ABSTRACT
Background
Cervical dystonia (CD) involves clinical and motor manifestations, and visual and cognitive dysfunctions may also be frequent.
Objective
To evaluate functional vision, visual attention, and cognitive aspects in patients with CD compared with a control group.
Methods
Fifty patients with CD were assessed using the Useful Field of View Test (UFOV), the Mini Mental State Examination (MMSE), and the Trail Making Tests (TMT‐A and TMT‐B), and compared with an identical number of health controls matched by sex, age, and educational level.
Results
No differences were seen between the groups in terms of MMSE score (P = 0.481), but the CD patient group had poorer scores for the TMA‐A (P = 0.004) and TMT‐B (P = 0.004). For the UFOV subtests, a decrease was found for visual processing speed (P < 0.001), divided attention (P < 0.001), and selective attention (P = 0.001), as well as higher frequency in the categories with higher risk index in the UFOV test (P < 0.001).
Conclusion
Patients with CD may exhibit decreased functional vision and visual attention, as well as higher risk in performing complex activities.
Keywords: dystonia, cervical dystonia, activities of daily living, visual perception, cognition
Dystonia is a movement disorder characterized by abnormal movements and/or postures due to sustained or intermittent muscle contractions,1 leading to abnormal posture patterns and repetitive movements.1 Cervical dystonia (CD) is a common form of focal dystonia, characterized by the presence of involuntary movements of the cervical region in different planes and directions that result in abnormal postures of the head, neck, and shoulders.1, 2 The phenomenology of CD is complex, often involving postures such as torticollis (rotational deviation of the neck around the vertical axis), laterocollis (lateral neck flexion), and antero or retrocollis (flexion of the neck in the anteroposterior plane), in isolation or, more commonly, in combination.2, 3 The classic definition of dystonia primarily addresses motor disorders, but there is a good amount of evidence pointing to additional non‐motor features such as sensory, behavioral, neuropsychiatric, cognitive, and sleep‐related abnormalities.4, 5 These manifestations, known as the non‐motor syndrome of primary dystonia, may be an intrinsic part of the pathophysiology of the disease, occasionally even predating the onset of the motor symptoms.6, 7
Cognitive functions are generally considered intact in patients with primary dystonia, although research on this topic is limited by small and heterogeneous samples that are often assessed with non‐standardized protocols.4 However, there are reports of subtle cognitive deficits with impairment of executive functions and attention,6, 7, 8, 9 indicating that this population can present a profile of selective cognitive impairment despite unchanged global cognitive functioning. Additionally, a number of studies have demonstrated the association between CD with sensory impairment and sensory‐motor integration,10, 11, 12, 13, 14, 15, 16 with damage to the visual system that could lead to difficulties with color discrimination, contrast perception,10 and slower motor response time to visual stimuli received.12, 13, 14, 15 Visual and spatial deficits have been observed in patients with CD during tasks involving walking, identifying targets, and exploring the environment, which are partially compensated by eye movements, raising questions about their driving ability.15 Finally, these visual aspects in CD have also been related to reduced quality of life and impacts on mobility‐related activities (such as walking, driving, crossing the street, and parking).16 Thus, the aim of this study was to evaluate whether motor and non‐motor disorders in patients with CD can alter functional vision and impair performance in complex everyday activities.
Methods
Selection of Patients and Controls
Data were collected from a convenience sample consisting of 50 patients with CD who were followed at the Outpatient Movement Disorders Clinic at the Hospital de Clínicas of the Federal University of Paraná, between October 2017 and June 2019. Inclusion criteria were age > 18 years and dystonia affecting only the cervical region (focal dystonia). Exclusion criteria were secondary or heredodegenerative causes, treatment with botulinum toxin within the previous 120 days, previous stereotactic surgery, pharmacological treatment affecting dystonic movements or cognition, and clinical, neurological, ophthalmologic, or psychiatric illness that would make it impossible for the patient to take the tests.
The control group was composed of 50 subjects who accompanied patients seen at the same Outpatient Movement Disorders Clinic, matched by gender, age, and education level. This study was approved by the Ethics Committee on Human Research at the Hospital de Clínicas, Federal University of Paraná (process 74039717.6.0000.0096, ruling 2.323.684 of October 9, 2017). All study participants signed the terms of free and informed consent.
