ABSTRACT
Background
Parkinson's disease (PD) is multi‐symptom disease with variable progression.
Objectives
We performed a longitudinal study to address the evolution of motor symptoms (MS) and non‐motor symptoms (NMS), predictors of motor‐, cognitive‐, disability‐, and health‐related quality of life (HRQL) status and the relative usefullness of a battery of separate NMS scales (BSS) versus the Non‐Motor Symptom Scale (NMSS).
Methods
Seventy‐two patients were assessed at baseline and 4 years later with the NMSS and BSS. We assessed the following outcomes: cognition (Montreal Cognitive Assessment scale [MoCA]), disability (Unified Parkinson's Disease Rating Scale Part II [UPDRS II], Schwab and England [S&E]), motor dysfunction (Unified Parkinson's Disease Rating Scale Part III [UPDRS III], Hoehn and Yahr [HY]), and HRQL (EuroQol [EQ] EQ‐vertical visual analogue scale [VAS] and EQ‐Index). Statistical analysis included a comparison between scales scores at both time points and multivariate regression analysis to calculate the impact of each baseline symptom in outcomes. NMSS and BSS were introduced in separate models.
Results
NMSS Domain 4: perception/hallucinations, Parkinson's Psychosis Questionnaire, Apathy Scale, NMSS Domain 7: urinary, S&E, UPDRS II, HY, and MoCA scores worsened significantly. Dementia increased to a 4‐year prevalence of 39.8%. In the multivariate model using BSS, cognitive state variation was significantly predicted by baseline HY, EQ‐Index, and S&E. Using the NMSS, MoCA change was significantly associated with NMSS Domain 4: perceptions/hallucination score, cognitive status with UPDRS III score, HRQL with NMSS Domain 4: perception/hallucinations score, and S&E.
Conclusion
Our study suggests that NMS progress heterogeneously, BSS approach being more sensitive to change than NMSS. The multivariate analysis has shown that S&E and NMSS Domain 4: perception/hallucinations scores are the stronger predictors of HRQL and cognitive dysfunction variation, favoring NMSS over the BSS approach.
Keywords: Parkinson's disease, health‐related quality of life, psychosis, cognition
Parkinson's disease (PD) patients present with both motor symptoms (MS)1 and non‐motor symptoms (NMS).2 NMS are significantly correlated with health‐related quality of life (HRQL) and disability, more so than MS.3, 4 The need for a better characterization of NMS has led to validation in PD of several separate scales, and to the development of specific instruments, like the Non‐Motor Symptom Scale (NMSS),5 which aggregates several dimensions in one articulate system. PD being a progressive disorder, with varying outcomes, there is a need for longitudinal studies targeting both MS and NMS. Some studies6, 7, 8, 9, 10, 11, 12, 13, 14 have prospectively evaluated NMS in PD cohorts, with discrepant results, which might be explained by the use of different sets of scales for quantifying predictor variables.
In the present study, we have followed a cohort of non‐selected PD patients for a period of 4 years, with a variety of scales that are redundant for the various MS and NMS symptoms. In particular, we have used both the items of the NMSS and a battery of separate scales for each relevant NMS in PD. This has enabled us to evaluate several of the MS and NMS, as well as HRQL, motor dysfunction, and disability outcomes simultaneously with 2 different scales.
Our objectives were to (1) assess the progression of MS and NMS in a cohort of PD patients; (2) assess the predictors of cognitive, motor, disability, and HRQL deterioration; and (3) assess the predictive value of 2 different methods of assessing NMS (using NMSS versus a battery of separate scales).
Methods
Subjects
At baseline, we assessed all consecutive PD patients attending a movement disorders outpatient clinic in a tertiary referral center covering part of the metropolitan area of Lisbon, Portugal, during the period between March 2014 and March 2015. The United Kingdom (UK) Brain Bank diagnostic criteria were used for PD diagnosis. Patients were excluded if they presented with significant comorbidities that could interfere with assessment or represented an extra load of incapacity not related to PD, and/or signs and symptoms suggesting other causes for parkinsonism (multiple system atrophy, vascular parkinsonism, progressive supranuclear palsy, and iatrogenic parkinsonism). Inclusion and exclusion criteria were detailed elsewhere.3
Assessment
The following demographic‐ and disease‐related data were collected: age at study inclusion, age of disease onset, duration of disease (period, in years, between the first motor symptom experienced by the patient and the date of assessment), education (years of schooling), dopaminergic medication (expressed in levodopa equivalent daily doses [DED]).
Patients underwent structured assessment designed to cover all major aspects of MS and NMS, as well as disability and HRQL. Motor function was evaluated with the Unified Parkinson's Disease Rating Scale Part III (UPDRS III) and the Hoehn and Yahr scale (HY). Non‐motor function was assessed with the Parkinson's Psychosis Questionnaire (PPQ), Scales for Outcomes in Parkinson's Disease‐Sleep (SCOPA‐Sleep) (night‐time and daytime sleep), rapid eye movement (REM) Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Hospital Anxiety and Depression Scale, and the Apathy Scale. We used age‐ and education‐related cut‐offs for cognitive dysfunction, as provided by the Montreal Cognitive Assessment scale (MoCA) Portuguese validation study. The Pill Questionnaire was used to determine the impact of cognitive dysfunction on daily living activities. Patients with cognitive dysfunction and impact in daily living activities were classified has having dementia (PDD). Mild cognitive impairment was classified as cognitive dysfunction without impact in daily living activities (PD‐MCI). Patients without cognitive dysfunction were considered as cognitively normal (PD‐CN). Patients were also assessed with the NMSS, a scale designed for PD that allows to evaluate the following dimensions: Domain 1: cardiovascular symptoms; Domain 2: sleep/fatigue; Domain 3: mood/cognition; Domain 4: perception/hallucinations; Domain 5: attention/memory; Domain 6: gastrointestinal symptoms; Domain 7: urinary symptoms; Domain 8: sexual function symptoms; and Domain 9: miscellanea. Domains 2, 3, 4, and 5 partially overlap with those assessed by the other scales, which permits to evaluate some of the symptoms by 2 different instruments allowing a distinction between the effect of measurement and the symptom itself. HRQL was measured with the EuroQol (EQ), which yields 2 values: EQ‐vertical visual analogue scale (VAS) and EQ‐Index. Disability was assessed with UPDRS II and the Schwab and England Scale (S&E).
