Abstract
South Asia, encompassing many populous countries including India, Pakistan, and Bangladesh, is home to a wide variety of infectious diseases several of which are disproportionately prevalent, endemic or distinctive to the region. These result in considerable morbidity and mortality, which can be greatly reduced through public‐health measures, timely diagnosis and treatment. Some of these infectious diseases have neurological manifestations including movement disorders either due to the pathogen being neuroinvasive or via an immune‐mediated response. For diseases such as Japanese encephalitis, movement disorders are the primary manifestation while for others, they can be a presenting feature. Thus, recognizing these movement disorders is often crucial to the diagnosis of the particular infection, and/or to exclude infection as a cause and arrive at the correct alternate diagnosis. Once diagnosed, the infection‐related movement disorders are treated by targeting the infectious agent, or symptomatically. In this article, we describe and illustrate a variety of movement disorders that are seen in patients infected by viruses, bacteria and parasites in South Asia. This would be of value to neurologists practicing in the region and, with the increasing ease in movement of people and pathogens, those practicing elsewhere.
Keywords: infections, movement disorders, tropical, epidemics
Infectious diseases have afflicted humans since the start of agriculture and domestication of animals in ca.12,000 BCE, and are caused by microbes that once infected other animal hosts. Some infectious diseases, such as tuberculosis and malaria have existed for millennia, while others such as those caused by HIV, Zika, and certain influenza and coronaviruses have emerged within the last century and led to pandemics. The spread of a pathogen in the human population involves a complex interplay between the pathogen, the host(s) and the environment (Table 1).1
TABLE 1.
Terms related to infections (adapted from Dorland's medical dictionary and reference (1))
| Host: Human or another animal in which the infectious agent lives. Primary or definitive host is one in which the infectious agent can multiply sexually, for example, cats for the protozoa causing toxoplasmosis, humans for the tapeworm taeniasis. Intermediate host is one in which the infectious agent lives, and may multiply asexually but not sexually, for example, pigs for the tapeworm taeniasis. Amplifying host is one in which the infectious agent can multiply rapidly to high numbers, for example, pigs, birds and horses for Japanese encephalitis virus; Dead end or incidental host is one who cannot pass the infectious agent to another host, for example, humans for pathogens causing Japanese encephalitis, dengue, West Nile encephalitis and toxoplasmosis. |
| Zoonosis: Infections that are naturally transmitted from vertebrate animals to humans. |
| Vector: Organism that transmits an infection from person to person or from animals to humans. |
| Endemic: Prevalence of human infection in a defined geographical area. |
| Epidemic: High incidence of human infection in a population or geographical area. |
| Outbreak: High incidence of infection in a small geographical area. |
| Pandemic: Spread of infection, usually rapidly, over wide geographical areas or globally. |
| (Newly) emerging infections: Appearance of infection in a new host or increase in its incidence in a population or geographical area. Remerging infections refers to increase in incidence of infections that had previously declined, or appearance of resistant forms of the infection. |
South Asia, comprising India, Pakistan, Bangladesh, Nepal, Sri Lanka, Bhutan, and Maldives, is one the most populous regions of the world. It is home to 1.8 billion people, that is, approximately 25% of the human population. It has tropical and temperate climate, abundant fresh water, thriving agricultural practices, rich and diverse flora and fauna, and flourishing urban areas—all of which facilitate high human and animal population densities, and close proximity of humans with disease vectors and intermediary hosts. These in turn provide a conducive environment for infections to emerge, spread and persist leading to considerable disease prevalence, morbidity and mortality (Table 2).
TABLE 2.
Common and emerging infections in South Asia associated with movement disorders
| Disease | Global incidence (year) | Propensity to cause movement disordersa | Anatomical predilection of the infectionb | |||
|---|---|---|---|---|---|---|
| Basal ganglia | Brainstem | Accompanying video | ||||
| Arthropod‐borne viruses | Japanese encephalitis | 68,000 (2019)2 | +++3, 4 | Yes | Yes | Video 1, Video 2 |
| Dengue | 400 million (2019)5 | +4, 6, 7, 8, 9, 10 | No | No | Video 3 | |
| Zika | 270,000 (2019)11 | + | No | No | Video 4 | |
|
Congenital Zika syndrome |
− | +++12, 13 | ||||
| Neuroinvasive West Nile disease | >25,000 (1999–2019)c 14 | ++++15, 16 | Yes | Yes | − | |
| Chikungunya | 250,000 (2018)17 | +18 | No | No | − | |
| Other RNA viruses | Measles | 10 million (2018)19 | + | No | No | Video 5, Video 6 |
| SSPE | Few 1000s–10,000s /yeard | ++++20 | ||||
| MIBE | <100 (up to 2020)e 21 | +22 | ||||
| Nipah | 650 (1998–2018)e 23 | ++f 24 | No | Yes | − | |
| Rabies | 59,000 (2015)25 | ++++g 26 | No | Yes | Video 7 | |
| COVID‐19 | >100 million (2019–2021)e 27 | +28 | No | No | Video 8 | |
| DNA viruses | Herpes simplex encephalitis | h | +29 | No | No | Video 9 |
| Postinfection autoimmune encephalitis | − | +++29 | ||||
| Varicella zoster | 84 million (2019)11 | +30 | No | No | Video 9 | |
| Human retroviruses | HIV | 1.7 million (2019)31 | + (++)i 32, 33 | Yes | No | |
| Bacterial infections | Rheumatic chorea | − | ++++34 | No | No | Video 10 |
| Tetanus | 74,000 (2019)11 | ++++g 35 | No | No | Video 11 | |
| Tuberculosis | 10 million (2020)36 | +37 | No | No | Video 12 | |
| Syphilis | 14 million (2019)11 | +38 | No | No | − | |
| Parasitic infections | Malaria | 230 million (2019)39 | +40 | No | No | Video 13 |
| Toxoplasma encephalitis | − | +41, 42 | Yes | Yes | Video 13 | |
| Neurocysticercosis | 2.5–8 million prevalence43 | +44 | No | No | Video 13 | |
Fraction of symptomatic patients with movement disorders. ++++: >75%; +++: 50–75%; ++: 25%–50%; +: <25%.
Based on autopsy and neuroimaging studies.
Cumulatively about 25,000 cases were reported between 1999–2019 in the US alone.
Order of magnitude estimate based on a few tens of million annual cases of measles during this century19 with roughly one in 1000–10,000 developing SSPE.45
Cumulative case numbers for the given range of years.
As seen in the Malaysia outbreak in 1998–99.
Including spasms.
Over 90% of adults over 50 years have been infected.
HIV‐related movement disorders are more frequent in advanced AIDS.
Several infections are associated with movement disorders. These accounted for 20% of non‐neurodegenerative disorders seen by movement disorder specialists at a center in South Asia; a larger proportion than reported outside the region.46, 47, 48, 49 Infection‐related neuronal injury or dysfunction results either from direct neuro‐invasion or release of neuro‐toxins by the pathogen, or are induced by the immune response.35, 50, 51 More than one mechanism can be operative. Movement disorders may develop during the acute phase of the illness, or be delayed by months or even decades.3, 52 Infections also differ in their propensity for causing movement disorders. For instance, among the infections common in South Asia, movement disorders are seen frequently in patients with Japanese encephalitis and rheumatic chorea, occasionally in patients with herpes simplex encephalitis, and rarely in patients with influenza or malaria (Table 2). Knowledge of the spectrum, the timeline, and the frequency of movement disorders can aid diagnosis and patient management.
In this video anthology we cover viral, bacterial and parasitic infections based on whether they are common or emergent in South Asia and their propensity to cause movement disorders. Infections that are rare in the region (eg, Whipple's disease53) or do not often cause movement disorders (eg, leptospirosis54) are not discussed. The included videos of 41 cases show the range and characteristics of movement disorders associated with each of the selected infection. And in the text, we outline, for each pathogen, the mode of transmission, burden of the disease and the clinical syndrome with a focus on neurological complications, particularly movement disorders. In Table 1 we define some of the infectious disease terminology used in the article; Table 2 presents an overview of the prevalence of the discussed infectious diseases and their propensity to cause movement disorders; Figure 1 outlines the signs and investigations, while Figure 2 shows the characteristic neuroimaging abnormalities, that can help diagnose a patient with suspected infection‐related movement disorders.
FIG 1.
