The Task Force of the International Parkinson and Movement Disorder Society defines tremor as an “involuntary, rhythmic, oscillatory movement of a body part”.1 According to Task Force consensus, tremor is classified into two main axes: clinical features (axis‐1) and etiology (axis‐2). We followed this classification in approach to a case which led to discovery of a rare new etiology for the tremor syndrome.
Case Report
A 27‐year‐old right‐handed Kurdish woman was referred to our center to be considered for deep brain stimulation (DBS) owing to severe disabling tremor. Her tremor started in the right upper extremity at the age of 13 years, gradually spread to the contralateral side and head. Due to positive familial history and the absence of other neurological features, she was diagnosed as essential tremor (ET). Primidone, gabapentin, propronolol, levodopa and clonazepam showed no appreciable benefit. Gradually, the tremor became disabling interfering with daily activities. At the age of 20, she developed voice tremor and imbalance. Her parents were cousins and her mother had mild head tremor (see pedigree in Figure S1).
On examination, mini‐mental‐state‐examination score was 29 out of 30. Speech had a scanning quality. There was severe side‐to‐side head tremor. Extra‐ocular‐movement examination was normal. There was postural tremor along with intention tremor (but no rest component) in upper extremities more severe on the right side. Finger‐to‐nose and heel‐to‐shin tests suggested mild dysmetria. Gait was mildly ataxic with mild difficulties performing tandem‐walking (Video 1). Deep‐tendon‐reflexes were brisk and plantar reflexes were flexor bilaterally (SARA‐score = 14).
Video 1.
In this video patient exhibits scanning speech, severe side‐to‐side head tremor and postural tremor in upper extremities more severe on the right side. Finger‐to‐nose and heel‐to‐shin tests shows obvious dysmetria. There is no hypo/bradykinesia. Gait is mildly ataxic and she is not able to tandem‐walk.
According to the consensus classification, the patient has an adolescence‐onset, familial, segmental, kinetic (with intentional component) and postural tremor of hands accompanied by limb and truncal ataxia. Therefore, in axis‐1 the tremor syndrome is a “combined tremor syndrome” or “tremor syndrome with additional signs”, “ataxia” in this case.1, 2
Brain MRI was normal, without any evidence of cerebellar atrophy (Fig. 1).
FIG. 1.
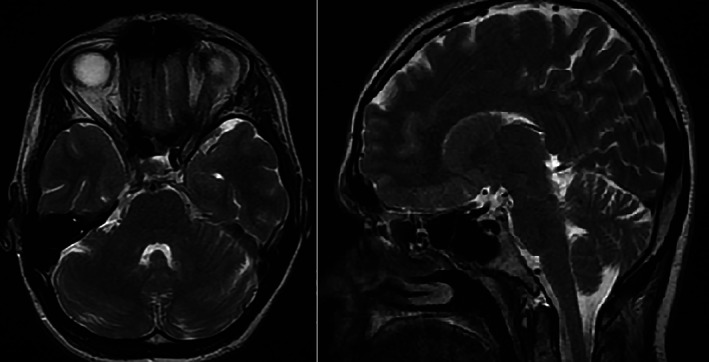
Axial (left) and sagittal (right) T2‐weighted brain MRI showed no cerebellar atrophy.
The possible etiologies (e.g. hyperthyroidism and Wilson disease) were ruled out by history, appropriate examinations and laboratory tests.
Due to presence of ataxia with tremor and strong familial propensity, we suspected SCA subtypes. Unfortunately, a SCA panel (e.g. SCA1, SCA2, SCA3, SCA6 and SCA12) was not performed for this patient. Genetic study through whole exome sequencing revealed a missense novel mutation in CCDC88C gene (NM_001080414: exon 30: c.T5922G: p.S1974R) confirming the diagnosis of SCA40. This was predicted as a variant of uncertain significance (VUS). The mutation was not located in a protein functional domain including Myosin_tail_1, RILP‐like or DUF2570. The affected family members were not available for segregation analysis and the pedigree was obtained through the history (see Supplementary materials regarding the methodology of genetic study). DBS is widely used in the treatment of different types of tremor, but in the presence of ataxia, although it may help the tremor component, it is reported to have no effect on or even exacerbate the dysmetria.2 As our experience regarding DBS in patients with ataxia has been concordant with these reports, we did not consider DBS for this patient.
Discussion
A wide range of disorders may be misdiagnosed as ET, among which, tremor‐dominant Parkinson disease, enhanced physiological tremor, spinocerebellar ataxia (SCA), fragile‐X tremor‐ataxia syndrome (FXTAS), dystonic tremor, cortical tremor (a form of rhythmic cortical myoclonus), neuropathic tremor, drug‐induced tremor, orthostatic tremor with postural upper extremity tremor while seated, and functional tremor, should be highlighted.3
We should keep in mind that many cases who are diagnosed as ET (up to 50%) will eventually turn out to be genetic dystonic or cerebellar disorders with tremor presentation.3 The acceptance of a few additional findings or soft signs in the context of ET syndrome (designated as ET plus)1 may compromise the diagnosis of disorders presenting similar to ET, such as dystonia, SCA, FXTAS or other etiologically distinct disorders.4 Therefore, it seems appropriate to see ET as a syndrome in Axis‐1 and then to begin searching for an etiology in Axis‐2.