The same protocol was applied at baseline and to all patients available for assessment at follow‐up, at the completion of a 4‐year period.
Data Analysis
Comparisons between patients included and not included at follow‐up were performed with 2‐sided independent samples, student t or Mann Whitney tests, depending on variable distribution. Proportions in cognitive status at baseline and follow‐up were compared with the McNemar test. Prevalence of dementia at 4 years was calculated as the sum of the number of cases with dementia at baseline and all new cases divided by the mean population during the middle of the observation period; 95% confidence intervals (95% CI) were calculated.
To evaluate the progression of symptoms and variation in disability and HRQL, baseline and follow‐up data were compared with paired sample t tests (for continuous variables) or the McNemar test (dichotomous variable). The magnitude of changes was calculated with relative changes ([mean test at follow‐up − mean test at baseline]/mean test at follow‐up) and effect size ([mean test at baseline − mean test at follow‐up]/SD test at baseline). To test the effect of disease duration in symptom progression, patients were divided in 3 groups according to disease duration at baseline (1–5, 6–10, and above 10 years duration) and compared using repeated measures ANOVA. For evaluating the variables that best predicted changes in cognition, motor function, HRQL, and disability, linear regression models (univariate followed by multivariate) were used. The outcome variable with the greater variation was chosen over the variable with the smaller variation whenever there were more than 2 scales for the same dimension, motor function (HY and UPRDS III), HRQL (EQ‐Index and EQ‐VAS), and disability (UPDRS II and S&E). The dependent variables were, therefore, the absolute change (score at follow‐up – score at baseline) in MoCA, HY, EQ‐Index, and S&E, respectively (these variables were tested separately). Predictors in the univariate model were demographic, disease‐related variables, DED, UPDRS III, and the NMS variables. To avoid collinearity and to account for possible bias created by the use of different scales for the same symptom, 2 multivariate models were used with 2 sets of predictor variables, 1 including NMSS dimensions and the other using the battery of separate non‐motor symptom scales. Demographic, disease‐related data, DED, and UPDRS III were used in both models. Predictor variables were included in the multivariate model if the univariate association showed a p value below 0.20. Backward selection method was used, removing variables individually up to 0.10 significance level. A similar model, but using logistic regression, was used to evaluate predictors for cognitive status change (excluding those patients that were demented at baseline). In this case, the outcome variable was change in cognitive status, considered as a dichotomous variable: worsening (changes from PD‐CN to PD‐MCI or PDD, change from PD‐MCI to PDD) versus maintenance or improvement (PD‐MCI to PD‐MCI or PD‐CN, PD‐CN to PD‐CN). Significance was held at p < 0.05.
Ethics
All patients signed informed consent forms, and the investigation protocol was approved by Hospital Egas Moniz ethics committee.
Results
Of the initial 134 patients, 72 (54%) were reassessed at follow‐up. Sixty‐two patients were lost for follow‐up, 37 refused being reassessed, were unavailable, or could not be found, 22 died during follow‐up period, and 3 patients were considered to have a different diagnosis. Patients not included at follow‐up differed significantly at baseline from included patients regarding age, age of onset, UPDRS II, NMSS Domain 4: perception/hallucinations, RBDSQ scores (higher in the excluded patients), MoCA, and S&E (lower). Patients that refused being reassessed were significantly older and had lower MoCA scores than the patients who accepted (Table S1).
Demographic and disease‐related data at baseline of the patients assessed at both time points were the following: 34 (46.6%) females, mean age 70.22 years (SD 9.09, limits 42–88), mean age of onset 63.63 (10.22, 38–86), disease duration 7.08 years (5.36, 1–25), mean DED 536.26 (394.62, 0–1600.00).
Table 1 compares baseline and follow‐up data of NMS. There was significant worsening regarding MoCA (medium effect size), PPQ (large effect size), apathy (medium effect size), NMSS Domain 4: perceptions/hallucinations (large effect size) and NMSS Domain 7: urinary (medium effect size) scores. In Figure 1, we depict the relative changes in NMS separately for NMSS and the battery of non‐motor scales.
TABLE 1.
Change in non‐motor symptoms in PD
| Baseline | Follow‐up | p | Relative change (%) | Effect size | |
|---|---|---|---|---|---|
| Mean (standard deviation) | Mean (standard deviation) | ||||
| MoCA | 20.33 (5.57) | 17.89 (6.26) | 0.0001**** | −14.0 | 0.40 |
| RBDSQ | 6.79 (3.32) | 5.96 (3.77) | 0.056 | −0.14 | 0.25 |
| HADS‐anxiety | 7.99 (4.38) | 7.99 (4.12) | 0.826 | 0 | 0 |
| HADS‐depression | 8.03 (4.61) | 9.06 (4.62) | 0.230 | 0.12 | −0.22 |
| SCOPA‐sleep daytime | 3.90 (3.29) | 4.08 (3.66) | 0.648 | 0.04 | −0.05 |
| SCOPA‐sleep night‐time | 4.23 (3.92) | 4.49 (4.29) | 0.531 | 0.06 | 0.07 |
| PPQ | 2.61 (2.93) | 5.37 (6.77) | 0.001*** | 0.51 | −0.94 |
| Apathy | 12.13 (8.29) | 16.25 (10.13) | 0.004** | 0.25 | −0.50 |
| NMSS affection/cognition | 15.83 (17.40) | 20.44 (22.10) | 0.125 | 0.23 | −0.26 |
| NMSS perception/hallucinations | 1.07 (2.49) | 2.90 (5.16) | 0.003** | 0.63 | −0.73 |
| NMSS sleep/fatigue | 10.63 (8.72) | 9.24 (7.98) | 0.135 | −0.15 | 0.16 |
| NMSS cardiovascular | 1.87 (3.12) | 2.22 (3.82) | 0.521 | 0.16 | −0.11 |
| NMSS attention/memory | 7.52 (7.74) | 9.49 (10.74) | 0.167 | 0.21 | −0.25 |
| NMSS gastrointestinal | 4.72 (6.15) | 6.33 (8.51) | 0.095 | 0.25 | −0.26 |
| NMSS urinary | 7.33 (8.97) | 11.00 (11.25) | 0.013* | 0.34 | −0.41 |
| NMSS sexual function | 3.93 (6.57) | 4.97 (6.91) | 0.275 | 0.21 | −0.16 |
| NMSS miscellanea | 7.23 (8.53) | 9.94 (11.10) | 0.055 | 0.26 | −0.32 |
Abbreviations: MoCA, Montreal Cognitive Assessment scale; RBDSQ, REM Sleep Behavioral Disorder Symptom Questionnaire; HADS, Hospital Anxiety and Depression Scale, SCOPA, Scale for Outcomes in Parkinson's Disease; PPQ, Parkinson's Psychosis Questionnaire; NMSS, Non‐Motor Symptom Scale. ***p<0.001;**p<0.01;*p<0.05
FIG. 1.