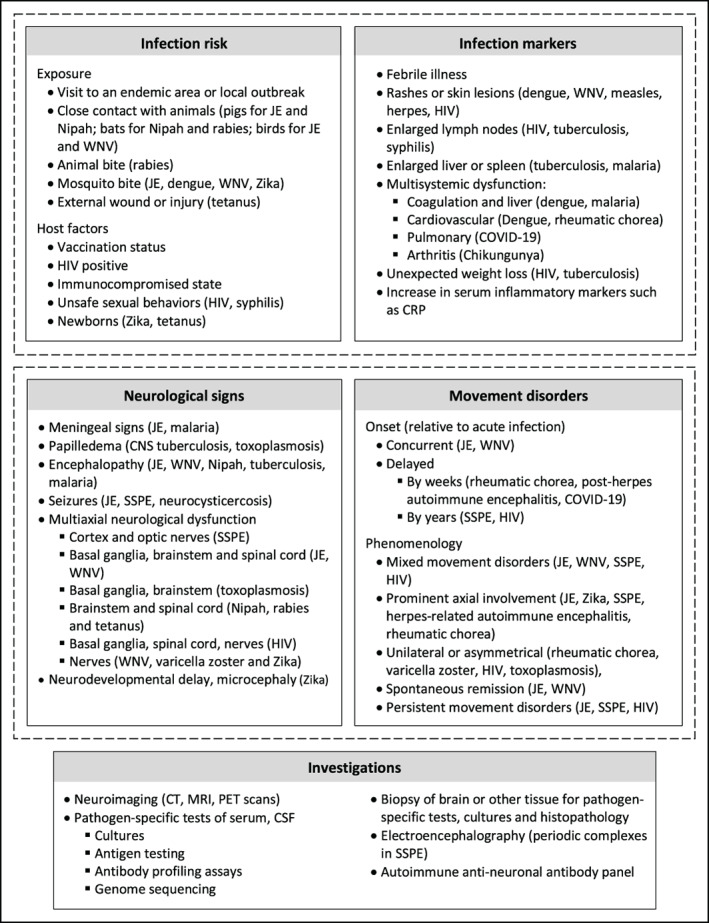
Diagnostic evaluation of patients with suspected infection‐related movement disorders. Infection susceptibility and signs (current or in patient‐history) are combined with neurological and movement disorders evaluations to reach a diagnosis, which is confirmed through imaging and laboratory studies. Examples of diagnoses to consider are given in parenthesis. Abbreviations: CRP, C‐reactive protein; CSF, cerebrospinal fluid; CT, computed tomography; JE, Japanese encephalitis; MRI, Magnetic resonance imaging; PCR, polymerase chain reaction; PET, positron emission tomography; SSPE, subacute sclerosing panencephalitis; WNV, West Nile virus or related encephalitis.
FIG 2.
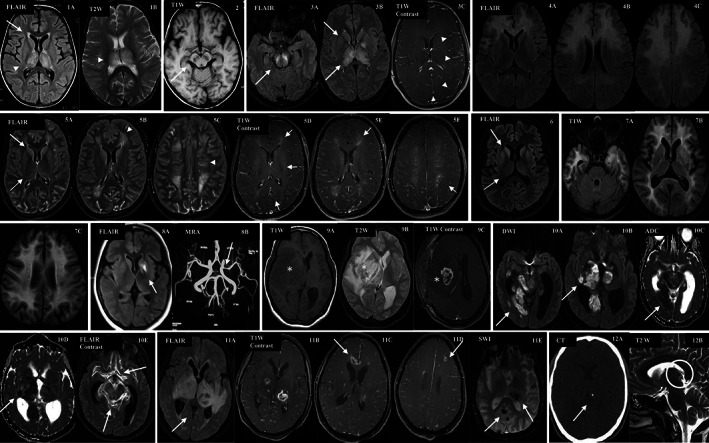
Neuroimaging abnormalities seen in infections common in South Asia. (1) and (2) Japanese encephalitis; (3) dengue; (4) SSPE; (5) COVID‐19; (6) rabies (7) HIV‐associated‐dementia; (8) post‐varicella arteriopathy; (9) and (10) CNS tuberculosis; (11) toxoplasmosis; and, (12) Neurocysticercosis (see Supplementary Material S1 for details).
Arthropod‐Borne RNA Viruses
Arthropods (insects, tics etc.) are vectors of transmission for a number of viruses in the tropical, subtropical and, to an extent, temperate zones. Many of the viruses that are of clinical significance in South Asia are spread by human‐adapted Aedes and Culex mosquitoes, and belong to the genus Flavivirus. The flaviviruses include the Japanese encephalitis, Dengue, Zika and West Nile viruses. All of these except dengue are highly neurotropic, entering the CNS using a diverse set of pathways, and sometimes targeting the basal ganglia or the developing brain.50, 55
Japanese Encephalitis
Japanese encephalitis virus (JEV) is a zoonotic pathogen transmitted by the Culex and Aedes mosquitoes.56, 57 JEV was first described in Japan in the 1870s but over the last three decades it has become endemic in South and Southeast Asia, and in the Western Pacific countries.58 Two billion people in these regions are at risk of JEV infection. In the endemic regions, there are approximately 50–175,000 cases of Japanese encephalitis (JE) annually, which makes JEV the commonest cause of viral encephalitis.58 Of these approximately 20–30% of patients die, while 30–50% suffer long‐term neurological and cognitive disability.2, 58
In most patients JEV infection is either asymptomatic or mild, but in approximately 1:250 it causes severe disease. Symptoms vary from a brief febrile illness, aseptic meningitis, encephalitis to flaccid quadriplegia (poliomyelitis). Children under 15 years are most susceptible to developing encephalitis.2, 58 In JE the virus enters the brain likely through a Trojan horse mechanism, causing extensive neuroinflammation and blood brain barrier disruption.58 Thalamus, basal ganglia and the brainstem are particularly affected, possibly due to JEV's affinity to dopaminergic cells.55, 59
Movement disorders, usually associated with brainstem signs, are observed in approximately 50–70% of patients with Japanese encephalitis.3, 4 These are seen early in the illness or develop concurrently as coma abates.3, 4, 60 Some patients have a biphasic clinical course, of initial encephalitis and recovery, followed by movement disorders, behavioral changes and brainstem signs after 2–3 weeks.4, 61 Painful dystonia, often generalized, is common. Oromandibular dystonia with mutism and dysphagia is a useful diagnostic clinical feature in children.4, 62, 63 Parkinsonism has been reported early in the illness and is usually self‐remitting. However, in some cases with severe nigral necrosis, it is persistent and sometimes responsive to levodopa (Video 1; Video 2).3, 4, 60, 62, 64
Video 1.
Movement disorders and brainstem signs in children with acute Japanese encephalitis.
Video 2.
Persistent and delayed‐onset movement disorders in children with Japanese encephalitis. Also shown are dopamine‐responsive parkinsonism and dystonia.
Movement disorders such as tremor, perioral dyskinesias, chorea, myoclonus, stereotypies, tics and catatonia have also been described. Brainstem signs are frequent and include pupillary changes, oculogyric crisis, opsoclonus, oculomotor paresis, decerebrate posturing, cardiac arrhythmias and disordered breathing patterns. Cognitive problems, hypothalamic dysfunction, pyramidal signs and anterior horn cell degeneration have been reported (Video 1; Video 2).3, 4, 57, 60, 61, 62, 63
Dengue
Dengue is the most rapidly expanding communicable disease with a 400% increase between 2000 and 2013. It is caused by the dengue virus and transmitted by the Aedes mosquito. Approximately 400 million people are afflicted every year, 75% of whom are in Asia.65 Three billion people residing in South Asia and other tropical and subtropical regions, where the Aedes mosquito is common, are at risk of acquiring the infection.5, 65
Dengue virus infection is asymptomatic in 75% of cases.5 Among those with symptoms, the clinical spectrum ranges from a self‐remitting febrile illness, often with a rash, to a potentially fatal dengue shock syndrome. Dengue virus leads to a multisystemic disease but rarely infects the brain. Neurological complications are reported in 0.5–20% of cases.6, 66 They include encephalopathy due to multiorgan failure, brain hemorrhage due to coagulopathy, and postinfection neurological syndromes.5, 6, 58, 66
Movement disorders are rare but postinfection (immune mediated) disorders, such as opsoclonus‐myoclonus‐ataxia syndrome, parkinsonism, dystonia and tremor have been reported (Video 3).6, 7, 8, 9, 10 Recently a rapidly progressive dementia with PSP‐like phenocopy was reported in a patient with chronic dengue encephalitis.67
Video 3.
Movement disorders in patients with dengue.
Zika
Zika is a viral disease transmitted by the Aedes aegypti mosquito, person‐to‐person contact (infected mother to fetus and through sexual intercourse) or via blood transfusion. The Zika virus was originally described in rhesus monkeys in Uganda in 1947, and later was serologically detected among humans in Africa and Asia. Significant Zika outbreaks with febrile illness and high infection rates were seen in the Pacific Islands in 2007–16; cases of postinfection Guillain‐Barre syndrome were recorded. In 2015–17, a mutated Zika virus emerged and caused a pandemic in South America, the Caribbean and Africa. In addition to febrile illness, it now led to microcephaly in infants born to infected mothers.68, 69 Infection clusters have been detected in India and Southeast Asia recently, and Zika is an emerging threat in all Aedes‐infested areas.68, 69, 70, 71
A majority of patients with Zika infection are asymptomatic (50–80%) while others experience a self‐remitting febrile illness; case fatality rate is <0.01%.69 However, Zika virus is highly neurotropic in fetal brains where it destroys the neural progenitor cells. Zika infection during pregnancy results in intrauterine death, congenital Zika syndrome, or neurodevelopmental impairments in 20–30% of fetuses and neonates.68, 69
Congenital Zika syndrome is characterized by severe microcephaly, complex brain malformations, cortical–subcortical and basal ganglia calcifications, ophthalmic abnormalities, contractures and intrauterine growth restriction. Movement disorders (dystonia, chorea) have been reported in 40 to 95% of these infants.12, 13, 72, 73, 74 They include dystonic postures of fingers (eg, “one‐two‐five” and “swan neck”) and toes, extensor axial hypertonia, internal rotation of the shoulder, and dyskinesia of mouth and tongue (Video 4).12, 13 Also described are retained primitive reflexes and persistence of patterned movements of arms, legs and tongue that are normally seen only in fetal and early postnatal period.12, 74
Video 4.