Clinical Evaluation
Diagnosis of CD was confirmed in all patients by neurologists specialized in movement disorders. The CD motor assessment was performed using the Brazilian Portuguese adaptation of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS). The total score for this scale (0–85 points) combines the scores for intensity, disability, and pain, with higher scores indicating greater impairment.17, 18
The control group underwent the same assessments as the CD group, except for the TWSTRS. Global cognitive assessment was measured using the Mini Mental State Examination (MMSE) and the Trail Making Test (TMT). The maximum MMSE score, indicating satisfactory performance in all evaluated domains, is 30 points.19, 20 The TMT is composed of two parts, A and B. The TMT‐A tests visual search and motor speed abilities and prepares the subject for the TMT‐B, which assesses executive functions.21, 22 In both sections, the subject receives brief instructions before starting the test and is asked to connect 25 circles (a numeric sequence in part A, and interspersed numbers and letters in part B), which are randomly distributed on a paper sheet, as quickly as possible, without lifting the pencil from the paper surface.23 The main variable of interest is the total time to complete the test, in seconds.
The visual assessment was performed in two parts, first with visual function screening tests (Hamilton‐Veale, the Snellen chart search test, confrontation visual field exam, and the saccade test)24, 25, 26, 27, 28, 29 to identify visual problems. The second part involves the Useful Field of View Test (UFOV) version 6.1.4; this computerized test consists of three subtests that assess the speed of visual processing, divided attention, and selective attention through tasks that gradually become more complex. Using both eyes, the subject must detect, identify, and locate targets that are quickly presented along the visual field, as the speed increases progressively by milliseconds. Subjects who normally use corrective lenses are permitted to use them during the test. The UFOV test lasts approximately 15 minutes, and before each subtest the subject does a practice run to ensure they understand the task. The score is automatically calculated for each subtest according to the subject's performance. The risk categories, which can simulate the degree of risk in performing everyday activities, are generated automatically by combining the scores for the three subtests: very low, low, low to moderate, moderate to high, and high.30
Statistical Analysis
The data were extracted for statistical analysis using IBM SPSS Statistics software for Windows (version 23). To verify the normality of the sample, we used the Kolmogorov–Smirnov test with Lilliefors correction, considering the variables age and education level. The Mann–Whitney U test was used to analyze the pairing of continuous variables between the groups. The chi‐square test of independence was used to determine whether the results between the groups were correlated according to categorical variables, and the bivariable correlation test was used to determine the existence of correlation between the test results for the CD group. Since this sample does not have a normal distribution, we used the Spearman correlation. Values of P < 0.05 were considered significant.
Results
Mean patient age for the CD group was 49.14 ± 10.86 years, and they had an average of 9.46 ± 4.16 years of education. Isolated neck deviation postures were seen in 35 (70%): 29 (58%) with torticollis and 6 (12%) laterocollis. The remaining 15 participants (30%) had deviations involving combined postures. In the control group, mean age was 48.04 ± 10.31 years, with an average education of 10.4 ± 3.87 years.
Table 1 shows the pairing between the groups and the clinical characteristics. The results indicate statistically similar performance on the MMSE (P = 0.481) and a significant difference in the TMT‐A (P = 0.004), TMT‐B (P = 0.004), and the three subtests comprising the UFOV test: processing speed (P = 0.000), divided attention (P = 0.000), and selective attention (P = 0.001), all favoring the control group.
TABLE 1.
