent features of parkinsonism.
A 4‐Hz pseudo‐rhythmic pattern of involuntary movements has been described with cerebello‐rubro‐thalamic pathway lesions, while the coexistence of tremor at rest is usually described as HT.4 Levodopa‐responsive tremor with features resembling HT has been reported.5 The present report suggests that whenever such a “HT‐like” tremor is responsive to levodopa, it is unlikely due to a rubral lesion, while it more likely reflects a dystonic tremor due to severe dopaminergic denervation. No cerebellar sign was observed and HT worsens from posture to kinetic, which is not the case here. We speculate that a dramatic acute dopaminergic deafferentation was the main cause of the hemidystonia (prevalent segmental facial and upper limb dystonia), whose pattern (tonic vs. phasic tremor‐like) may vary according to the long‐ and short‐duration response to levodopa. When these patients are treated with levodopa, the appearance of dystonic tremor only in periods of subtherapeutic levodopa effect (just prior to the full ON and OFF states) should be considered a diphasic dystonic tremor and managed accordingly.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
MM: 1B, 1C, 3A, 3B;
SB: 1B, 3B
AM: 1C, 3B;
FB: 1C, 3B;
LP: 1C, 3B;
GG: 1C, 3B;
AC: 1A, 1B, 1C, 3A, 3B;
RC: 1A, 1B, 1C, 3A, 3B
Disclosures
Ethical Compliance Statement
The patient provided signed informed consent to video recording and publication for scientific purposes. The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The present study was neither sponsored nor funded. All the authors report no conflicts of interest.
Financial Disclosures for the Previous 12 Months
RC has received fees for speaking at conferences from Zambon, Bial, UCB, and Lusofarmaco. AC has received fees for consultancies and for speaking at conferences from IPSEN and Merz. The other authors have no financial disclosures.
Acknowledgments
SB and RC would like to thank the “Fondazione Grigioni per il Morbo di Parkinson” (Milan, Italy) for supporting clinical research.
Anna Castagna and Roberto Cilia equally contributed to this work.
References
- 1.Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord 1994;9(5):493–507. [DOI] [PubMed] [Google Scholar]
- 2.Loher TJ, Krauss JK. Dystonia associated with pontomesencephalic lesions. Mov Disord 2009;24(2):157–167. [DOI] [PubMed] [Google Scholar]
- 3.Rabuffetti M, Marzegan A, Crippa A, et al. The LAMB gait analysis protocol: definition and experimental assessment of operator‐related variability. Proc Inst Mech Eng 2019;233(3):342–353. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Raethjen J, Lindemann M, Krack P. The pathophysiology of tremor. Muscle Nerve 2001;24(6):716–735. [DOI] [PubMed] [Google Scholar]
- 5.Alqwaifly M. Treatment responsive Holmes tremor: case report and literature review. Int J Health Sci 2016;10(4):558–562. [PMC free article] [PubMed] [Google Scholar]