Movement disorders in patients with congenital Zika syndrome.
West Nile Virus
West Nile virus is a zoonotic virus transmitted by Culex mosquitoes. First identified in Uganda in 1937, the virus is now endemic in North America, parts of Europe, Russia, Venezuela and Australia. Over the last two decades, small outbreaks have been identified in India and it is an emerging threat in South Asia.14, 75
West Nile virus infection is asymptomatic in 75% of patients and causes a brief febrile illness in most of the others. Only 1% of patients, particularly those above 50 years of age and those who are immunocompromised, develop a neuroinvasive disease.15 The virus enters the brain possibly using the Trojan horse, transcellular, paracellular and axonal transport routes.50 Clinically, West Nile virus related neuroinfection manifests with meningitis, encephalitis or acute flaccid paralysis (poliomyelitis).15 Mortality of up to 18% has been reported.16
Movement disorders are common in patients with encephalitis. Tremor is reported in as much as 80–100% of patients. It is usually coarse, postural, asymmetric and seen in the upper limbs.16 Parkinsonism has been observed in two‐thirds and myoclonus (usually involving the face and upper limbs) in one‐third of patients. Cognitive problems, cerebellar signs, cranial nerve palsies, and, rarely, chorea have also been described.15, 16, 75 Approximately 40–80% of patients with West Nile virus infection, particularly those with neuroinvasive syndromes, experience symptoms that persist for months and years after the initial illness.15, 16, 76 Residual symptoms include tremor, parkinsonism, impaired concentration, insomnia, fatigue. Postinfection opsoclonus‐myoclonus, ataxia, and Guillain Barré syndrome have been reported.15, 16
Chikungunya
Chikungunya is an emerging viral zoonotic disease that is spread by the Aedes aegypti mosquito. First identified in humans in Tanzania in 1952, it subsequently caused epidemics in Africa, South and Southeast Asia, and, more recently, the Americas.17 Co‐infections with other Aedes‐borne arboviruses, Zika and dengue, have been reported.18
In most instances, the chikungunya virus leads to self‐remitting fever. Neurological complications are reported in 5–10% of patients, and include encephalitis, myelopathy and neuropathies. In a systematic review of patients with chikungunya encephalitis, 50% showed good recovery, 20% had residual neurological deficits and 30% died.18
Movement disorders, however, are uncommon. Tremor, dystonia, opsoclonus, stimulus‐sensitive myoclonus, oromandibular and limb dyskinesia, parkinsonism, behavioral changes, cerebellar ataxia and worsening of pre‐existing neurological disability have been reported during the acute phase of the disease, or a few weeks or months later.18, 77
Other RNA Viruses
Measles
Measles (rubeola) is a highly contagious viral disease that spreads through respiratory droplets and small‐particle aerosols. Before the introduction of widespread vaccination in 1963, it resulted in over 2.6 million deaths every year.78 In 2018 there were 140,000 measles‐related deaths.19 Most of these were in children under 5 years in sub‐Saharan Africa and in South and Southeast Asia.11, 19, 78
Classically, measles leads to a self‐remitting fever with rash. Usually the measles virus is efficiently cleared by the immune system after the rash and patients develop lifelong immunity to reinfection. However, if measles infection is acquired at a young age, there is risk of persistence of the virus and subsequent development of fatal subacute sclerosing panencephalitis (SSPE) characterized by widespread encephalitis, neurodegeneration and high anti‐measles antibody response.45, 79, 80 This happens in 1:10,000 people infected with measles, 6–10 years (range: 2 to 30 years) after the primary infection. Measles infection at a younger age (<5 years) is associated with increased risk of SSPE and a shorter latency period.45, 80
Patients usually present with SSPE in childhood though adult‐onset disease has also been reported.81 Early symptoms are innocuous and include drop in scholastic performance, irritability, behavioral changes, vision problems and subtle myoclonus. Overt myoclonus and other movement disorders develop later in the illness, followed by progressive encephalopathy, coma and death. Average survival is 4 years from disease onset though instances of longer‐term survival with natural remissions are reported in 10% of patients.20, 80 Fulminant course, leading to death within a few months, has been reported in up to 10% of patients. This is often associated with measles infection at an early age (<2 years), concurrent HIV, or other conditions that compromise the immune system (Video 5; Video 6).80, 82
Video 5.
Movement disorders in patients with subacute sclerosing panencephalitis (SSPE).
Video 6.
Clinical course of fulminant SSPE. Movement disorders in a child with measles inclusion body encephalitis (MIBE).
Myoclonic jerks (positive and/or negative) are seen in almost all patients with SSPE. They manifest as clumsiness, head drops, near‐falls and falls. The frequency and intensity of the myoclonus increase with excitement and disease progression.20, 80, 81 Slow myoclonus and characteristic periodic complexes on EEG are diagnostic markers for SSPE.52 Other movement disorders, such as tremors, dystonia, generalized choreoathetosis, parkinsonism, Pisa syndrome, and acute neuroleptic malignant syndrome, are often seen in mid‐to‐late stages of the disease though they can sometimes be the presenting feature.52, 80, 81, 83, 84 In the terminal stage, myoclonic jerks abate and most patients develop mutism and an akinetic rigid syndrome (Video 5; Video 6).80, 85
Brainstem signs, cognitive and behavioral problems include periodic catatonia, stereotypic repetitive behaviors (hand clapping, pelvic thrusting, foot‐tapping, muttering), self‐mutilation, obsessive cleanliness, and visual and auditory hallucinations.86, 87
Recently, measles inclusion‐body encephalitis (MIBE) has been identified as a rare fatal complication of measles infection. Fewer than 100 cases have been reported mainly among people with congenital immunodeficiency syndromes, HIV, hematological malignancies or among transplant recipients.21, 79 Akin to SSPE, MIBE is associated with persistence of the measles virus in the brain. However, unlike SSPE, MIBE develops within weeks of the measles infection, is associated with minimal brain inflammation, and does not raise measles antibodies in CSF and serum.79 MIBE manifests with intractable seizures, movement disorders, focal neurological signs and ultimately leads to coma and death. Myoclonus, tremor, chorea, dystonia and akathisia have been reported (Video 6, segment 2).21, 22, 79
Nipah
Nipah is a viral zoonotic disease that is emergent in South Asia. Outbreaks of Nipah encephalitis have been reported in Malaysia, Singapore, Bangladesh, India and the Philippines. Humans are infected by consuming food contaminated with secretions of fruit bat, the natural host for the virus; through close and prolonged contact with infected pigs; or, through person‐to‐person transmission. The average case fatality rate for Nipah has been estimated to be 60% though with wide variation across outbreaks.23, 88
Most patients infected with the Nipah virus develop encephalitis with prominent brainstem and spinal cord dysfunction characterized by segmental myoclonus or rhythmic jerking of muscles (reported in 30% of cases), autonomic failure, oculomotor signs and flaccid areflexic quadriplegia.24 Most survivors report protracted fatigue, cognitive problems, cranial nerve palsies, ataxia, focal weakness, myoclonus, cervical dystonia and bradykinesia. New‐onset cervical dystonia, oculomotor dysfunction, cerebellar signs, and myoclonus have been observed months after the acute illness.24, 89
Rabies
Rabies is a viral disease transmitted to humans through a bite of an infected animal. It is endemic in Asia and Africa where the main animal reservoir is dogs. Elsewhere, canine rabies has been well controlled and the diseases are acquired only infrequently, primarily through bites of wild animals, such as bats, foxes, and racoons.25, 90 Rabies leads to over 59,000 deaths annually.25, 91
Following an animal bite, the rabies virus spreads from the wound to the spinal cord and brain along neuroanatomical pathways.26 After a brief febrile prodrome, a majority of patients develop brainstem encephalitis (also called “encephalitic” or “furious rabies,” as opposed to “paralytic rabies” that results from spinal cord dysfunction). These patients exhibit a fluctuating sensorium with delirium alternating with lucid periods. Soon painful and distressing inspiratory spasms involving the oro‐pharyngeal, neck and respiratory muscles emerge. Initially, the spasms are triggered by blowing air (aerophobia), drinking water or even the sight of water (hydrophobia), but later occur spontaneously (Video 7). Autonomic failure, coma and death usually occur within days.26, 90, 92 The rare survivors are left with severe disability. Cerebellar signs, severe dystonia‐parkinsonism, generalized choreoathetosis, ballism and mutism have been reported among these (Video 7).93
Video 7.