Comparative analysis between groups
| Variable* | Patients with cervical dystonia (n = 50) | Controls (n = 50) | P ** |
|---|---|---|---|
| Sex (M/F) | 18/32 | 18/32 | 1 |
| Age (years) | 49.14 ± 10.86 | 48.04 ± 10.31 | 0.528 |
| Education (years) | 9.46 ± 4.16 | 10.4 ± 3.87 | 0.231 |
| TWSTRS total score (0–85) | 25.5 ± 13.38 | ‐ | ‐ |
| Severity score (0–35) | 14.38 ± 4.47 | ‐ | ‐ |
| Disability score (0–30) | 6.04 ± 4.45 | ‐ | ‐ |
| Pain score (0–20) | 7.95 ± 4.89 | ‐ | ‐ |
| MMSE score (0–30) | 27.8 ± 2.12 | 28.26 ± 1.56 | 0.481 |
| TMT‐A (sec) | 61.2 ± 47.61 | 39.78 ± 11.95 | 0.004 |
| TMT‐B (sec) | 171.2 ± 98.24 | 120.94 ± 89.63 | 0.004 |
| UFOV ‐ processing speed (ms) | 38.24 ± 66.02 | 15.24 ± 1.05 | 0.000 |
| UFOV ‐ divided attention (ms) | 113.90 ± 158.53 | 33.02 ± 37.48 | 0.000 |
| UFOV ‐ selective attention (ms) | 191.81 ± 150.65 | 101.62 ± 66.84 | 0.001 |
MMSE, Mini Mental State Examination; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; UFOV, Useful Field of View Test.
The groups were compared using the Mann–Whitney U test, except for sex and dominant hand, which used the chi‐square test.
The categorical results, which show the frequency of each risk level for complex everyday activities obtained for subjects in both groups according to the UFOV test, are presented in Fig. 1. The results for the UFOV test risk categories differed significantly between the groups, with the CD patients demonstrating higher risk categories more frequently than the control group.
FIG. 1.
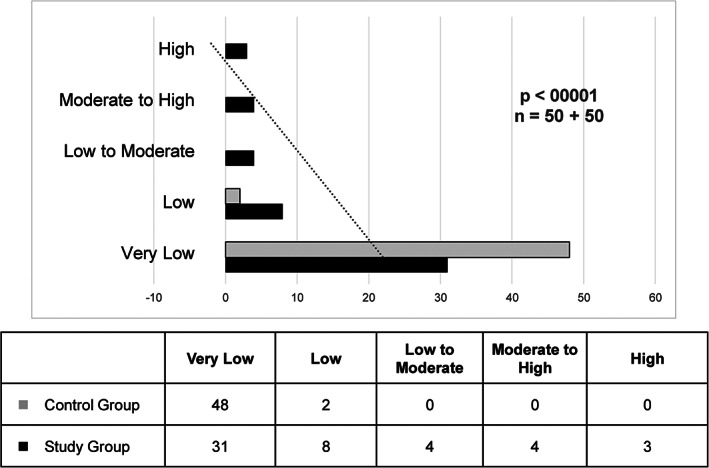
UFOV risk categories for complex everyday activities, cd group and control group. Significance was obtained using the chi‐square test and considering the likelihood ratio, since the number of frequencies lower than 5 exceeded 20%.
The results of bivariable correlation analysis are highlighted in Table 2. Moderate correlations were found between the cognitive tests and the UFOV subtests. As for the severity of dystonia and the cognitive aspects assessed, a weak positive correlation was found between the total TWSTRS score and the UFOV selective attention subtest (ρ = 0.277; P < 0.01), and between the TMT‐B and the intensity subscale (ρ = 0.271; P < 0.01) and the total TWSTRS score (ρ = 0.281; P < 0.01). Table 2 also presents correlations found between the different parts of one assessment, which were expected.
TABLE 2.
Correlations between ratings for the study group
| Variable | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 TWSTRS Severity |
14.38 (4.47) | 1 | |||||||||
| 2 TWSTRSDisability | 6.04 (4.45) | .536 ** | 1 | ||||||||
| 3 TWSTRS Pain | 7.95 (4.89) | .384** | .445 ** | 1 | |||||||
| 4 TWSTRS Total | 28.37 (10.93) | .801 ** | .800 ** | .759 ** | 1 | ||||||
| 5 MMSE | 27.8 (2.12) | −.055 | −.17 | −0.02 | −.116 | 1 | |||||
| 6 TMT‐A | 61.2 (47.61) | .226 | .124 | −0.053 | .139 | −.565** | 1 | ||||
| 7 TMT‐B | 171.2 (98.24) | .271* | .181 | 0.087 | .281* | −.619** | .607 ** | 1 | |||
| 8 UFOV ‐ PS | 38.24 (66.02) | −.15 | −.099 | −.141 | −.132 | −.341** | .23 | .428** | 1 | ||
| 9 UFOV ‐ DA | 113.90 (158.53) | .077 | .004 | 0.208 | .171 | −.362** | .443** | .509** | .573 ** | 1 | |
| 10 UFOV ‐ SA | 191.818 (150.65) | .206 | .133 | 0.211 | .277* | −.498** | .667** | .655** | .420 ** | .744 ** | 1 |
Moderate correlations are in bold and highlighted in dark gray. Correlations between parts of the same assessment are in italics and highlighted in light gray.