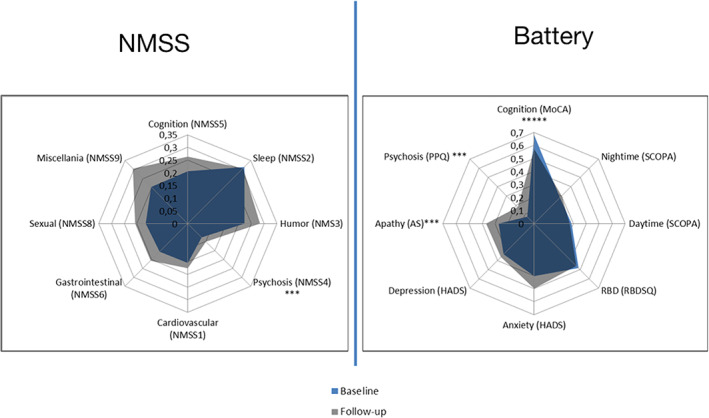
Variation in non‐motor symptoms according to NMSS and a battery of separate scales. NMSS, Non‐motor symptoms scale; MoCA, Montreal cognitive assessment scale; SCOPA, scale for outcomes in Parkinson's disease; SCOPA daytime, daytime sleepiness; SCOPA night‐time, night‐time sleep complaints; RBD, REM sleep behavior disorder; RSBDSQ, REM Sleep Behavior Disorder Screening Questionnaire; HADS, Hospital Anxiety and Depression Scale; AS, Apathy Scale; PPQ, Parkinson's Psychosis Questionnaire. For comparison, scale scores were normalized ([value‐minimum]/[maximum‐minimum]), varying from 0 to 1. Values in graphic represent means. *****p<0.0001; ***p<0.005
Number of patients in each disease duration group were: 0–5 years = 59, 6–10 = 48, and >10 = 24. Disease duration group was significantly related with symptom progression regarding RSBDSQ score (F = 5.735, p= 0.005) and UPDRS II (F = 3.560, p = 0.034) (more significant progression in longer duration groups).
Table 2 shows changes in outcome variables. There was a significant decrease both in S&E and UPDRS II, but the difference was more expressive regarding S&E. Effect size was higher for EQ‐Index than for EQ‐VAS but differences did not reach significance in neither scale. HY increased significantly (small effect size) but UPDRS III variation was not significant. MoCA decreased significantly, as alluded above. There was a significant increase in PDD, with a concomitant decrease in PD‐CI cases. Of the 134 assessed at baseline, 22 (15.4%) patients presented with dementia. At follow‐up, 19 of 72 patients that were reassessed (27.5%) had criteria for dementia, yielding a 4‐year prevalence of 39.8% (95% CI = 35.4–44.2). Figure 2 depicts the transition in cognitive status. A total of 40.8% percent of the PD‐CN patients evolved either to PD‐MCI (22.4%) or directly into PDD (18.4%). A total of 58.8% of the PD‐MCI patients maintained their cognitive status, 23.5% evolved to PDD, and 17.6% improved to PD‐CN. All the PDD patients at baseline maintained their status at follow‐up.
TABLE 2.
Change in outcome variables
| Baseline | Follow‐up | P | Relative change (%) | Effect size | |
|---|---|---|---|---|---|
| Mean (standard deviation) or frequency (%) | Mean (standard deviation) or frequency (%) | ||||
| Schwab and England | 71.27 (15.96) | 79.86 (22.68) | 0.001** | 10.77 | −0.38 |
| UPDRSII | 11.84 (9.47) | 14.19 (8.97) | 0.031* | 15.77 | −0.25 |
| EQ‐Index | 0.550 (0.28) | 0.61 (0.23) | 0.066 | 10.28 | −0.28 |
| EQ‐VAS | 63.67 (19.85) | 58.94 (25.59) | 0.178 | 8.03 | 0.23 |
| UPDRS III | 25.93 (15.98) | 27.41 (14.80) | 0.485 | 5.40 | 0.09 |
| Hoehn and Yahr | 2.25 (0.79) | 3.33 (3.83) | 0.021* | 32.43 | −0.28 |
| PD‐NC/PD‐MCI/ PDD | 49 (66.7)/17(23.6)/6 (8.7) | 32 (44.0)/21 (29.2)/19 (27.5) | 0.001** | ||
UPDRS, Unified Parkinson's Disease Rating Scale; EQ, EuroQol; MoCA, Montreal Cognitive Assessment Scale; PD‐NC, Parkinson's patients with normal cognition; PD‐MCI: Parkinson's patients with mild cognitive impairment; PDD, Parkinson's disease patients with dementia.
FIG. 2.
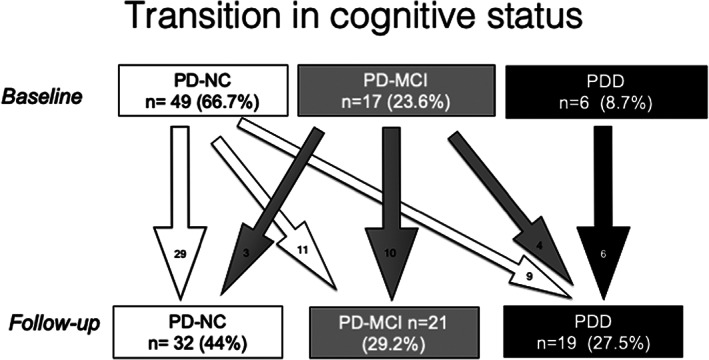
Transition in cognitive status. Digits represent number (percentage) of patients in each cognitive state at baseline and follow‐up (boxes) or number of patients transiting between states (arrows). PD‐CN, Parkinson's disease, cognitively normal; PD‐MCI, Parkinson's disease mild cognitive impairment; PDD, Parkinson's disease dementia.
