Brain regeneration from an evolutionary perspective: Brain regeneration (the full restoration of tissue after loss from injury or disease) is the most sought after goal for researchers working in developmental neurobiology. It also appears to be the most challenging to achieve when considering the mammalian brain. Whereas remarkable regenerative capacities can be present in the central nervous systems of many non-mammalian vertebrates (e.g., fish, amphibians), these kinds of processes appear to be dramatically reduced in mammals (Bonfanti, 2011). The reasons for such differences across animal classes are not completely understood, yet, some clear aspects have emerged from the study of well-established models like the teleost fish brain (Lange and Brand, 2020), which has: i) multiple, widespread stem cell niches that provide continuous, physiological cell renewal, as well as regeneration after lesioning; ii) additional neural elements that can de-differentiate after injury and re-acquire stem cell properties; iii) the ability to re-activate developmental programs in order to provide regenerative capacity. Studies on regeneration in various tissues and organs across animal species indicate that physiological and lesion-induced regeneration requires the coexistence of some (if not all) of the above-mentioned aspects, which, in the mammalian brain, are either absent or restricted to very small neurogenic niches. The most intuitive explanation for differences in brain regeneration across animal classes, apart from causal reasons, is the need for more neuroanatomical complexity linked to increased computational capabilities that often occurs in parallel with increased brain size. The “complexity” of large brains appears to be incompatible with substantial cell renewal/regeneration, a process that would be biologically expensive and somehow in contrast with the requirement for “stability” of the neural circuits (e.g., to retain long-term memories related to multiple previous experiences in long-living organisms). The current state of knowledge is still a mix of evidence and theories that are blurred by the frequently irregular patterns of evolution, but it does point to an important, underestimated issue: phylogenetic variations in the location, amount, rate, and type of brain plasticity in mammals.
Stem cell-driven adult neurogenesis and brain plasticity in mammals: Since its initial discovery, adult neurogenesis has been considered a turning point in our understanding of brain regeneration. Most mammalian brains host at least two active neurogenic sites (three, considering the hypothalamus) where multipotent neural stem cells generate new neurons capable of maturing and undergoing functional integration within restricted brain regions. Initially, this discovery was viewed in terms of a typical stem cell system, such as those existing in skin and blood, and was interpreted as a possible source of new neurons that could join pre-existing elements to replace neuronal cells damaged/lost in neurodegenerative disorders. However, it is now evident that mammalian neural stem cell niches produce only a few types of neurons, and that these are selectively integrated in very specific neural circuits. Moreover, neurogenic sites hosting the stem cells progressively decrease in number and activity across the lifespan of the animals. This decrease occurs very early in large-brained mammals, including humans, where it leads to substantial exhaustion of the stem cell niches in adolescence (Parolisi et al., 2018).
The disappointment regarding the potential of stem cells to contribute to brain regeneration is understandable, especially considering the huge effort by the scientific community over the years. In addition, the substantial differences emerging between mice and humans have important implications for the use of rodent models to study brain plasticity in a translational perspective. Nevertheless, mammalian brains possess other forms of plasticity that are not confined within small, restricted regions but ubiquitously present, and congruent with the need for stability in the number/type of neurons, e.g., the well-known synaptic and dendritic/axonal plasticity. These structural changes consist of adjustments of small components of pre-existing cells and do not provide “true” brain regeneration in terms of neuronal replacement and/or neural tissue reconstruction (Figure 1A, top).
Figure 1.