Abbreviations: DA, Divided Attention; MMSE, Mini Mental State Examination; PS, Processing Speed; SA, Selective Attention; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale.
Significant correlation at 0.01.
Significant correlation at 0.05.
The analysis also demonstrated that the dominant movement type (torticollis, anterocollis, retrocollis, or laterocollis) did not affect the cognitive assessment results. When the CD group was subdivided into patients with isolated postures (n = 35) or combined postures (n = 15), a significant difference was only seen between these subgroups in the intensity subscale (P = 0.026) and the total TWSTRS score.
Discussion
The UFOV test measures processing speed related to visual aspects (such as visual acuity and contrast sensitivity) and cognitive abilities (executive functioning, attention, and memory). This test evaluates the skills necessary to perform everyday tasks in an experimental environment.31, 32, 33, 34 In the present study, a significant difference was found between the CD group and the control group for the results of the three UFOV subtests, as well as more frequent findings of higher degrees of risk in performing everyday activities in the CD patient group.
These findings may also reflect the pathophysiological substrate of CD impacting visual function, which has been discussed in the literature. One previous study found impairment in visual discrimination of color and contrast perception in patients with idiopathic focal dystonia, indicating that the pathophysiology of these syndromes is not restricted to basal ganglia dysfunction.10 In addition, the role of the cerebellum in the pathophysiology of dystonia has been the subject of attention in recent years,11 as this structure may have a role in the integration of received visual feedback information with motor responses when predicting future actions.12 Furthermore, a recent study showed that CD patients demonstrated an overall increase in reaction time to locate peripheral targets.13 This function depends on perception in comparison with head alignment and eye position in the orbit14; for this reason, disorders such as CD may have a subtle impact on how visual information is processed.13, 14
Bradnam et al. (2020) have demonstrated that dystonia could affect performance of everyday activities related to vision, such as walking, crossing the street, driving, and parking a car, which could impact quality of life in these individuals.16 Visual/spatial deficits have been observed in patients with CD during tasks that involve exploring the environment and identifying targets, when patients exhibited saccadic eye movements to compensate for head rotation; whether this compensation is a learned strategy to overcome the physical restriction imposed by the dystonic posture of the head while exploring the environment or is the result of a cerebellar dysfunction associated with the dystonia is still the subject of debate.15 In isolated patients, these authors also found a pattern of eye movement compatible with that seen in people with chronic visual field deficits: some patients with head rotation were unable to locate targets on the side opposite the deviation.15 Bradnam et al. stressed the clinical importance of these findings, since visual/spatial deficits could lead to functional losses in mobility‐related activities.15
In this regard, the UFOV has been proven to be sensitive in predicting risk of involvement in traffic accidents for different age groups, particularly the selective attention subtest.31, 35 No previous studies on CD or idiopathic other forms of dystonia have used the UFOV test, but it has been used to assess other neurological diseases; in the area of movement disorders, it has been used to demonstrate driving difficulties among patients with Parkinson's disease (PD).36, 37, 38, 39 These studies found that UFOV results were correlated with the number of errors committed and with failing scores on a practical driving test,37 and that tests such as the UFOV and TMT can provide important data related to the abilities needed for driving.38
The results of the cognitive tests used in this study demonstrated that participants with CD exhibited equivalent global cognitive performance (as measured by the MMSE) but significantly different performance on assessments related to specific cognitive skills, such as selective attention, divided attention (UFOV subtests), and executive functions (TMT‐B), compared to healthy controls.