Table 3 shows the association between predictors and outcome variables in the univariate model. Variables associated with outcome at p < 0.02 level were included in the multivariate models.
TABLE 3.
Predictors of cognitive, disability, HRQL, and motor change in univariate analysis
| MoCA | Cognitive state | Disability | HRQL | Motor function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | parameter estimate (95% CI) | P | |
| Age | −2.443 (−3.656 to −1.229) | 0.0001 | 0.954 (0.888 to 1.025) | 0.202 | −.347 (−0.158 to −1.332) | 0.187 | 0.003 (−0.005 to 0.010) | 0.484 | 0.034 (−0.135 to 0.067) | 0.504 |
| Education | .183 (−0.122 to 0.488) | 0.235 | 1.047 (0.893 to 1.226) | 0.573 | .569 (−0.674 to 1.186) | 0.352 | 0.004 (−0.017 to 0.018) | 0.573 | 0.058 (−0.293 to 0.177) | 0.624 |
| Age of onset | −0.058 (−0.108 to 0.065) | 0.351 | 0.977 (0.918 to 1.041) | 0.477 | −.369 (−0.847 to 0.109) | 0.128 | 0.001 (−.006 to 0.009) | 0.678 | 0.024 (−0.119 to 0.070) | 0.609 |
| Duration | 0.027 (−0.210 to 0.264) | 0.821 | 0.886 (0.791 to 0.992 | 0.036 | 0.256 | 0.584 | 0.005 (−0.009 to 0.019) | 0.485 | −0.017 (−0.164 to 0.199) | 0.850 |
| Gender | 0.895 (−1.545 to 3.335) | 0.467 | 2.970 (0.836 to 10.545) | 0.092 | 4.729 (−4.810 to 14.268) | 0.326 | −0.022 (−.158 to 0.114) | 0.748 | −0.016 (−1.838 to 1.871) | 0.986 |
| DED | −.001 (−0.005 to 0.002) | 0.379 | 0.999 (0.997 to 1.000 | 0.057 | −0.000 (−0.013 to 0.012) | 0.946 | 0.000 (0.000 to 0.000) | 0.904 | −0.001 (−0.003 to 0.002) | 0.647 |
| Hoehn and Yahr | 0.440 (−1.101 to 1.981) | 0.571 | 0.360 (0.136 to 0.954) | 0.040 | 1.358 (−4.689 to 7.406) | 0.656 | 0.146 (0.059 to 0.232) | 0.001 | ||
| UPDRS III total | 0.020 (−0.057 to 0.097) | 0.598 | 0.945 (0.900 to 0.992) | 0.024 | 0.116 (−0.188 to 0.421) | 0.448 | 0.008 (0.004 to 0.012) | 0.0003 | −0.037 (−0.022 to 0.095) | 0.215 |
| UPDRSII | .038 (−0.092 to 0.167) | 0.566 | 0.902 (0.829 to 0.981) | 0.016 | 0.108 (−0.398 to 0.614) | 0.671 | 0.017 (0.009 to 0.025) | 0.0001 | −0.040 (−0.057 to 0.137) | 0.415 |
| Schwab England | −.026 (−0.102 to 0.051) | 0.506 | 1.054 (1.009 to 1.102) | 0.019 | −0.010 (−0.014to −0.006) | 0.00002 | 0.018 (−0.076 to 0.041) | 0.544 | ||
| MoCA | 1.050 (0.930 to 1.186) | 0.430 | −0.059(−0.642 to 1.780) | 0.894 | −0.013 (−0.026 to 0.001) | 0.065 | 0.046 (−0.215 to 0.124) | 0.592 | ||
| EQ‐VAS | −.003 (−0.063 to 0.057) | 0.917 | 1.021 (0.990 to 1.053) | 0.187 | 0.001 (−0.244 to 0.245) | 0.996 | −0.006 (−0.009 to −0.002) | 0.001 | 0.016 (−0.063 to 0.031) | 0.498 |
| EQ‐Index | 0.270 (−3.970 to 4.510) | 0.899 | 11.442 (0.800 to 163.698) | 0.442 | 1.016 (−16.128 to 18.160) | 0.906 | 1.652 (−4.941 to 1.637) | 0.320 | ||
| RBDSQ | 0.004 (−0.368 to 0.376) | 0.982 | 0.915 (0.765 to1.094) | 0.328 | 0.222 (−1.232 to 1.676) | 0.761 | .004 (−0.017 to 0.025) | 0.692 | 0.151 (−0.430 to 0.128) | 0.283 |
| HADS ‐ anxiety | −0.124 (−0.403 to 0.156) | 0.381 | 1.079 (0.936 to 1.244 | 0.295 | −0.007 (−1.123 to 1.109) | 0.991 | .010 (−0.006 to 0.025) | 0.211 | 0.023 (−0.239 to 0.192) | 0.829 |
| HADS ‐ depression | 0.062 (−0.198 to 0.323) | 0.634 | 1.034 (0.910 to 1.175) | 0.609 | 0.346 (−0.711 to 1.403) | 0.516 | 0.019 (0.005 to 0.033) | 0.010 | −0.114 (−0.089 to 0.317) | 0.265 |
| SCOPA‐sleep daytime | .032 (−0.339 to 0.403) | 0.864 | 0.943 (0.788 to 1.128) | 0.519 | 0.400 (−1.256 to 1.222) | 0.586 | −.011 (−0.032 to 0.011) | 0.315 | −.054 (−0.228 to 0.336) | 0.704 |
| SCOPA‐sleep night‐time | .012 (−0.295 to 0.319) | 0.939 | 1.038 (0.884 to 1.218) | 0.651 | −.017 (−1.058 to 1.858) | 0.978 | 0.021 (0.005 to 0.038) | 0.013 | −0.049 (−0.191 to 0.288) | 0.687 |
| PPQ | −.164 (−0.576 to 0.249) | 0.431 | 0.804 (0.642 to 1.007) | 0.058 | 0.479 (−4.689 to 7.406) | 0.558 | .018 (−0.006 to 0.042) | 0.148 | −.018 (−0.296 to 0.332) | 0.911 |
| Apathy scale | −.009 (−0.154 to 0.13 | 0.902 | 1.011 (0.936 to 1.092) | 0.787 | 0.052 (−0.538 to 0.643) | 0.860 | .008 (0.000 to 0.016) | 0.042 | −0.025 (−0.089 to 0.139) | 0.661 |