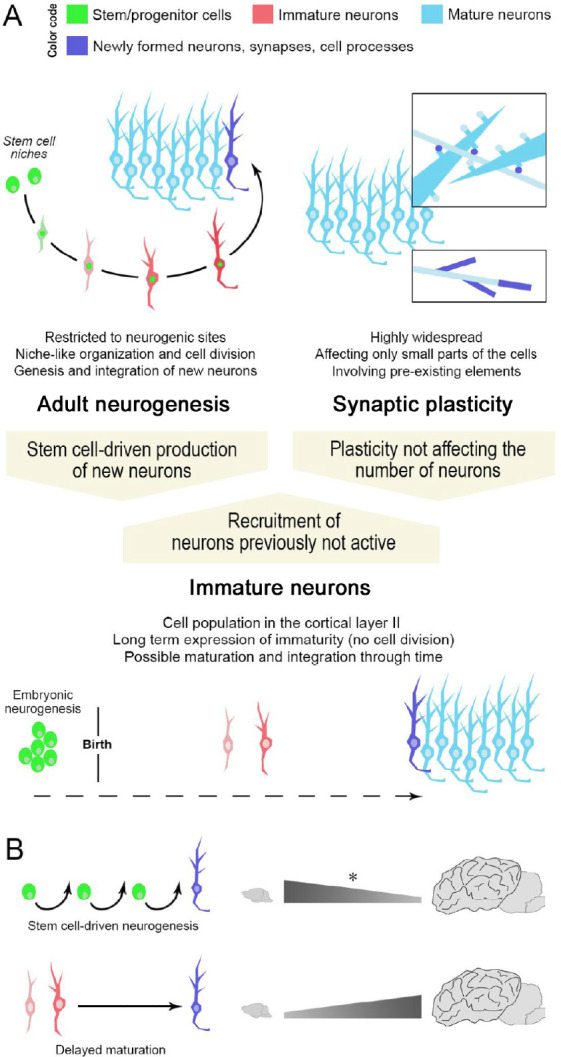
Different ways for achieving structural plasticity in the adult mammalian brain.
Stem cell-driven genesis of new neurons (adult neurogenesis) and synaptic/axonal plasticity (A, top) represent two extremes of plastic events in the brain. Non-newly generated “immature” neurons (A, bottom), as a form of delayed neurogenesis without division, might be considered as an intermediate form of plasticity providing new elements for the pre-existing neural circuits in the absence of active stem cell niches/neural progenitors. Note that a similar outcome (the addition of a new neuron in the circuits) can be obtained through different plastic processes, not all of which involve stem/progenitor cells (high top: color code indicating different maturational states of neurons; dark blue indicates newly formed elements). (B) Specific types of plasticity such as “classic” adult neurogenesis (top) or “immature” neurons (bottom) can coexist, yet, with highly different distributions and amounts. The numbers of immature neurons can vary remarkably in mammals, with phylogenetic variation between small-brained and large-brained species (La Rosa et al., 2020b). Asterisk, the reduction in adult neurogenesis rates across mammalian species has not yet been assessed through systematic, comparable approaches.
From the evolutionary point of view, it makes sense to shift from adult neurogenesis to forms of structural modification that meet the increasing, intrinsic need for stability; however, this comes at the cost of lost regenerative capacity. Why, then, are neurogenic niches maintained in the mammalian brain? A possible answer can be found in the knowledge accumulated over the last thirty years, showing that the newly generated neurons in the olfactory bulb and hippocampus support the physiological need to learn from life experience. Although most scientists still consider adult neurogenesis to be a potential source of neurons for brain repair, it appears to be a physiological “tool” that allows the neural circuits involved in survival-related tasks (food search/recognition, identification of predators, learning, memory, reproduction) to adapt to a changing environment. Though the endogenous neural stem cells in rodents do react after injury/inflammation (reactive or lesion-induced neurogenesis), the result is not regeneration or substantial cell replacement, because most of the resulting cells undergo aberrant or abortive fate. This view is supported by the recent interpretation of adult hippocampal neurogenesis as a protracted developmental process that allows for progressive maturation of hippocampal circuits and refining/sharpening of the related cognitive functions, even in mice (Semënov, 2019). Hence, adult neurogenesis in mammals appears to belong in the wide chapter of “structural plasticity” rather than “brain regeneration” and, accordingly, in that of physiological plasticity rather than mechanisms for brain repair. Large-brained, long-living species seem to maintain different kinds of structural plasticity to provide progressive brain maturation rather than regeneration, with large variations depending on age and brain region.