Mobility in the environment, whether this means walking in the street, using public transport, or (especially) driving a vehicle, requires various simultaneous body functions34, 40, 41 Executive functions are essential for decision making, planning, execution, and behavior in specific situations.42 In this present study, the results of the TMT‐B exhibited moderate positive correlations with the three UFOV subtests: processing speed, divided attention, and selective attention, which is corroborated by the data from previous studies.
Cognitive involvement in primary dystonia is a controversial topic in the literature, often cited as a primary part of the phenotype, unrelated to motor manifestations,4 or secondary to motor symptoms.6, 7 In a retrospective study, Foley et al. (2017) compared cognitive aspects and mood between 38 patients with primary dystonia (25 with CD and 13 with generalized dystonia) and 50 healthy controls. The participants underwent a battery of neuropsychological tests and were evaluated on global cognition, memory, attention, and executive function. No significant differences were found in the results between the patients with different clinical forms of dystonia, but when all patients with dystonia were combined into one group and compared with the controls, a significant difference was seen in cognitive tests such as the TMT‐A and TMT‐B. Additionally, these authors pointed out that the cognitive deficits found were not related to disability or severity of motor symptoms.6 Similarly, Duane (2004) stated that deficits in executive functions and attention are most prominent in CD, and these deficits were not influenced by the TWSTRS pain or disability scores, suggesting that they may not be secondary to motor symptoms. Based on these data and his own observations, this same author speculate on the possibility that the patterns of cognitive dysfunction in dystonia precede onset of motor symptoms, potentially indicating a neural system that is intrinsically at risk for dystonia.7
Conversely, a literature review by Stamelou et al. (2012) found little evidence that cognitive deficits are present in primary dystonia, and argued that subtle alterations may be related to motor symptoms and pain. These authors maintain that the investigation of cognitive aspects is limited by the small samples and heterogeneous populations in these studies.4
This present study found weak positive correlations between the total TWSTRS score and the UFOV selective attention subtest, and between the TMT‐B and the intensity subscale and the total TWSTRS score, which does not challenge the findings of Foley et al. or Duane. Our sample excluded patients still affected by botulinum toxin therapy (patients were included only at least 120 days after the last application); new research with a broader sample including patients receiving and experiencing the effects of this therapy could help clarify the relationship between motor symptoms and cognitive performance.
This present study includes a broad evaluation protocol, which was intended to compare the performance of participants with and without CD in functions that are important for everyday activities, and presents results that are significant for the study of this health condition, creating possibilities for discussion in this field.
Besides the difficulties addressed previously, some limitations of this study should also be taken into consideration. Even though a history of psychiatric illness was an exclusion factor, because a depression scale was not applied this variable cannot be entirely ruled out, and consequently may have influenced the results of the cognitive assessments. Other aspects that were not considered were the presence of tremor (since the scale chosen for evaluation did not include this factor) and the use of sensory tricks when the tests were being applied.