| NMSS affection/cognition | −.006 (−0.075 to 0.061) | 0.871 | 1.001 (0.962 to 1.040) | 0.485 | −0.012 (−0.293 to 0.269) | 0.934 | 0.006 (0.002 to 0.002) | 0.002 | −0.004 (−0.050 to 0.059) | 0.872 |
| NMSS perception/hallucinations | −.333 (−0.818 to 0.152) | 0.175 | 0.785 (0.626 to 0.985) | 0.036 | −0.568 (−2.500 to 1.363) | 0.559 | −.025 (−0.053 to 0.003) | 0.084 | −0.017 (−0.357 to 0.391) | 0.928 |
| NMSS sleep/fatigue | −.124 (0.261 to 0.013) | 0.075 | 0.958 (0.894 to 1.026) | 0.218 | −0.348 (0.895 to 0.199) | 0.208 | 0.003 (−0.005 to 0.010) | 0.498 | 0.005 (−0.112 to 0.102) | 0.922 |
| NMSS cardiovascular | −.090 (−0.475 to 0.296) | 0.643 | 0.961(0.776 to 1.190) | 0.715 | −0.605 (−2.118 to 0.907) | 0.427 | 0.016 (−0.005 to 0.037) | 0.137 | −0.041 (−0.252 to 0.335) | 0.779 |
| NMSS attention/memory | −.118 (−0.274 to 0.039) | 0.139 | 0.919 (0.845 to 1.000) | 0.051 | .135 (−0.487 to 0.758) | 0.666 | .010 (0.001 to 0.018) | 0.027 | −.059 (−0.060 to 0.179) | 0.326 |
| NMSS gastrointestinal | −.048 (−0.247 to 0.151) | 0.633 | 1.011 (0.892 to 1.146) | 0.863 | −0.235 (−1.017 to 0.548) | 0.552 | 0.012 (0.002 to 0.023) | 0.024 | 0.003 (−0.155 to 0.148) | 0.964 |
| NMSS urinary | −.111 (−0.242 to 0.021) | 0.097 | 0.960 (0.896 to 1.029) | 0.249 | −0.189 (−0.732 to 0.354) | 0.490 | −0.0003 (−0.008 to 0.007) | 0.935 | 0.092 (−0.195 to 0.011) | 0.079 |
| NMSS sexual function | −.098 (−0.288 to 0.092) | 0.306 | 0.995 (0.909 to 1.088) | 0.911 | −0.270 (−1.044 to 0.504) | 0.488 | −0.007 (−0.018 to 0.003) | 0.181 | 0.139 (−0.286 to 0.007) | 0.062 |
| NMSS – miscellanea | −5.516 (−11.681 to 0.649) | 0.079 | 0.934 (0.869 to 1.004) | 0.065 | −20.285 (45.378 to 4.809) | 0.111 | 0.113 (−0.244 to 0.470) | 0.531 | −1.389 (−0.080 to 0.144) | 0.576 |
DED, dopa equivalent doses; UPDRS, Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment Scale; EQ, EuroQol; RSBDQ, REM Sleep Behavior Disorder Questionnaire; HADS, Hospital Anxiety and Depression Scale; SCOPA, Scale for Outcomes in Parkinson's Disease; SCOPA Daytime, Daytime Sleepiness; SCOPA Night‐time, Night‐time sleep complaints; PPQ, Parkinson's Psychosis Questionnaire; NMSS, Non‐motor symptoms scale.
Table 4 presents the multivariate model using the battery of separate non‐motor scales as predictors. Cognitive state variation was significantly predicted by HY stage. HRQL was significantly predicted by S&E. There were no significant associations (p<0.02) between the battery of non‐motor scale predictors and MoCA, disability, and motor function variation in univariate analysis, so no variables was carried to multivariate analysis regarding these ouctomes.
TABLE 4.
Predictors of cognitive, disability, HRQL and motor change in multivariate analysis (model using battery of separate non‐motor symptom scales)
| MoCA | Cognitive state | Disability | HRQL | Motor function | |||
|---|---|---|---|---|---|---|---|
| Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | ||||
| Age | – | – | – | – | |||
| Age of onset | – | – | – | – | |||
| Gender | – | – | – | – | |||
| Hoehn and Yahr stage | 0.302 (0.104 to 0.876) | 0.028 | NS | NS | |||
| UPDRS III total | NS | NS | NS | NS | |||
| MoCA | – | – | NS | NS | |||
| RBDSQ | – | – | – | – | |||
| HADS ‐ anxiety | – | – | – | – | |||
| HADS ‐ depression | – | – | NS | NS | |||
| SCOPA‐sleep daytime | – | – | – | – | |||
| SCOPA‐sleep night‐time | – | – | NS | NS | |||
| PPQ | 0.800 (0.626 to 1.022) | 0.074 | NS | NS | |||
| Apathy | – | – | NS | NS | |||
| EQ‐index | – | – | |||||
| EQ‐VAS | NS | NS | −0.003 (−0.006 to 0.0004) | 0.071 | |||
| UPDRS II | NS | NS | NS | NS | |||
| Schwab and England | NS | NS | −0.008 (−0.013 to −0.003) | 0.001 | |||
UPDRS: Unified Parkinson's Disease Rating Scale MoCA, Montreal Cognitive Assessment scale; RBDSQ, REM Sleep Behavioral Disorder Symptom Questionnaire; HADS, Hospital Anxiety and Depression Scale, SCOPA, Scale for Outcomes in Parkinson's Disease; PPQ, Parkinson's Psychosis Questionnaire; EQ, EuroQol.