Rabies‐related phobic inspiratory spasms. Movement disorders in a long‐term survivor of rabies.
Bat‐related rabies has been associated with chorea in the bitten limb during the prodrome, and focal brainstem signs, myoclonus, opsoclonus, tremors, hemichorea, hemiparesis and seizures during the later stages.90, 93
Coronaviruses
Within the last 20 years, three novel human coronaviruses have caused significant outbreaks:
The severe acute respiratory syndrome coronavirus (SARS‐CoV) that emerged in 2002–03, likely from the civet cat, and caused a near‐pandemic with about 8000 reported cases and 800 deaths.
The Middle East respiratory syndrome coronavirus (MERS‐CoV) that emerged in 2012 from dromedary camels, and led to about 2500 cases and 800 deaths; and
SARS‐CoV2 that emerged in 2019 and led to an ongoing global pandemic of COVID‐19 with about 200 million reported cases and over 4 million deaths by June 2021.1, 27, 94
All these coronaviruses typically lead to respiratory illness and spread from person‐to‐person mainly through droplets and aerosols. However, SARS‐CoV‐2, and to a certain extent MERS, can affect a variety of systems.1, 94 In particular, neurological symptoms develop in 20–80% of patients infected with SARS‐CoV‐2 and in isolated cases of SARS and MERS.95, 96
Movement disorders are uncommon and, to date, only in a few dozen cases have been reported in patients with COVID‐19. The most frequent being myoclonus, ataxia and opsoclonus due to systemic complications, structural lesions or as part of a postinfection syndrome (Video 8).28 Also reported are tremors, parkinsonism and hyperekplexia.28, 95, 97, 98 Given the recency of the COVID‐19 pandemic, the potential for delayed‐onset movement disorders remains but current evidence indicates that they are relatively rare.
Video 8.
Movement disorders in patients with COVID‐19.
DNA Viruses
Herpes Simplex Viruses
Herpes simplex virus (HSV) causes mucocutaneous infections, and less commonly CNS and visceral infections in humans. Infection is usually acquired in childhood through direct contact with lesions or fluids, or during birth through infected mothers. Once acquired, the virus persists lifelong in sensory ganglia. HSV is highly contagious and more than 90% of the adults have been infected by the fifth decade of life.99
The incidence of HSV encephalitis is 2–4 cases/million population/year and it is associated with 10–25% mortality.51, 99 In South Asia, it is one of the commonest acute encephalitis (following Japanese encephalitis).100 HSV encephalitis has a bimodal age distribution and usually affects children under 3 years and adults over 50 years of age.99 In 15–25% children and 7–15% adults there is neurological relapse within 3 months of HSV encephalitis.29 Though some of these relapses are due to HSV reinfection, a majority result from immune‐mediated encephalitis.29, 51, 99
There are only isolated reports of movement disorders or basal ganglia involvement in patients with HSV encephalitis. However, in patients with autoimmune encephalitis following HSV encephalitis, movement disorders are common and often the initial feature (Video 9, segment 1). Most of these patients develop chorea, choreoathetosis and ballism, usually with oro‐facial involvement. Myoclonus, dystonia, tremor, stereotypies, Kluver‐Bucy‐like syndrome, have also been described. Psychosis and behavioral problems are common in older children and adults.29, 51
Video 9.
Movement disorders related to herpes simplex and varicella zoster viruses.
Varicella Zoster Virus
Varicella zoster virus is a highly contagious virus spread through the respiratory route that causes chickenpox (varicella), a self‐remitting febrile illness with characteristic rash in children. It is seen worldwide, mainly in tropical and temperate regions, in nearly all people by late‐childhood to mid‐adulthood if they have not been vaccinated.101
After an initial viremia during varicella, the virus remains dormant in the dorsal root ganglia and on reactivation causes herpes zoster (also called shingles).102 Neurological symptoms are rare, and seen during primary infection or following viral reactivation.103 They include cerebellitis, aseptic meningitis, encephalitis, transverse myelitis, neuritis, and vasculopathy that commonly affects the small penetrating lenticulo‐striatal arteries.103, 104 Movement disorders such as hemichorea or hemidystonia due to contralateral basal ganglia infarcts (due to focal cerebral arteriopathy) have been described (Video 9, segment 2).30, 104
Human Retroviruses
HIV
Human immunodeficiency virus (HIV), causes acquired immunodeficiency syndrome (AIDS). The virus is transmitted primarily through sexual contact, and also via blood, blood products and from mother to infants (intrapartum, perinatally and through breast milk). Since the identification of the first patient with HIV infection in 1981, the global AIDS epidemic has affected about 75 million people and led to about 33 million deaths. As of 2019, 38 million people are living with AIDS of whom two‐thirds are in Africa and 12% in South and Southeast Asia.31
HIV infection is associated with a wide spectrum of neurological symptoms, which are seen in 20–60% of patients.32 Multiple mechanisms contribute to the neurological problems, including the HIV infection itself, opportunistic infections, neoplasms and treatment‐related adverse effects. In the CNS, the basal ganglia and the substantia nigra are particularly affected by HIV.33 Neuroleptic‐sensitivity is also seen.32, 33
Movement disorders are reported in 4–50% of patients infected with HIV, with the higher incidence observed in cohorts of patients with advanced AIDS.32 Movement disorders develop both early in the disease course, or later as a complication of opportunistic infections or HIV‐associated‐dementia (HADS; also called AIDS‐dementia‐complex or HIV encephalopathy).33, 105 Before the introduction of combination antiretroviral therapy (cART), the onset of movement disorders such as tremor, parkinsonism or myoclonus were poor prognostic signs.33 Tremor (postural or rest) and parkinsonism are the most common movement disorders and are almost invariably present in patients with late‐stages of HAD. Isolated tremor, rest or postural, can herald HAD. Also described are rubral tremors following tubercular or toxoplasma lesions in the midbrain.32, 33, 106 Other movement disorders including hemichorea, hemiballism, generalized chorea, dystonia, and myoclonus are rare and observed with structural lesions (including herpes zoster radiculitis causing segmental myoclonus) and HADS.32, 33 Dopamine receptor blocker induced tremor, dystonia and parkinsonism; trimethoprim‐sulfamethoxazole induced tremors; and, protease induced parkinsonism have been reported.33
Bacterial Infections
Rheumatic Chorea
Acute rheumatic fever is an immune‐mediated multisystemic disease caused by group A streptococcus bacteria. It usually affects children aged 5–15 years. Globally, 3–6% of the population is susceptible to acute rheumatic fever, 95% of whom reside in sub‐Saharan Africa, Pacific nations, Australasia, and Central and South Asia.107, 108
Rheumatic or Sydenham chorea develops in 10–30% of patients with rheumatic fever, 6 weeks to 6 months after the acute group A streptococcal pharyngitis.34 It is the commonest cause of chorea in the endemic regions. The chorea is of jerky or tic‐tac character; may be unilateral or generalized; and, is often associated with emotional lability and obsessive–compulsive behaviors.34, 109 Dysarthria (due to lingual and oromandibular chorea) and imbalance are common. Ballism, tics, dystonia, other movement disorders and executive dysfunction may also occur (Video 10).109 Symptoms usually remit over few weeks to 6 months. Recurrences are uncommon.34, 109
Video 10.
Phenomenology of chorea in patients with rheumatic chorea.
Tetanus
Tetanus is caused by the bacteria Clostridium tetani. It is vaccine‐preventable but due to under‐vaccination approximately 75,000 people develop tetanus and 35,000 deaths are reported annually, primarily in low‐ to middle‐income countries.11
Tetanus spores enter the body through wounds or deep abrasions, germinate and release the tetanus toxin, which is a potent neurotoxin. The toxin blocks presynaptic inhibition of the motor neurons in the spinal cord and brainstem leading to skeletal muscle spasms, rigidity and autonomic dysfunction.35
Cranial muscle involvement in tetanus results in trismus (lock jaw), or laryngeal spasms (affected neonates may experience difficulty in sucking milk).35 Painful axial muscle spasms leading to opisthotonus posturing and abdominal wall rigidity are typical (Video 11). Laryngeal spasms or cardiovascular instability (due to autonomic failure) can be fatal.35, 110
Video 11.
Tetanus‐related lockjaw and muscle spasms. And demonstration of spatula test.