This study demonstrated that patients with CD exhibit significant impairment of specific cognitive functions and visual planning. Additional research could further investigate the pathophysiological relationship with the deficits found in our sample and compare results in test subjects undergoing botulinum toxin therapy. The tools used in this study are usually employed to evaluate complex everyday activities such as driving, but no studies or epidemiological data have demonstrated an elevated frequency of traffic accidents among patients with CD. Future studies could investigate the influence of CD on the skills needed to perform this and other types of complex tasks.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
MSCB: 1B, 1C, 3A
RN:1A, 2A, 2B, 2C, 3B
CHFC: 3B
HAGT: 2C, 3B
Disclosures
Ethical Compliance Statement
This study was approved by the Ethics Committee on Human Research at the Hospital de Clínicas, Federal University of Paraná (process 74039717.6.0000.0096, ruling 2.323.684 of October 9, 2017). Written informed consents were obtained from the patients for the report of their clinical findings. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors declare that there are no funding sources or conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain 1998;121(4):547–560. [DOI] [PubMed] [Google Scholar]
- 3.Comella CL. Cervical dystonia. In: Warner TT, Bressman SB, eds. Clinical and Management of Dystonia. London: Informa Healthcare; 2007:73–79. [Google Scholar]
- 4.Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non‐motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain 2012;135(6):1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munhoz RP, Teive HAG, Della Coletta MV, et al. Frequency of obsessive and compulsive symptoms in patients with blepharospasm and hemifacial spasm. Arq Neuropsiquiatr 2005;63(2A):213–216. Erratum in: arq Neuropsiquiatr 2005; 63(2b):552. [DOI] [PubMed] [Google Scholar]
- 6.Foley JA, Vinke RS, Limousin P, Cipolotti L. Relationship of cognitive function to motor symptoms and mood disorders in patients with isolated dystonia. Cogn Behav Neurol 2017;30(1):16–22. [DOI] [PubMed] [Google Scholar]
- 7.Duane DD. Re: executive cognitive deficits in primary dystonia. Mov Disord 2004;19(1):116–117. author reply 117. [DOI] [PubMed] [Google Scholar]
- 8.Relja M, Miletic V. Assessment of non‐motor symptoms and cognitive functions in patients with isolated adult‐onset cervical dystonia previously not treated with botulinum toxin: a single‐Centre case‐controled study. Neurology 2018;90(Suppl 15):P5.043. [Google Scholar]
- 9.Scott RB, Gregory R, Wilson J, et al. Executive cognitive deficits in primary dystonia. Mov Disord 2003;18(5):539–550. [DOI] [PubMed] [Google Scholar]
- 10.Büttner T, Kuhn W, Dietz M, et al. Impaired visual function in focal idiopathic dystonia. Eur Neurol 1999;41(2):94–98. [DOI] [PubMed] [Google Scholar]
- 11.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord 2013;28(7):958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filip P, Lungu OV, Shaw DJ, Kasparek T, Bares M. The mechanisms of movement control and time estimation in cervical dystonia patients. Neural Plast 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amlang CJ, Hubsch C, Rivaud‐Pechoux S, et al. Contributions of visual and motor signals in cervical dystonia. Brain 2017;140(1):e4. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh AG, Zee DS, Crawford JD, Jinnah HA. Reply: contributions of visual and motor signals in cervical dystonia. Brain 2017;140(1):e5. [DOI] [PubMed] [Google Scholar]
- 15.Bradnam L, Chen CS, Callahan R, Hoppe S, Rosenich E, Loetscher T. Visual compensation in cervical dystonia. J Clin Exp Neuropsychol 2019;41(7):769–774. [DOI] [PubMed] [Google Scholar]
- 16.Bradnam L, Chen C, Graetz L, Loetscher T. Reduced vision‐related quality of life in people living with dystonia. Disabil Rehabil 2020;42(11):1556–1560. [DOI] [PubMed] [Google Scholar]
- 17.Consky ES, Lang AE. Clinical assessments of patients with cervical dystonia. In: Jancovic J, Hallett M, eds. Therapy with Botulinum Toxin. New York: Marcel Dekker; 1994:211–237. [Google Scholar]
- 18.Sekeff‐Sallem FA. Tradução para o Português e validação da escala de avaliação de torcicolo espasmódico de Toronto (Toronto Western Spasmodic Torticollis Rating Scale) [Translation to Portuguese and validation of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS)] [dissertation]. [São Paulo(BR)]: Faculdade de Medicina da Universidade de São Paulo; 2015. p. 183 Portuguese.
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 20.Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. The mini‐mental state examination in an outpatient population: influence of literacy. Arq Neuropsiquiatr 1994;52(1):1–7. Portuguese. [PubMed] [Google Scholar]
- 21.Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc 2006;1(5):2277–2281. [DOI] [PubMed] [Google Scholar]
- 22.Jenner B. Appendix: driver evaluation tools. In: Eby DW, Molnar LJ, Kartje PS, eds. Maintaining Safe Mobility in an Aging Society. New York: CRC Press; 2009:155–169. [Google Scholar]
- 23.Lezak MD, Howieson DB, Bigler ED, Tranel D. Chapter 9, orientation and attention. In: Lezak MD, Howieson DB, Bigler ED, Tranel D, eds. Neuropsychological Assessment. 5th ed.New York: Oxford University Press; 2012:529–573. [Google Scholar]
- 24.Charlton J, Koppel S, O'Hare M, et al. Influence of Chronic Illness on Crash Involvement of Motor Vehicle Drivers. Clayton: Monash University Accident Research Centre; 2010. Report No 300.