In the multivariate model using the NMSS (Table 5), MoCA change was significantly associated with baseline NMSS Domain 4: perceptions/hallucination score. Cognitive status was predicted by UPDRS III score (mood/cognition, attention/memory, and NMSS Domain 9: miscellanea were kept in the model at trend values). HRQL change was significantly associated with NMSS Domain 4 perception/hallucinations score and very significantly associated with S&E (there as an association with NMSS Domain 3: mood/cognition at trend value). None of the models showed significant predictors of motor function and disability change.
TABLE 5.
Predictors of cognitive, disability, HRQL ad motor change in multivariate analysis (model using the NMSS)
| MoCA | Cognitive state | Disability | HRQL | Motor function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | Parameter estimate (95% CI) | P | |
| Age | NS | NS | – | – | NS | NS | – | – | – | – |
| Age of onset | – | – | – | – | NS | NS | – | – | – | – |
| Gender | – | – | NS | NS | NS | NS | – | – | – | – |
| Hoehn and Yahr stage | – | – | NS | NS | – | – | NS | NS | ||
| UPDRS III total | – | – | 0.929 (0.872 to 0.990) | 0.023 | – | – | NS | NS | – | – |
| NMSS affection/cognition | – | – | 1.066 (0.997 to 1.140 | 0.062 | – | – | 0.003 (0.0003 to 0.007 | 0.077 | – | – |
| NMSS perception/hallucinations | −0.565 (−1.055 to −0.075) | 0.024 | – | – | – | – | −0.040 (−0.064 to −0.016) | 0.001 | – | – |
| NMSS sleep/fatigue | NS | NS | – | – | – | – | NS | NS | – | – |
| NMSS cardiovascular | – | – | – | – | – | – | NS | NS | – | – |
| NMSS attention/memory | NS | NS | 0.884 (0.775 to 1.007) | 0.063 | – | – | NS | NS | – | – |
| NMSS gastrointestinal | – | – | – | – | – | – | NS | NS | – | – |
| NMSS urinary | NS | NS | – | – | – | – | – | – | NS | NS |
| NMSS sexual function | – | – | – | – | – | – | NS | NS | 0.139 (−0.007 to 0.286) | 0.062 |
| NMSS ‐ miscellanea | – | – | 0.023 (0.0004 to 1.257) | 0.065 | −24.385 (−51.375 to 2.605) | 0.076 | – | – | – | – |
| EuroQoL 5D | – | – | – | – | – | – | – | – | ||
| EQ‐VAS | – | – | NS | NS | – | – | NS | NS | – | – |
| UPDRS II | – | – | NS | NS | – | – | NS | NS | – | – |
| Schwab and England | – | – | NS | NS | – | – | −.010 (−0.014 to −0.005) | <0.00001 | – | – |
UPDRS, Unified Parkinson's Disease Rating Scale; NMSS, Non‐Motor Symptom Scale; EQ, EuroQol.
Discussion
Progression in NMS
We found a heterogeneous progression of NMS. Psychosis, apathy, NMSS Domain 7: urinary symptoms, and cognitive dysfunction, as assessed by MoCA, increased significantly. This differed from other studies using the NMSS.6, 9, 14 Using other scales, some authors7 found worsening in cognition, depression, autonomic symptoms, and impulsive–compulsive symptoms, and others8 showed stability in cognitive scores, worsening in autonomic function, sleepiness, and RBD symptoms, but improvement in global NMS burden and depression (all studies performed in de novo cohorts). In cohorts not selected for disease stage10 sleep, gastrointestinal, attention/memory, hyperhidrosis, and seborrhea domains prevalence increased whereas psychiatric, cardiovascular, and respiratory ones decreased. These discrepancies could be ascribed to differences in assessment scales in some cases and in disease duration in others. Our sample was not limited to de novo patients. Longer disease duration patients are expected to have more severe symptoms, meaning that ceiling effects could have blunt the variation of symptoms in our study, particularly regarding the dimensions that had high scores at baseline (e.g., affect and sleep).3 Some NMS symptoms, like depression, anxiety, RBD, and night‐time sleep, respond better to medication (which could explain its stability in ours and inclusively its improvement in some of the previous studies8, 9) than apathy and cognitive dysfunction, whereas psychosis can be worsened by dopamine dose increase. Apathy occurs concomitantly with cognitive dysfunction and depression, from which is frequently hard to differentiate.15 Previous work in PD was able to distinguish between the presence of apathy and reactive psychological conditions related to the incapacity,16 suggesting that this syndrome could be caused by neurodegenerative changes intrinsic to PD, possibly related to ongoing disturbance of dopaminergic and serotoninergic fronto‐striatal pathways.17 That this symptom has worsened significantly in our cohort, and more so than mood symptoms, hints at neurodegenerative cause for apathy.
It should be noted that, regardless of their differences, all studies have revealed a heterogeneous progression of NMS, with differing domains varying at different paces, which probably reflects the multitude of systems being affected in different ways at each neuropathological stage.
Relative Use of the 2 Different Methods of NMS Assessment
Psychosis increased significantly in both scales, with large effect sizes. Cognitive function, however, evolved differently depending on the assessment method: cognitive complaints, as assessed by NMSS Domain 5: attention/memory, did not increase significantly, whereas objective measurement with MoCA showed significant worsening. Although it is a physician‐completed questionnaire, the NMSS depends on the subjective judgment of the patient regarding his cognitive state, whereas MoCA score is given by the objective results of several separate tasks. The discrepancy between MoCA and NMSS Domain 5: attention/cognition scores could, therefore, be considered in accordance with segregation between subjective cognitive complaints and objective findings in PD, as suggested by our baseline study.3, 18 Our findings, therefore, suggest that MoCA could be more sensitive to change than NMSS regarding cognitive worsening. Items regarding apathy are not separately evaluated in NMSS, being included in the affect/cognition item, which in our study did not change significantly. Some authors have found apathy to be distinct from depression in PD patients,16 which might explain the difference between apathy progression according to the Apathy Scale and that assessed with the NMSS. Using a separate scale for this symptom might be useful for longitudinal assessment of apathy in PD patients.
These findings point to the general conclusion that longitudinal assessment results vary depending on the scales that are used. Globally, the battery of separate scales was more sensitive to change than the NMSS.