Tremors with ataxia (cerebellar tremors) are generally caused by lesions in the cerebellothalamic pathway. A wide range of disorders may be responsible for this syndrome, among which SCAs are classically a consideration.1, 2
SCAs are a heterogeneous group of autosomal dominant (AD) disorders that classically present with progressive cerebellar ataxia and a variable combination of other features including ocular movement abnormality, dysarthria, pyramidal signs, dystonia, tremor, myoclonus, parkinsonism, chorea, pigmentary retinopathy, optic atrophy, peripheral neuropathy, amyotrophy, seizures, cognitive dysfunction and psychiatric symptoms. Among the known subtypes, SCA5, 12, 14, 15, 16, 19, 20, 22, 23, 27, 35 have been associated with tremor. SCA12, 15 and 27, similar to ET, show more prominent upper limb postural tremor.5, 6 SCA40 has been reported only in two families with the clinical picture including progressive cerebellar ataxia, vertical gaze ophthalmoplegia, pyramidal signs, action tremor (and/or intention tremor), dementia and parkinsonism.7, 8 In this report, we have tried to elucidate another clinical presentation of SCA40, describing an ET syndrome presentation with mild and later‐onset cerebellar signs, which may have been present in the previously reported cases but were not emphasized on from this perspective.8
Interestingly, the pontocerebellar atrophy, a common MRI finding in SCAs, may not be a consistent feature, as in the Polish family with SCA408 and our case. When encountering an ET syndrome with AD inheritance, even in the absence of obvious cerebellar ataxia and other neurological features, one should consider SCA subtypes especially 12, 15, 27 and 40 in the differential diagnosis. We have to include a caution statement at the end; as the most frequent SCA subtypes are caused by repeat expansions and a SCA panel was not performed for this patient and considering that this patient has a VUS in the CCDC88C gene, common causes of action tremor with ataxia, like SCA12 are not ruled out assuredly (Table S1 and materials).
To conclude, we emphasize the usefulness of clinical application of the two‐step consensus classification, especially in cases presenting with an ET syndrome. Although there are ambiguities and limitations with this approach, it helps to consider ET as a syndrome and look further for the etiologic causes, many of which have yet to be identified, as was the case for our patient.
Author Roles
(1). 1Research project: A. Conception, B. Organization, C. Execution, (2). Statistical Analysis: A. Design, B. Execution, C. Review and Critique, (3). Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
ME: 1B, 2B, 3A, 3B
SA: 1C, 2B
FM: 1C, 3A
RPM: 1C, 3B
AEL: 1C, 3B
AA: 1C, 3B
MR: 1A, 1B, 1C, 2B, 3B
Disclosures
Ethical Compliance Statement
Iran University of Medical Sciences ethical committee and review board has approved the use of human data for this study to be published as a case report. The patient has consented in written informed consent form for the publication of the report and scientific use of the video. All authors have read and complied with the Journal's Ethical Publication Guidelines. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
No specific funding was received for this work. We declare that the authors have no conflict of interest.
Financial Disclosures for the Previous 12 months
The authors declare that there are no additional disclosures to report.
Supporting information
Figure S1 The pedigree shows the proband and family members suffering similar symptoms. Note the difference in major symptoms of some family members mentioned in the pedigree.
Table S1 The list of the genes tested for the patient is available in this table. These genes included neurodegenerative disorders causative genes including ataxia, dystonia, HSP, parkinsonism, ALS, NBIA, and neuropathies.
Supplementary file: Legend: Detailed description of genetic testing methodology is available in this document.
Acknowledgments
The authors acknowledge the patient and her family for consenting to participate in this study.
References
- 1.Bhatia K, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED. Diagnosis and management of tremor. Continuum 2016;22(4):1143–1158. [DOI] [PubMed] [Google Scholar]
- 3.Espay AJ, Lang AE, Erro R, Merola A, Fasano A, Berardelli A, Bhatia KP. Essential pitfalls in “essential” tremor. Mov Disord 2017;32(3):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano A, Lang AE, Espay AJ. What is “essential” about essential tremor? A diagnostic placeholder. Mov Disord 2018;33(1):58–61. [DOI] [PubMed] [Google Scholar]
- 5.Sun YM, Lu C, Wu ZY. Spinocerebellar ataxia: relationship between phenotype and genotype–a review. Clin Genet 2016;90(4):305–314. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Hodaie M, Munhoz RP, Rohani M. SCA 35 presenting as isolated treatment‐resistant dystonic hand tremor. Parkinsonism Relat Disord 2017;37:118–119. [DOI] [PubMed] [Google Scholar]
- 7.Tsoi H, Yu ACS, Chen ZS, et al. A novel missense mutation in CCDC88C activates the JNK pathway and causes a dominant form of spinocerebellar ataxia. J Med Genet 2014;51(9):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leńska‐Mieciek M, Charzewska A, Królicki L, et al. Familial ataxia, tremor, and dementia in a polish family with a novel mutation in the CCDC88C gene. Mov Disord 2019;34(1):142–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The pedigree shows the proband and family members suffering similar symptoms. Note the difference in major symptoms of some family members mentioned in the pedigree.
Table S1 The list of the genes tested for the patient is available in this table. These genes included neurodegenerative disorders causative genes including ataxia, dystonia, HSP, parkinsonism, ALS, NBIA, and neuropathies.
Supplementary file: Legend: Detailed description of genetic testing methodology is available in this document.