- 25.Hamilton Veale [homepage on the internet] . Introducing the Hamilton Veale contrast sensitivity test. https://www.contrast-sensitivity-test.com/
- 26.Reed K, Liu K, Kalu PN. An assessment of the physical factors that influence older adult driving ability. Int Health Journal 2018;1:10–24. [Google Scholar]
- 27.Bicas HEA . Visual acuity: measurements and notations. Arq Bras Oftalmol 2002;65(3):375–384. Portuguese. [Google Scholar]
- 28.Gutman SA, Schofeld AB. Screening Adult Neurologic Populations. 2nd ed.Bethesda, MD: AOTA Press; 2009. [Google Scholar]
- 29.Fedeger AM. Avaliação de condutores de automóveis com Doença de Parkinson: um estudo em Curitiba‐Pr [Evaluation of car drivers with Parkinson's Disease: a study in Curitiba‐PR] [dissertation]. [Curitiba(BR)]: Universidade Federal do Paraná; 2016, p. 149. Portuguese.
- 30.Visual Awareness Research Group . UFOV® Users Guide Version 6.1.4. Punta Gorda; 2009, p. 28.
- 31.Selander H, Wressle E, Samuelsson K. Cognitive prerequisites for fitness to drive: norm values for the TMT, UFOV and NorSDSA tests. Scand J Occup Ther 2020;27(3):231–239. [DOI] [PubMed] [Google Scholar]
- 32.Aust F, Edwards JD. Incremental validity of useful field of view subtests for the prediction of instrumental activities of daily living. J Clin Exp Neuropsychol 2016;38(5):497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woutersen K, Guadron L, Van den Berg AV, et al. A meta‐analysis of perceptual and cognitive functions involved in useful‐field‐of‐view test performance. J Vis 2017;17(14):1–20. [DOI] [PubMed] [Google Scholar]
- 34.Kay LG, Bundy AC, Clemson L, Cheal B, Glendenning T. Contribution of off‐road tests to predicting on‐road performance: a critical review of tests. Aust Occup Ther J 2012;59(1):89–97. [DOI] [PubMed] [Google Scholar]
- 35.McManus B, Cox MK, Vance DE, Stavrinos D. Predicting motor vehicle collisions in a driving simulator in young adults using the useful field of view assessment. Traffic Inj Prev 2015;16(8):818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Classen S. Consensus statements on driving in people with Parkinson's disease. Occup Ther Health Care 2014;28(2):140–147. [DOI] [PubMed] [Google Scholar]
- 37.Classen S, McCarthy DP, Shechtman O, et al. Useful field of view as a reliable screening measure of driving performance in people with parkinson's disease: results of a pilot study. Traffic Inj Prev 2009;10(6):593–598. [DOI] [PubMed] [Google Scholar]
- 38.Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson's disease. Ann Neurol 2006;60(4):407–413. [DOI] [PubMed] [Google Scholar]
- 39.Amick MM, Grace J, Ott BR. Visual and cognitive predictors of driving safety in Parkinson's disease patients. Arch Clin Neuropsychol 2007;22(8):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathias JL, Lucas LK. Cognitive predictors of unsafe driving in older drivers: a meta‐analysis. Int Psychogeriatr 2009;21(4):637–653. [DOI] [PubMed] [Google Scholar]
- 41.Asimakopulos J, Boychuck Z, Sondergaard D, Poulin V, Ménard I, Korner‐Bitensky N. Assessing executive function in relation to fitness to drive: a review of tools and their ability to predict safe driving. Aust Occupa Ther J 2012;59(6):402–427. [DOI] [PubMed] [Google Scholar]
- 42.Organização Mundial da Saúde [World Health Organization] . CIF: Classificação Internacional de Funcionalidade, Incapacidade e Saúde [ICF: International Classification of Functioning, Disability and Health), São Paulo(BR), 2015, p. 336. Portuguese.