Variation in Outcome Measures
Cognition, motor function, and disability worsened significantly, whereas HRQL did not. The magnitude of changes in each outcome was scale dependent. HY increased significantly, but not UPDRS III, which might be related to HY relying strongly on axial symptoms, which are less responsive to dopaminergic treatment than appendicular signs. Regarding disability, the S&E scale showed to be more sensitive to change than UPDRS II. UPDRS II has been criticized for including several items that assess impairments, but not functional status.19 Other studies have also found HRQL not to worsen at follow‐up9, 10 or even to improve.13 HRQL depends on various factors, some of them responsive to medication, which could account for the heterogeneity in the results of this outcome variable.
As expected, there was a significant number of patients whose cognitive status worsened. The prevalence of dementia was lower than in other 4‐year longitudinal studies performed in populations not selected for disease stage,20 which could be ascribed to differences in population at baseline (Aarsland patients were younger and had less disease duration) or in the method used for defining cognitive status. In our study, several PD‐CN progressed directly to PPD, without intermediate MCI status, differently to what has been reported in newly diagnosed patients.21 Dementia appears to be a definite state, because no PDD patients improved at follow‐up, as also described in Aarsland et al.20 PD‐MCI patients, however, were less prone to change, and some improved to PD‐CN. This has been described previously21 and could be ascribed to several factors: treatment of comorbidities that affect cognition, redraw of prejudicial drugs, and beneficial effect of dopaminergic treatment.
Predictors of Motor, Cognitive, Disability, and HRQL Outcomes
MoCA change was significantly predicted by NMSS Domain 4: perception/hallucinations, which is in line with several studies revealing psychosis to be a strong predictor of cognitive change in PD20, 22 probably linked to concomitant dysfunction of parieto‐occipital and hippocampal structures that control cognition and visual perception.23 Worsening of cognitive status, however, was not predicted by hallucinations but by baseline motor dysfunction. The methodologic difference between the 2 cognitive assessment approaches used in our study lies on the use of the Pill Questionnaire to determine the transition to PDD status (because the transition between PD‐CI and PD‐MCI is determined solely by MoCA score increase). Although that scale was proposed for determining incapacity precisely for its theoretical advantage over scales that more overtly rely on motor function,24 its positive predictive value has been challenged in subsequent work.25 Moreover, our results are in accordance with studies that have shown that motor dysfunction, particularly axial symptoms, is a risk factor for dementia.11, 12, 15, 19, 26 Reduction in HRQL was strongly determined by baseline disability status as measured by S&E, but also by psychosis scores. Previous studies6, 27 also found S&E to be very significantly related to HRQL change, but not psychosis. Again, a longer duration of symptoms in our cohort could explain the difference, because psychosis, a symptom that usually appears later, could be more discriminant as the disease progresses. None of the models could significantly predict motor dysfunction or disability progression. This could eventually be explained by partial compensation of motor and disability progression by increased dopaminergic doses.
The Predictive Value of NMSS and the Separate Battery of NMS Scales
Our study suggests that NMSS is more useful as a predictor than the battery of separate scales, because none of the scales in the latter was significantly associated with any of the outcomes in multivariate analysis. Conversely, NMSS Domain 4: perception/hallucinations proved useful both for predicting cognitive change and loss of HRQL. This is in contrast with PPQ, which did not show significant predictive value. PPQ could be less specific than NMSS, because it includes not only hallucinations and delusions items but also questions related to sleep disturbance and orientation. Discrepancies in findings obtained with the NMSS and the battery of separate scales are not surprising, because NMSS aims at being comprehensive regarding the entire spectra of NMS in PD, whereas separate scales aim at more in‐depth assessing of each function. As discussed recently,28 the correlation between NMSS items and other scales, although acceptable, is not strong in every case and could be influenced by the composite nature of some of the items.
Limitations and Strong Points
Our study suffered a relatively high attrition rate caused by death and refusal to be reassessed, which diminishes the representativeness of our final sample. Patients that refused to be reassessed were older and had higher levels of cognitive dysfunction than those that accepted, suggesting that these variables could influence the attrition rates in this type of studies. Cognitive dysfunction could affect patient reasoning, contribute to a diminished willingness in study participation, or make dislocation to the study site more difficult. Although MoCA suitability for assessing cognition in PD has been shown,29 we did not perform in‐depth neuropsychological examination, which must be taken in account when analyzing cognitive status data. Finally, our sample could be criticized for being heterogeneous, because it was not restricted to early stage or de novo patients. We should note, however, that disease duration at baseline did not influence the progression in most scores, when comparing groups with different disease durations at study inclusion. (RBD score was an exception, which could be related with a higher prevalence of this disorder in long duration disease, as reported previously).30 Our results also suggest that disability progression, as assessed with the UPDRS II, is also influenced by disease duration. Our study has the advantages of presenting a longitudinal analysis of a wide set of features in PD, and providing a detailed analysis of NMS, using more than 1 scale for the same symptoms and outcomes, which permits to compare the performance of different assessment methods in the same cohort. Our study has the advantage of evaluating several outcomes simultaneously, including HRQL, which has seldom been evaluated in a longitudinal manner.