Tuberculosis
Tuberculosis is a respiratory disease caused (most commonly) by the bacteria Mycobacterium tuberculosis and the foremost cause of death among infectious diseases. About one‐fourth of the world population is thought to be infected, of which 5–10% develop active disease. South Asia accounts for 40% of the cases. In 2019 there were 10 million new cases and 1.4 million deaths attributable to tuberculosis (these have reduced by 9% and 14% respectively since 2015).36
Tuberculosis is spread through droplet infection. The bacteria seeds the lung during the primary infection. Spread to CNS is hematogenous and occurs in 1–5% of the infected patients. The risk is higher in children under five (particularly in the endemic areas), those with HIV, and those who are otherwise immunocompromised. Clinically, CNS tuberculosis presents most frequently with meningitis (often complicated by cranial nerve palsies, vasculitis, and hydrocephalous) and space‐occupying lesions (tuberculomas and tubercular abscesses); and, less commonly, as a spinal disorder. Untreated CNS tuberculosis is fatal.111
Movement disorders are reported in 0.5–20% of patients with CNS tuberculosis, either early or later in the course of the disease.37, 112 A wide spectrum of movement disorders including chorea, ballism, tremor, dystonia (including status dystonicus), parkinsonism, opsoclonus‐myoclonus, bobble‐head syndrome, cerebellar ataxia and behavioral problems have been described (Video 12).37, 112, 113, 114, 115
Video 12.
Movement disorders and neurological signs due to CNS tuberculosis.
Syphilis
Syphilis is a sexually‐transmitted disease that can also be transferred from mother to child. It is caused by the bacteria Treponema pallidum. Globally, there are about 14 million new infections every year, mainly in sub‐Saharan Africa, and South East Asia and South America. The incidence of syphilis decreased dramatically after the introduction of penicillin in the 1940s; however, the number of cases has been increasing since 2000.11
Syphilis is a chronic multisystemic disease, characterized by periods of activity interspersed with periods of latency lasting months or years. CNS invasion generally occurs in the first few weeks of infection. Neurosyphilis is more common among patients with HIV coinfection.
Movement disorders are rare. Most reports predate penicillin and two‐thirds of the cases reported are in men. Over half had associated Argyll Robertson pupils (bilateral small pupils that constrict when focusing on a near object but do not constrict when exposed to light).38 Orolingual dyskinesias were frequent and of varied phenomenology. One such dyskinesia, the “Candy sign” that resembles the natural movement of sucking a candy, is considered diagnostic of syphilis. Tremor, chorea, athetosis, ballism, myoclonus (cortical and/or subcortical), dystonia, tremulous parkinsonism, PSP‐like and CBS‐like phenocopies, and cerebellar ataxia have been reported.38, 116
Parasitic Infections
Malaria
Malaria is the most important human protozoal infection. It is caused by the protozoa Plasmodium (particularly, P. vivax and P. falciparum) and transmitted by Anopheles mosquitoes. Malaria is endemic in tropical and subtropical regions, with half the world population at risk. In 2019 there were 230 million cases and 410,000 deaths attributed to the disease. Two‐thirds of the cases and deaths were in children under 5 years. Cases in South Asia reduced by 70% between 2000 and 2019, and now account for only 3% of the worldwide incidence. The largest absolute reduction was in India, while Sri Lanka was declared malaria‐free in 2015.39
Malaria is often clinically indistinguishable from other febrile illnesses. Cerebral malaria is a complication of severe falciparum malaria that manifests with diffuse encephalopathy, generally without focal neurological signs.
Movement disorders are rare in malaria. Decorticate, decerebrate and opisthotonus posturing related to increased intracranial pressure have been described in children with cerebral malaria and are associated with high mortality.117 Psychosis, rigidity, opsoclonus‐myoclonus, cerebellar ataxia, and brainstem signs have also been reported during the acute illness and can persist.40, 118, 119 Movement disorders may also occur as part of an immune‐mediated post‐malaria neurological syndrome, a self‐remitting illness reported after recovery from severe cerebral malaria in around 1:1000 of survivors. Features of the neurological syndrome include rigidity, tremors, myoclonus, choreoathetosis, catatonia, cerebellar dysfunction (mainly midline), visual or auditory hallucinations, psychosis, hemiparesis and seizures (Video 13, segment 1).119, 120
Video 13.
Movement disorders related to parasitic infections: malaria, toxoplasmosis, and neurocysticercosis.
Toxoplasmosis
Toxoplasmosis is a food borne disease caused by the protozoa Toxoplasma gondii. One‐third of the world population is estimated to be infected, mainly by consumption of undercooked meat or shellfish, or food contaminated with cat feces.121 Infection can also spread from mother to fetus, which is seen in 1:1000–10,000 live births.122
Coinfection with HIV, or other immunocompromised states, can result in reactivation of the latent toxoplasma infection and lead to toxoplasma encephalitis (TE). TE is the most common opportunistic infection‐related disorder among patients with HIV. It developed in 30–40% of these patients before the introduction of cART; the risk has halved since then.123 In patients without HIV, TE is rare.121
TE in patients with HIV leads to focal or multifocal abscesses, primarily in the brain stem and basal ganglia. Clinical features include fever, headache, altered sensorium, seizures, cranial nerve paresis, hemiparesis, cerebellar signs, psychosis, dementia and, in some, unilateral movement disorders.121 Chorea, athetosis, ballism are the commonest movement disorders. Tremor (rest, kinetic and rubral), rigidity, parkinsonism, dystonia, akasthisia, tics and facial dyskinesia have also been reported (Video 13, segments 2, 3). These usually remit with treatment of TE.41, 42, 121, 124, 125 In patients without HIV, TE usually presents with diffuse meningoencephalitis. Movement disorders are rare in these patients.126
Taeniasis
Taenia solium is a cestode (tapeworm) for which humans are the definitive host and pigs, the intermediary host. The adult form is present in the intestines of infected humans. The larval forms (cysticerci) is usually found in pigs but can also infect various tissues in humans and in other animals (cysticercosis). It is estimated that neurocysticercosis (cysticerci infection of the CNS) affects about 2.5–8 million people worldwide. The disease is highly prevalent in Asia, Africa and South America.43
Cysticerci can be present in the brain, spinal cord, ventricles and meninges. They can also be present within skeletal muscles, where they may be palpable. Disseminated neurocysticerci, described as “Van Gogh's starry sky” on brain scans, may be asymptomatic or manifest as uncontrolled seizures, dementia, and signs of raised intracranial pressure.127 Cysticerci are often present in basal ganglia but because of their small size, chronicity, slow growth and the limited inflammatory response to intact cysts, movement disorders are rare.128 There are only isolated reports of movement disorders such as parkinsonism, dystonia, tremor, PSP‐like syndrome, hemichorea, hemiballism, myoclonus, lingual dyskinesia or hemifacial spasm (Video 13, segment 4).44, 128, 129, 130, 131, 132
Conclusion
This article has focused on infectious diseases in South Asia. Such regional focus is useful since, as discussed earlier, the set of prevalent pathogens and diseases can differ widely by population and geography. Furthermore, even the constellation of symptoms, including movement disorders, can differ between regions and outbreaks. This was the case, for example, with Zika, where the recent outbreaks in South America were highly neurotropic in neonates and associated with congenital Zika syndrome, which earlier outbreaks in Asia, Africa and the Pacific Islands were not known to be. The mortality rate of an infectious disease can also differ significantly between regions, as has been observed with Nipah and, arguably, COVID‐19. Factors that may explain such regional differences in disease syndrome and mortality are not well understood but may include genetic variations of the pathogen; differences in the age, demographics, comorbidities, vaccination and immunological status, etc. of the population; along with various environmental, societal, economic and health‐system differences.
Knowledge of the locally prevalent infectious diseases and the associated neurological syndrome can help a movement disorder specialist correctly diagnose and treat a patient. Some of the infection‐related movement disorders can mimic those induced by strokes, drugs, neurodegeneration or autoimmune disorders and, therefore, looking at the complete set of symptoms, test‐results, and patient‐history is crucial to differential diagnosis. Since the onset of the movement disorders can be significantly delayed from the time of exposure to the infectious pathogen (for example, SSPE may develop decades after a measles infection), the original infection may be discovered only by directed inquiry and testing motivated, in part, by the regional epidemiology. Finally, it is sometimes necessary to eliminate infection as a possibility cause before settling on an alternate diagnosis.
While region‐based considerations can be useful, it is important not to regard them too rigidly. Pathogens, their hosts and carriers, and hence infectious diseases are constantly crossing national and regional borders. And, as has been amply illustrated through recent outbreaks of chikungunya, Ebola, and various influenza and coronavirus pandemics, new pathogens are constantly emerging and can spread globally within months. Population growth, globalization and climate change are expected to increase human and animal migration and interaction, and thus hasten the emergence of new infectious diseases. Along with faster vaccine and treatment development and deployment, pathogen testing and genomic surveillance, and other therapeutic and public‐health measures, improved and interdisciplinary clinical knowledge, practices and communication can help us mitigate these risks.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.A.: 1A, B, C; 3A, B
S.A.: 1C; 3B
M.B.: 1A, B, C; 3B
Disclosures
Ethical Compliance Statement
ICH GCP guidelines were followed and patients were managed as per standard‐of‐care and Institutional guidelines. Informed patient consent was obtained at time of recording the patient video and/or photographs. All authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interests
No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Supporting information
Supplementary Material S1. Detailed description of Figure 2.
Supplementary Material S2. Transcript for video narration.