Non-newly generated “immature” neurons: a smart choice? A new element has been recently introduced in the landscape of brain plasticity: the so-called cortical “immature” neurons. These cells, first discovered in the layer II of the paleocortex of rodents, are generated before birth but continue to express immaturity marker molecules into adulthood (Bonfanti and Nacher, 2012). Currently, the mechanisms that allow these young neurons to maintain their immaturity and remain in “standby mode” are not known and far from being understood. We do know that these cells have one of two morphologies that appear to represent different stages of maturation: they occur as either small, bipolar, more immature Type 1 cells, or as large, ramified, less immature Type 2 cells (Bonfanti and Nacher, 2012; Piumatti et al., 2018). Because the number of cortical immature neurons in layer II decreases with age, Type 2 cells are considered to be more complex elements derived from the progressive maturation of Type 1 cells. Recently, this hypothesis was confirmed by experiments in transgenic mice expressing DCX-CreERT2/Flox-EGFP, in which DCX- expressing cells were visualized with green fluorescent protein and followed through time, revealing that the immature cells “do not vanish in the course of aging, but progressively resume their maturation into glutamatergic neurons” (Benedetti et al., 2019). After months of progressive maturation, characterized by increasing soma size and increasing complexity of their dendritic arborization, Type 1 cells become Type 2 cells (“complex cells”). Patch clamp experiments showed that the complex cells appeared to be functionally integrated into piriform cortex circuits, though having different properties with respect to layer II principal neurons (Benedetti et al., 2019). The occurrence of a population of non-proliferative, dormant neuronal precursors, which share markers of immaturity with those continuously produced in the adult stem cell niches, may represent a reservoir of young neurons for the cerebral cortex. In rodents, cortical immature neurons are restricted to the piriform and entorhinal cortices (paleocortex). Yet, by studying a wide range of animal species, belonging to several mammalian orders and including species with different brain sizes, gyrencephaly, life history and socioecological features, we recently showed that these neurons are widely distributed and remarkably abundant in the whole cerebral cortex (including the neocortex) of large-brained, more gyrencephalic mammals (Piumatti et al., 2018; La Rosa et al., 2020). This trend suggests that cortical immature neurons might have been chosen during evolution to provide a reservoir of plastic cells within the relatively stable cerebral cortex (not endowed with stem cells/stem cell niches), particularly the neocortex, which has undergone remarkable expansion in most mammals with large brains (Figure 1B). This reservoir of young, undifferentiated neurons with varying degrees of maturation that can progressively integrate into the layer II neural circuits, might be considered a form of “neurogenesis without division”, representing a plastic process between two extremes of stem cell-driven, adult neurogenesis and structural modifications affecting small parts of pre-existing elements (Figure 1A). For highly complex and relatively stable cerebral cortices of large-brained mammals, reliance on pre-existing neurons that can be added functionally throughout life might be an evolutionarily advantageous, energetically inexpensive solution for overcoming the lack of stem/progenitor cells.
Interestingly, the occurrence of DCX+ , immature neurons does not appear to be restricted to the cortical mantle: very similar cells have been described in subcortical regions such as the amygdala, claustrum and white matter. Also in this case, they seem to be particularly abundant in large brains (Piumatti et al., 2018) including humans (Sorrells et al., 2019), though wider comparative studies, including comparable quantitative analyses and further phenotypic characterization, are still lacking. The recent identification of DCX+ neurons in the adult human hippocampus, in the absence of significant cell division and without a typical stem cell niche organization, strongly suggests that immature-like elements might also persist in the neurogenic sites after the decrease/ending of their activity (references and discussion in Parolisi et al., 2018; Seki, 2020). The occurrence of immature neurons arrested in an intermediate state of differentiation and already present in their final anatomical location could be seen as a response to the need for neurogenic-like plasticity in nervous systems that have very reduced capacity for producing new neurons, as well as for functionally integrating exogenously-grafted neural stem cells.