Conclusions
Our study suggests that the progression of NMS in PD patients is heterogeneous. Worsening is more significant regarding psychosis, apathy, and objective cognitive dysfunction. In this regard, scales that isolate these symptoms specifically, instead of aggregating them in the same score, could be more useful. Different outcomes varied differently and conclusions about the progression of the same outcome could depend on the scale used. Finally, the multivariate analysis has shown that S&E and NMSS Domain 4: perception/hallucinations scores are the stronger predictors of HRQL and cognitive dysfunction variation.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
P.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
F.L.:1C, 3B
R.B.: 1C, 3B
J.P.M.: 1C, 3B
C.B.: 1C, 3B
L.C.: 1C, 3B
M.S.: 1C, 3B
M.S.: 1C, 3B
M.F.: 1C, 3B
B.M.: 1C, 3B
Disclosures
Ethical Compliance Statement
The study protocol was approved by Centro Hospitalar de Lisboa Ocidental ethics committee. Informed consent was obtained from all patients. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors declare that there are no funding sources or conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Supporting information
Table S1. Baseline comparison between included and non‐included patients. MoCA, Montreal Cognitive Assessment scale; RBDSQ, REM Sleep Behaviour Disorder Symptom Questionnaire; HADS, Hospital Anxiety and Depression Scale, SCOPA, Scale for Outcomes in Parkinson's Disease; PPQ, Parkinson's Psychosis Questionnaire; NMSS, Non‐Motor Symptom Scale
Potential conflict of interest:
References
- 1.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 2010;25(15):2493–2500. 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- 3.Bugalho P, Lampreia T, Miguel R, Mendonça MD, Caetano A, Barbosa R. Non‐motor symptoms in Portuguese Parkinson's disease patients: correlation and impact on quality of life and activities of daily living. Sci Rep 2016;6:32267. 10.1038/srep32267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez‐Martin P, Rodriguez‐Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group . The impact of non‐motor symptoms on health‐related quality of life of patients with Parkinson's disease. Mov Disord 2011;26(3):399–406. 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22(13):1901–1911. 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 6.Prakash KM, Nadkarni NV, Lye WK, Yong MH, Tan EK. The impact of non‐motor symptoms on the quality of life of Parkinson's disease patients: a longitudinal study. Eur J Neurol 2016;23(5):854–860. 10.1111/ene.12950. [DOI] [PubMed] [Google Scholar]
- 7.Simuni T, Caspell‐Garcia C, Coffey CS, et al. Baseline prevalence and longitudinal evolution of non‐motor symptoms in early Parkinson's disease: the PPMI cohort. J Neurol Neurosurg Psychiatry 2018;89(1):78–88. 10.1136/jnnp-2017-316213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollenhauer B, Zimmermann J, Sixel‐Döring F, et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 2016;87(2):168–177. 10.1212/WNL.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erro R, Picillo M, Vitale C, et al. The non‐motor side of the honeymoon period of Parkinson's disease and its relationship with quality of life: a 4‐year longitudinal study. Eur J Neurol 2016;23(11):1673–1679. 10.1111/ene.13106. [DOI] [PubMed] [Google Scholar]
- 10.Antonini A, Barone P, Marconi R, et al. The progression of non‐motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol 2012;259(12):2621–2631. 10.1007/s00415-012-6557-8. [DOI] [PubMed] [Google Scholar]
- 11.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol 2017;16(1):66–75. 10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams‐Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA. The CamPaIGN study of Parkinson's disease: 10‐year outlook in an incident population‐based cohort. J Neurol Neurosurg Psychiatry 2013;84(11):1258–1264. 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 13.Mollenhauer B, Zimmermann J, Sixel‐Döring F, et al. Baseline predictors for progression 4 years after Parkinson's disease diagnosis in the De novo Parkinson cohort (DeNoPa). Mov Disord 2019;34(1):67–77. 10.1002/mds.27492. [DOI] [PubMed] [Google Scholar]
- 14.Ou R, Yang J, Cao B, et al. Progression of non‐motor symptoms in Parkinson's disease among different age populations: a two‐year follow‐up study. J Neurol Sci 2016;360:72–77. 10.1016/j.jns.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry 2008;79(10):1088–1092. 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch‐Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology 2006;67(1):33–38. 10.1212/01.wnl.0000230572.07791.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dujardin K, Sgambato V. Neuropsychiatric disorders in Parkinson's disease: what do we know about the role of dopaminergic and non‐dopaminergic systems? Front Neurosci 2020;14:00025. 10.3389/fnins.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa RP, Mendonça MD, Caetano AP, Lampreia TM, Miguel R, Bugalho PM. Cognitive complaints in Parkinson's disease patients: from subjective cognitive complaints to dementia and affective disorders. J Neural Transm (Vienna) 2019;126(10):1329–1335. 10.1007/s00702-019-02042-8. [DOI] [PubMed] [Google Scholar]
- 19.Shulman LM, Armstrong M, Ellis T, et al. Disability rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2016;31(10):1455–1465. 10.1002/mds.26649. [DOI] [PubMed] [Google Scholar]
- 20.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Arch Neurol 2003;60(3):387–392. 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013;70(5):580–586. 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 22.Anang JB, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83(14):1253–1260. 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson's progression markers initiative. J Neurol Neurosurg Psychiatry 2017;88(4):325–331. 10.1136/jnnp-2016-314832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord 2007;22(16):2314–2324. 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 25.Martinez‐Martin P. Dementia in Parkinson's disease: usefulness of the pill questionnaire. Mov Disord 2013;28(13):1832–1837. 10.1002/mds.25649. [DOI] [PubMed] [Google Scholar]
- 26.Arie L, Herman T, Shema‐Shiratzky S, Giladi N, Hausdorff JM. Do cognition and other non‐motor symptoms decline similarly among patients with Parkinson's disease motor subtypes? Findings from a 5‐year prospective study. J Neurol 2017;264(10):2149–2157. 10.1007/s00415-017-8605-x. [DOI] [PubMed] [Google Scholar]
- 27.Forsaa EB, Larsen JP, Wentzel‐Larsen T, Herlofson K, Alves G. Predictors and course of health‐related quality of life in Parkinson's disease. Mov Disord 2008;23(10):1420–1427. 10.1002/mds.22121. [DOI] [PubMed] [Google Scholar]
- 28.Skorvanek M, Goldman JG, Jahanshahi M, et al. Global scales for cognitive screening in Parkinson's disease: critique and recommendations. Mov Disord 2018;33(2):208–218. 10.1002/mds.27233. [DOI] [PubMed] [Google Scholar]
- 29.van Wamelen DJ, Martinez‐Martin P, Weintraub D, et al. The non‐motor symptoms scale in Parkinson's disease: validation and use. Acta Neurol Scand 2021;143(1):3–12. 10.1111/ane.13336. [DOI] [PubMed] [Google Scholar]
- 30.Sringean J, Stefani A, Marini K, et al. REM sleep behavior disorder and REM sleep without Atonia are more frequent in advanced versus early Parkinson's disease. Sleep 2021;zsab067. 10.1093/sleep/zsab067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline comparison between included and non‐included patients. MoCA, Montreal Cognitive Assessment scale; RBDSQ, REM Sleep Behaviour Disorder Symptom Questionnaire; HADS, Hospital Anxiety and Depression Scale, SCOPA, Scale for Outcomes in Parkinson's Disease; PPQ, Parkinson's Psychosis Questionnaire; NMSS, Non‐Motor Symptom Scale