Acknowledgments
The authors acknowledge and thank their colleagues for sharing their experience and patient videos for this article: Prof. Ravindra Kumar Garg, King George's Medical University, Lucknow, India (Video 1, segments 1–3; and, Video 3, segments 1 and 2); Prof. Orlando Barsottini, Federal University of São Paulo, Brazil; Prof. Marcelo Masruha, Escola Paulista de Medicina, Brazil and Dr. Mariana Krueger, Universidade de Fortaleza, Brazil (Video 4); Prof. Anaita Udwadia‐Hegde, Jaslok Hospital and Research Centre, Mumbai, India (Video 5, segments 1 and 5; Video 10, segment 1; and, Video 12, segments 1 and 4); Prof. Bhim Sen Singhal, Bombay Hospital Institute of Medical Sciences, Mumbai, India (Video 5, segment 4); Prof. Charulata Sankhla, P D Hinduja Hospital & Medical Research Centre, Mumbai, India (Video 6, segment 1); Dr. Pradnya Gadgil, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, India (Video 6, segment 2); Dr. Dhananjay Duberkar, Sahyadri Specialty Hospital, Nashik, India (Video 7, segment 2); Dr. Shrikant Deshmukh, Medicover Hospital, Aurangabad, India (Video 11, segment 1); Prof. Marcelo Merello, Raúl Carrea Institute for Neurological Research, Buenos Aires, Argentina (Video 13, segment 1); Dr. Anand Diwan, Narayani Hospital, Nashik, India (Video 13, segment 2).
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Annu Aggarwal, Email: annu.aggarwal@gmail.com.
Mohit Bhatt, Email: drmbhatt@gmail.com.
References
- 1.Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID‐19. Cell 2020;182(5):1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Japanese encephalitis 2019. https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis.
- 3.Sarkari NB, Thacker AK, Barthwal SP, et al. Japanese encephalitis (JE). Part I: clinical profile of 1,282 adult acute cases of four epidemics. J Neurol 2012;259(1):47–57. [DOI] [PubMed] [Google Scholar]
- 4.Misra UK, Kalita J. Spectrum of movement disorders in encephalitis. J Neurol 2010;257(12):2052–2058. [DOI] [PubMed] [Google Scholar]
- 5.WHO . Dengue and severe dengue fact sheet 2020. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 6.Prabhat N, Ray S, Chakravarty K, et al. Atypical neurological manifestations of dengue fever: a case series and mini review. Postgrad Med J 2020;96(1142):759–65. [DOI] [PubMed] [Google Scholar]
- 7.Fong CY, Hlaing CS, Tay CG, Ong LC. Post‐dengue encephalopathy and parkinsonism. Pediatr Infect Dis J 2014;33(10):1092–1094. [DOI] [PubMed] [Google Scholar]
- 8.Bopeththa B, Ralapanawa U. Post encephalitic parkinsonism following dengue viral infection. BMC Res Notes 2017;10(1):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.do Rosário MS, Giovanetti M, de PAP J, et al. Opsoclonus‐myoclonus‐ataxia syndrome associated with chikungunya and dengue virus co‐infection. Int J Infect Dis 2018;75:11–14. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A, Pandey S. Generalized dystonia/parkinsonism and double‐doughnut sign in dengue encephalitis. Mov Disord Clin Pract 2020;7(5):585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira H, Dos Santos SP, Amancio A, et al. Neurological outcomes of congenital Zika syndrome in toddlers and preschoolers: a case series. Lancet Child Adolesc Health 2020;4(5):378–387. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden H, Silveira‐Moriyama L, van der Linden V, et al. Movement disorders in children with congenital Zika virus syndrome. Brain Dev 2020;42(10):720–729. [DOI] [PubMed] [Google Scholar]
- 14.CDC . West Nile virus: cumulative maps and data for 1999–2019 2020. https://www.cdc.gov/westnile/statsmaps/cumMapsData.html#two.
- 15.Lenka A, Kamat A, Mittal SO. Spectrum of movement disorders in patients with neuroinvasive west Nile virus infection. Mov Disord Clin Pract. 2019;6(6):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, et al. West Nile virus neuroinvasive disease. Ann Neurol 2006;60(3):286–300. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Chikungunya fact sheet 2020. https://www.who.int/news-room/fact-sheets/detail/chikungunya.
- 18.Mehta R, Gerardin P, de Brito CAA, Soares CN, Ferreira MLB, Solomon T. The neurological complications of chikungunya virus: a systematic review. Rev Med Virol 2018;28(3):e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel M, Dumolard L, Nedelec Y, et al. Progress toward regional measles elimination‐worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep 2019;68(48):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prashanth LK, Taly AB, Ravi V, Sinha S, Rao S. Long term survival in subacute sclerosing panencephalitis: an enigma. Brain Dev 2006;28(7):447–452. [DOI] [PubMed] [Google Scholar]
- 21.Baldolli A, Dargere S, Cardineau E, et al. Measles inclusion‐body encephalitis (MIBE) in a immunocompromised patient. J Clin Virol 2016;81:43–46. [DOI] [PubMed] [Google Scholar]
- 22.Aldecoa I, Archilla I, Herrero L, et al. Measles inclusion body encephalitis. Clin Neuropathol 2020;39(4):148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma V, Kaushik S, Kumar R, Yadav JP, Kaushik S. Emerging trends of Nipah virus: a review. Rev Med Virol 2019;29(1):e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh KJ, Tan CT, Chew NK, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med 2000;342(17):1229–1235. [DOI] [PubMed] [Google Scholar]
- 25.WHO . United against rabies collaboration: first annual report 2019. https://rabiesalliance.org/resource/first-annual-progress-report-global-strategic-plan-end-human-deaths-dog-mediated-rabies.
- 26.Hemachudha T, Ugolini G, Wacharapluesadee S, et al. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol 2013;12(5):498–513. [DOI] [PubMed] [Google Scholar]
- 27.Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis 2020;20(5):533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JL, Murphy KA, Sarna JR. Myoclonus and cerebellar ataxia associated with COVID‐19: a case report and systematic review. J Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nosadini M, Mohammad SS, Corazza F, et al. Herpes simplex virus‐induced anti‐N‐methyl‐d‐aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Dev Med Child Neurol 2017;59(8):796–805. [DOI] [PubMed] [Google Scholar]
- 30.Bulder MM, ten Houten R, Klijn CJ, Braun KP. Unilateral movement disorder as a presenting sign of paediatric post‐varicella angiopathy. BMJ Case Rep 2013;2013:bcr2013009437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNAIDS . Global HIV & AIDS statistics‐2020 fact sheet 2020. https://www.unaids.org/en/resources/fact-sheet.
- 32.Carroll E, Sanchez‐Ramos J. Hyperkinetic movement disorders associated with HIV and other viral infections. Handb Clin Neurol 2011;100:323–334. [DOI] [PubMed] [Google Scholar]
- 33.Tse W, Cersosimo MG, Gracies JM, Morgello S, Olanow CW, Koller W. Movement disorders and AIDS: a review. Parkinsonism Relat Disord 2004;10(6):323–334. [DOI] [PubMed] [Google Scholar]
- 34.Tumas V, Caldas CT, Santos AC, Nobre A, Fernandes RM. Sydenham's chorea: clinical observations from a Brazilian movement disorder clinic. Parkinsonism Relat Disord 2007;13(5):276–283. [DOI] [PubMed] [Google Scholar]
- 35.Yen LM, Thwaites CL. Tetanus. Lancet 2019;393(10181):1657–1668. [DOI] [PubMed] [Google Scholar]
- 36.WHO . Global tuberculosis report 2020. 2020. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020.
- 37.Alarcon F, Duenas G, Cevallos N, Lees AJ. Movement disorders in 30 patients with tuberculous meningitis. Mov Disord 2000;15(3):561–569. [DOI] [PubMed] [Google Scholar]
- 38.Pitton Rissardo J, Fornari CA. Neurosyphilis‐associated movement disorder: a literature review. Ann Mov Disord 2020;3(3):129–144. [Google Scholar]
- 39.WHO . WHO world malaria report 2020. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/9789240015791-eng.pdf?sfvrsn=d7a8ec53_3&download=true.
- 40.Kochar DK, Kumawat BL, Kochar SK, et al. Cerebral malaria in Indian adults: a prospective study of 441 patients from Bikaner, north‐west India. J Assoc Physicians India 2002;50:234–241. [PubMed] [Google Scholar]
- 41.Micheli F, Granana N, Scorticati MC, Giannaula RJ, Reboredo G. Unilateral postural and action tremor resulting from thalamic toxoplasmosis in a patient with acquired immunodeficiency syndrome. Mov Disord 1997;12(6):1096–1098. [DOI] [PubMed] [Google Scholar]
- 42.Carrazana E, Rossitch E Jr, Martinez J. Unilateral "akathisia" in a patient with AIDS and a toxoplasmosis subthalamic abscess. Neurology 1989;39(3):449–450. [DOI] [PubMed] [Google Scholar]
- 43.WHO . Taeniasis/cysticercosis fact sheet 2020. https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis.