Conclusions and future perspectives: True brain regeneration occurs at some levels in the nervous systems of some non-mammalian vertebrates and is greatly reduced in the mammalian brain, especially in adult humans. The occurrence of neural stem cells in adult mammals is restricted to specific region and mostly linked to the progressive completion of the olfactory bulb and hippocampus during postnatal development, based on cues coming from environmental conditions/life experiences. In large-brained species, it appears that the remarkable expansion of the neocortex was paralleled by an increase in non-newly generated, immature neurons (also in subcortical regions) that form during embryogenesis and cannot divide postnatally, but retain undifferentiated features through time. Studies carried out in rodents show that this reservoir of immature cells can provide new neurons through progressive maturation and integration into cortical circuits. Many questions remain regarding this novel form of plasticity in the mammalian brain, especially concerning the physiological role(s) of these cells, their ultimate fate, as well as the mechanisms that underly their “quiescence” and elicit their maturation. We do not yet know whether they can react to lesion/pathology, or be modulated by external cues. Finally, we still do not know about their occurrence/abundance in the human brain, and this is difficult to predict, because we know that cortical immature neurons have evolved independently in different mammalian orders (La Rosa et al., 2020). If similar cells are widespread in Homo sapiens, as they are in other large-brained species, they might represent a substrate of the so-called “brain reserve” or “cognitive reserve” against the onset/impact of dementia and neurodegenerative diseases.
In conclusion, the recently discovered populations of immature neurons open new opportunities for preventive/therapeutic approaches in the extensive brain regions not involved in stem cell-driven adult neurogenesis, and provide a new vision in the field of brain plasticity, with a new twist on the traditional way to consider “brain regeneration”.
We thank Richard Vernell for overall English revision.
Additional file: Open peer review report 1 (73.4KB, pdf) .
Footnotes
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Gopi Margabandhu, University of Madras, India.
P-Reviewer: Margabandhu G; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Benedetti B, Dannehl D, König R, Coviello S, Kreutzer C, Zaunmair P, Jakubecova D, Weiger T M, Aigner L, Nacher J, Engelhardt M, Couillard-Després S. Functional integration of neuronal precursors in the adult murine piriform cortex. Cereb Cortex. 2019;30:1499–1515. doi: 10.1093/cercor/bhz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonfanti L. From hydra regeneration to human brain structural plasticity: a long trip through narrowing roads. ScientificWorldJournal. 2011;11:1270–1299. doi: 10.1100/tsw.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonfanti L, Nacher J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: the case of cortical layer II immature neurons. Prog Neurobiol. 2012;98:1–15. doi: 10.1016/j.pneurobio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Climent MA, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Martínez- Guijarro FJ, Nácher J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex. 2008;18:2229–2240. doi: 10.1093/cercor/bhm255. [DOI] [PubMed] [Google Scholar]
- 5.Seki T. Understanding the real state of human adult hippocampal neurogenesis from studies of rodents and non-human primates. Front Neurosci. 2020;14:839. doi: 10.3389/fnins.2020.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parolisi R, Cozzi B, Bonfanti L. Humans and dolphins: decline and fall of adult neurogenesis. Front Neurosci. 2018;12:497. doi: 10.3389/fnins.2018.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piumatti M, Palazzo O, La Rosa C, Crociara P, Parolisi R, Luzzati F, Lévy F, Bonfanti L. Non-newly generated, “immature” neurons in the sheep brain are not restricted to cerebral cortex. J Neurosci. 2018;38:826–842. doi: 10.1523/JNEUROSCI.1781-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange C, Brand M. Vertebrate brain regeneration – a community effort of fate-restricted precursor cell types. Curr Op Gen Dev. 2020;64:101–108. doi: 10.1016/j.gde.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 9.La Rosa C, Parolisi R, Bonfanti L. Brain structural plasticity: From adult neurogenesis to immature neurons. Front Neurosci. 2020a;14:75. doi: 10.3389/fnins.2020.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rosa C, Cavallo F, Pecora A, Chincarini M, Ala U, Faulkes CG, Nacher J, Cozzi B, Sherwood CC, Amrein I, Bonfanti L. Phylogenetic variation in cortical layer II immature neuron reservoir of mammals. eLife. 2020b;9:e55456. doi: 10.7554/eLife.55456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semënov MV. Adult hippocampal neurogenesis is a developmental process involved in cognitive development. Front Neurosci. 2019;13:159. doi: 10.3389/fnins.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorrells SF, Paredes MF, Velmeshev D, Herranz-Pérez V, Sandoval K, Mayer S, Chang EF, Insausti R, Kriegstein AR, Rubenstein JL, Garcia-Verdugo JM, Huang EJ, Alvarez-Buylla A. Immature excitatory neurons develop during adolescence in the human amygdala. Nat Commun. 2019;10:2748. doi: 10.1038/s41467-019-10765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