- 44.Alarcon F, Cedeno Y, de Yebenes JG. Parkinsonism and other movement disorders in 23 cases of neurocysticercosis. Parkinsonism Relat Disord 2017;42:47–53. [DOI] [PubMed] [Google Scholar]
- 45.Wendorf KA, Winter K, Zipprich J, et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clin Infect Dis 2017;65(2):226–232. [DOI] [PubMed] [Google Scholar]
- 46.Netravathi M, Pal PK, Indira DB. A clinical profile of 103 patients with secondary movement disorders: correlation of etiology with phenomenology. Eur J Neurol 2012;19(2):226–233. [DOI] [PubMed] [Google Scholar]
- 47.Del Brutto VJ, Tettamanti D, Del Brutto OH. Changing profile of 7,519 neurologic outpatients evaluated over 20 years. Eur Neurol 2012;68(6):381–390. [DOI] [PubMed] [Google Scholar]
- 48.Scott BL, Jankovic J. Delayed‐onset progressive movement disorders after static brain lesions. Neurology 1996;46(1):68–74. [DOI] [PubMed] [Google Scholar]
- 49.Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?–the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112(9):747–751. [DOI] [PubMed] [Google Scholar]
- 50.Cain MD, Salimi H, Diamond MS, Klein RS. Mechanisms of pathogen invasion into the central nervous system. Neuron 2019;103(5):771–783. [DOI] [PubMed] [Google Scholar]
- 51.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018;17(9):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson MC. Neurological complications of measles (Rubeola). Curr Neurol Neurosci Rep 2020;20(2):2. [DOI] [PubMed] [Google Scholar]
- 53.Bally JF, Méneret A, Roze E, Anderson M, Grabli D, Lang AE. Systematic review of movement disorders and oculomotor abnormalities in Whipple's disease. Mov Disord 2018;33(11):1700–1711. [DOI] [PubMed] [Google Scholar]
- 54.Singh R, Khurana D, Mehta S, Choudhary A, Petluri G, Lal V. Cerebellar ataxia due to Leptospirosis‐a case report. BMC Infect Dis 2016;16(1):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laureti M, Narayanan D, Rodriguez‐Andres J, Fazakerley JK, Kedzierski L. Flavivirus receptors: diversity, identity, and cell entry. Front Immunol 2018;9:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lord JS, Gurley ES, Pulliam JR. Rethinking Japanese encephalitis virus transmission: a framework for implicating host and vector species. PLoS Negl Trop Dis 2015;9(12):e0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar K, Arshad SS, Selvarajah GT, et al. Japanese encephalitis in Malaysia: an overview and timeline. Acta Trop 2018;185:219–229. [DOI] [PubMed] [Google Scholar]
- 58.Filgueira L, Lannes N. Review of Emerging Japanese Encephalitis Virus: new Aspects and Concepts about Entry into the Brain and Inter‐Cellular Spreading. Pathogens 2019;8(3):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar S, Kalita J, Saxena V, et al. Some observations on the tropism of Japanese encephalitis virus in rat brain. Brain Res 2009;1268:135–141. [DOI] [PubMed] [Google Scholar]
- 60.Pradhan S, Pandey N, Shashank S, Gupta RK, Mathur A. Parkinsonism due to predominant involvement of substantia nigra in Japanese encephalitis. Neurology 1999;53(8):1781–1786. [DOI] [PubMed] [Google Scholar]
- 61.Sarkari NB, Thacker AK, Barthwal SP, et al. Japanese encephalitis (JE) part II: 14 years' follow‐up of survivors. J Neurol 2012;259(1):58–69. [DOI] [PubMed] [Google Scholar]
- 62.Basumatary LJ, Raja D, Bhuyan D, Das M, Goswami M, Kayal AK. Clinical and radiological spectrum of Japanese encephalitis. J Neurol Sci 2013;325(1–2):15–21. [DOI] [PubMed] [Google Scholar]
- 63.Rayamajhi A, Singh R, Prasad R, Khanal B, Singhi S. Clinico‐laboratory profile and outcome of Japanese encephalitis in Nepali children. Ann Trop Paediatr 2006;26(4):293–301. [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal A, Udani V, Shah S, Bhatt M. Postencephalitic bilateral nigral necrosis with motor complications. Mov Disord 2006;21(S15):S526. [Google Scholar]
- 65.Wilder‐Smith A, Ooi E‐E, Horstick O, Wills B. Dengue. Lancet 2019;393(10169):350–363. [DOI] [PubMed] [Google Scholar]
- 66.Carod‐Artal FJ. Neurological manifestations of dengue viral infection. Res Rep Trop Med 2014;5:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson TP, Larman HB, Lee MH, Whitehead SS, Kowalak J, Toro C, et al. Chronic dengue virus panencephalitis in a patient with progressive dementia with extrapyramidal features. Ann Neurol 2019;86(5):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masmejan S, Musso D, Vouga M, et al. Zika Virus. Pathogens 2020;9(11):898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Musso D, Ko AI, Baud D. Zika virus infection—after the pandemic. N Engl J Med 2019;381(15):1444–1457. [DOI] [PubMed] [Google Scholar]
- 70.Gupta N, Kodan P, Baruah K, Soneja M, Biswas A. Zika virus in India: past, present and future. QJM. 2019;23:hcz273. [DOI] [PubMed] [Google Scholar]
- 71.Saxena SK, Kumar S, Sharma R, Maurya VK, Dandu HR, Bhatt MLB. Zika virus disease in India—Update October 2018. Travel Med Infect Dis 2019;27:121–122. [DOI] [PubMed] [Google Scholar]
- 72.Santos P, Nogueira F, Modenezi L, et al. Movement disorder development later after acute disseminated encephalomyelitis associated with zika virus: case report. Neurology 2017;88:P2.325. [Google Scholar]
- 73.Vhp L, Aragao MM, Pinho RS, et al. Congenital Zika virus infection: a review with emphasis on the spectrum of brain abnormalities. Curr Neurol Neurosci Rep. 2020;20(11):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Einspieler C, Utsch F, Brasil P, et al. Association of infants exposed to prenatal zika virus infection with their clinical, neurologic, and developmental status evaluated via the general movement assessment tool. JAMA Netw Open 2019;2(1):e187235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.David S, Abraham AM. Epidemiological and clinical aspects on West Nile virus, a globally emerging pathogen. Infect Dis (Lond) 2016;48(8):571–586. [DOI] [PubMed] [Google Scholar]
- 76.Murray KO, Garcia MN, Rahbar MH, et al. Survival analysis, long‐term outcomes, and percentage of recovery up to 8 years post‐infection among the Houston West Nile virus cohort. PLoS One 2014;9(7):e102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simon F, Barnay JL, Lannuzel A. The wide spectrum of neurological consequences of chikungunya disease. Rev Med Virol 2018;28(6):e1999. [DOI] [PubMed] [Google Scholar]
- 78.WHO . Measles fact sheet 2019. https://www.who.int/news-room/fact-sheets/detail/measles.
- 79.Griffin DE. Measles virus persistence and its consequences. Curr Opin Virol 2020;41:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mekki M, Eley B, Hardie D, Wilmshurst JM. Subacute sclerosing panencephalitis: clinical phenotype, epidemiology, and preventive interventions. Dev Med Child Neurol 2019;61(10):1139–1144. [DOI] [PubMed] [Google Scholar]
- 81.Prashanth LK, Taly AB, Ravi V, Sinha S, Arunodaya GR. Adult onset subacute sclerosing panencephalitis: clinical profile of 39 patients from a tertiary care centre. J Neurol Neurosurg Psychiatry 2006;77(5):630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sivadasan A, Alexander M, Patil AK, Balagopal K, Azad ZR. Fulminant subacute sclerosing panencephalitis in an individual with a perinatally acquired human immunodeficiency virus infection. Arch Neurol 2012;69(12):1644–1647. [DOI] [PubMed] [Google Scholar]
- 83.Pandey S, Tomar LR, Tater P. Pisa syndrome in a child with subacute sclerosing panencephalitis. JAMA Neurol 2018;75(2):255–256. [DOI] [PubMed] [Google Scholar]
- 84.Garg D, Reddy V, Singh RK, Dash D, Bhatia R, Tripathi M. Neuroleptic malignant syndrome as a presenting feature of subacute sclerosing panencephalitis. J Neurovirol 2018;24(1):128–131. [DOI] [PubMed] [Google Scholar]
- 85.Risk WS, Haddad FS. The variable natural history of subacute sclerosing panencephalitis: a study of 118 cases from the Middle East. Arch Neurol 1979;36(10):610–614. [DOI] [PubMed] [Google Scholar]
- 86.Sutar R, Rai NK. Revisiting periodic catatonia in a case of SSPE and response to intrathecal interferon: a case report. Asian J Psychiatr 2020;51:101996. [DOI] [PubMed] [Google Scholar]
- 87.Pandey S, Shukla T, Mishra A. The spectrum of repetitive behaviors associated with subacute sclerosing panencephalitis. Mov Disord. 2021;36(2):497–503. [DOI] [PubMed] [Google Scholar]
- 88.Thulaseedaran NK, Kumar KGS, Kumar J, et al. A case series on the recent Nipah epidemic in Kerala. J Assoc Physicians India 2018;66(10):63–67. [PubMed] [Google Scholar]
- 89.Sejvar JJ, Hossain J, Saha SK, et al. Long‐term neurological and functional outcome in Nipah virus infection. Ann Neurol 2007;62(3):235–242. [DOI] [PubMed] [Google Scholar]
- 90.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol 2002;1(2):101–109. [DOI] [PubMed] [Google Scholar]
- 91.Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 2015;9(4):e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhatt M, Wolters E, Tsui J, Snow BJ, Calne DB. A case of rabies in North America. Mov Disord 1990;5(1):40–43. [DOI] [PubMed] [Google Scholar]
- 93.Hu WT, Willoughby RE Jr, Dhonau H, Mack KJ. Long‐term follow‐up after treatment of rabies by induction of coma. N Engl J Med 2007;357(9):945–946. [DOI] [PubMed] [Google Scholar]
- 94.Chen B, Tian EK, He B, et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther 2020;5(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nath A. Neurologic complications of coronavirus infections. Neurology 2020;94(19):809–810. [DOI] [PubMed] [Google Scholar]
- 96.Aggarwal A, Singhal T, Bhatt M. Neurology and COVID‐19: acting now. Preparing for future. Ann Indian Acad Neurol 2020;23(4):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS‐CoV‐2. J Neurol Neurosurg Psychiatry 2020;91(9):1013–1014. [DOI] [PubMed] [Google Scholar]
- 98.Merello M, Bhatia KP, Obeso JA. SARS‐CoV‐2 and the risk of Parkinson's disease: facts and fantasy. Lancet Neurol 2021;20(2):94–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bradshaw MJ, Venkatesan A. Herpes simplex virus‐1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 2016;13(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar R, Kumar P, Singh MK, et al. Epidemiological profile of acute viral encephalitis. Indian J Pediatr 2018;85(5):358–363. [DOI] [PubMed] [Google Scholar]
- 101.Amjadi O, Rafiei A, Haghshenas M, et al. A systematic review and meta‐analysis of seroprevalence of varicella zoster virus: a nationwide population‐based study. J Clin Virol 2017;87:49–59. [DOI] [PubMed] [Google Scholar]
- 102.Gilden D, Nagel MA, Mahalingam R, et al. Clinical and molecular aspects of varicella zoster virus infection. Future Neurol 2009;4(1):103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steiner I, Kennedy PGE, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella‐zoster. Lancet Neurol 2007;6(11):1015–1028. [DOI] [PubMed] [Google Scholar]
- 104.Calabria F, Zappini F, Vattemi G, Tinazzi M. Pearls & Oy‐sters: an unusual case of varicella‐zoster virus cerebellitis and vasculopathy. Neurology 2014;82(2):e14–e15. [DOI] [PubMed] [Google Scholar]
- 105.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol 1986;19(6):517–524. [DOI] [PubMed] [Google Scholar]
- 106.Cardoso F. HIV‐related movement disorders: epidemiology, pathogenesis and management. CNS Drugs 2002;16(10):663–668. [DOI] [PubMed] [Google Scholar]
- 107.Zuhlke LJ, Beaton A, Engel ME, et al. Group A streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med 2017;19(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet. 2018;392(10142):161–174. [DOI] [PubMed] [Google Scholar]
- 109.Walker KG, de Vries PJ, Stein DJ, Wilmshurst JM. Sydenham chorea and PANDAS in South Africa: review of evidence and recommendations for management in resource‐poor countries. J Child Neurol 2015;30(7):850–859. [DOI] [PubMed] [Google Scholar]
- 110.Ergonul O, Egeli D, Kahyaoglu B, Bahar M, Etienne M, Bleck T. An unexpected tetanus case. Lancet Infect Dis 2016;16(6):746–752. [DOI] [PubMed] [Google Scholar]
- 111.Schaller MA, Wicke F, Foerch C, Weidauer S. Central nervous system tuberculosis: etiology, clinical manifestations and neuroradiological features. Clin Neuroradiol 2019;29(1):3–18. [DOI] [PubMed] [Google Scholar]
- 112.Rubio‐Hernandez M, Ortiz‐Alvarez A, Tello‐Martinez N, Vazquez‐Guevara D, Rodriguez‐Leyva I. Hemichorea‐hemiballism: an uncommon presentation of central nervous system tuberculosis. Mov Disord Clin Pract. 2020;7(Suppl 3):S77–S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garg RK, Singh SK, Malhotra HS, Singh MK. Abnormal head movement in a patient with tuberculous meningitis. BMJ Case Rep 2012;2012:bcr2012006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franzini A, Franzini A, Levi V, Cordella R, Messina G. An unusual surgical indication for cerebral tuberculosis: status dystonicus. Case report. Acta Neurochir 2018;160(7):1355–1358. [DOI] [PubMed] [Google Scholar]
- 115.Serrano‐Duenas M. Tuberculous meningitis and dystonia. Mov Disord 2001;16(3):582–583. [DOI] [PubMed] [Google Scholar]
- 116.Lenka A, Thota N, Stezin A, Pal PK, Yadav R. Orofacial involuntary movements in neurosyphilis: beyond the candy sign. Tremor Other Hyperkinet Mov (NY) 2017;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Idro R, Otieno G, White S, et al. Decorticate, decerebrate and opisthotonic posturing and seizures in Kenyan children with cerebral malaria. Malar J 2005;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bose K, Saha S, Islam MR, Chakraborty C, Laskar M. Opsoclonus myoclonus ataxia syndrome due to falciparum malaria in two Indian children. Indian J Ophthalmol 2016;64(11):852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kochar DK, Kumawat BL, Thanvi I, Agrawal N. Cerebellar syndrome in Plasmodium falciparum malaria. QJM 1999;92(4):233–234. [DOI] [PubMed] [Google Scholar]
- 120.Yadava SK, Laleker A, Fazili T. Post‐malaria neurological syndrome: a rare neurological complication of malaria. Infection 2019;47(2):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marra CM. Central nervous system infection with Toxoplasma gondii. Handb Clin Neurol 2018;152:117–122. [DOI] [PubMed] [Google Scholar]
- 122.Deigendesch N, Costa Nunez J, Stenzel W. Parasitic and fungal infections. Handb Clin Neurol 2017;145:245–262. [DOI] [PubMed] [Google Scholar]
- 123.Antinori A, Larussa D, Cingolani A, et al. Prevalence, associated factors, and prognostic determinants of AIDS‐related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 2004;39(11):1681–1691. [DOI] [PubMed] [Google Scholar]
- 124.Aquino CC, Felicio AC, Godeiro‐Junior C, et al. Tic disorder: an unusual presentation of neurotoxoplasmosis in a patient with AIDS. Case Rep Neurol 2010;2(3):145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carrazana EJ, Rossitch E Jr, Samuels MA. Parkinsonian symptoms in a patient with AIDS and cerebral toxoplasmosis. J Neurol Neurosurg Psychiatry 1989;52(12):1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pradhan S, Yadav R, Mishra VN. Toxoplasma meningoencephalitis in HIV‐seronegative patients: clinical patterns, imaging features and treatment outcome. Trans R Soc Trop Med Hyg 2007;101(1):25–33. [DOI] [PubMed] [Google Scholar]
- 127.Wadia N, Desai S, Bhatt M. Disseminated cysticercosis. New observations, including CT scan findings and experience with treatment by praziquantel. Brain 1988;111(Pt 3):597–614. [DOI] [PubMed] [Google Scholar]
- 128.Cosentino C, Velez M, Torres L, Garcia HH, Cysticercosis Working Group in P . Cysticercosis lesions in basal ganglia are common but clinically silent. Clin Neurol Neurosurg 2002;104(1):57–60. [DOI] [PubMed] [Google Scholar]
- 129.Puri V, Chowdhury V, Gulati P. Myoclonus: a manifestation of neurocysticercosis. Postgrad Med J 1991;67(783):68–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Revuelta Gutierrez R, Soto‐Hernandez JL, Suastegui‐Roman R, Ramos‐Peek J. Transient hemifacial spasm associated with subarachnoid brainstem cysticercosis: a case report. Neurosurg Rev 1998;21(2–3):167–170. [DOI] [PubMed] [Google Scholar]
- 131.Sharma P, Garg RK, Somvanshi DS, Malhotra HS. Progressive supranuclear palsy like syndrome: neurocysticercosis an unusual cause. Neurol India 2011;59(3):484–485. [DOI] [PubMed] [Google Scholar]
- 132.Wadia NH. Neurocysticercosis. In: Wadia NH, ed. Neurological Practice: An Indian Perspective. 1st ed.New Delhi, India: Elsevier; 2005:215–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Detailed description of Figure 2.
Supplementary Material S2. Transcript for video narration.


