Key Words: caudate-putamen nucleus, deep brain stimulation, dopamine, primary auditory cortex, sodium salicylate, sound, striatum, tinnitus
Abstract
Tinnitus can be described as the conscious perception of sound without external stimulation, and it is often accompanied by anxiety, depression, and insomnia. Current clinical treatments for tinnitus are ineffective. Although recent studies have indicated that the caudate-putamen nucleus may be a sensory gating area involved in noise elimination in tinnitus, the underlying mechanisms of this disorder are yet to be determined. To investigate the potential role of the caudate-putamen nucleus in experimentally induced tinnitus, we created a rat model of tinnitus induced by intraperitoneal administration of 350 mg/kg sodium salicylate. Our results revealed that the mean spontaneous firing rate of the caudate-putamen nucleus was increased by sodium salicylate treatment, while dopamine levels were decreased. In addition, electrical stimulation of the caudate-putamen nucleus markedly reduced the spontaneous firing rate of neurons in the primary auditory cortex. These findings suggest that the caudate-putamen nucleus plays a sensory gating role in sodium salicylate-induced tinnitus. This study was approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center (approval No. A2010031) on December 6, 2017.
Chinese Library Classification No. R444; R764.45; R338.8
Introduction
Tinnitus refers to the sensation of hearing sound without any external acoustic stimulation (Lanting et al., 2009). Epidemiological studies have reported that the global prevalence of tinnitus among adults is as high as 10–25% (Henry et al., 2005; Shargorodsky et al., 2010; Kim et al., 2015), and increases with age (Sindhusake et al., 2003; Rauschecker et al., 2010; Shargorodsky et al., 2010; Manche et al., 2016). Moreover, 1.5–7% of patients are severely affected by tinnitus, which has negative affect on their life quality (Nondahl et al., 2002; Bhatt et al., 2016), while 97% of patients present varying degrees of hearing loss (Manche et al., 2016). Tinnitus is often accompanied by anxiety, depression, insomnia, and even suicide in severe cases (Trevis et al., 2016, 2018; Bhatt et al., 2017; Martz et al., 2018; Chai et al., 2019). It is difficult to clinically treat tinnitus because the underlying neurophysiological and pathological mechanisms remain unclear.
Previous animal studies have suggested that cochlear lesions may cause central auditory gain (Eggermont, 2005; Salvi et al., 2016), which might explain the decreased tolerance to loudness and increased sensitivity to sound in patients with tinnitus. However, surgical removal of the auditory nerve does not alleviate tinnitus symptoms (Kameda et al., 2010). Increasing evidence indicates that tinnitus is a central plasticity disorder caused by peripheral lesions that are difficult to treat (Shore et al., 2016; Wu et al., 2016; Roberts, 2018). The underlying pathological process involves multiple neural networks, including the classical auditory (Sedley et al., 2015; Leaver et al., 2016), limbic (Kraus and Canlon, 2012; Leaver et al., 2016), cerebellar (Chen et al., 2017; Du et al., 2017), and basal ganglia (Ahsan et al., 2018; Perez et al., 2019) systems.
The striatum is the largest nucleus in the basal ganglia. It receives fiber projections from all cortices, including the auditory, motor, and sensory cortices (Hunnicutt et al., 2016; Miyamoto et al., 2018). The dorsal striatum, which comprises the caudate-putamen nucleus (CPu), serves as a sensory gating region in information transmission to the cerebral cortex and is suggested to play a crucial role in tinnitus (Lowry et al., 2004; Cheung and Larson, 2010; Larson and Cheung, 2013; Ahsan et al., 2018; Perez et al., 2019). Lowry et al. (2004) reported a case of chronic tinnitus that was cured after a cerebrovascular accident in the left corona radiata, including the caudate body and caudodorsal putamen. Subsequently, several studies have successfully used deep brain stimulation (DBS) to treat patients with Parkinson’s disease or essential tremor and tinnitus (Cheung and Larson, 2010; Larson and Cheung, 2013). These studies reported significant suppression or even complete alleviation of tinnitus and suggest that the caudate nucleus may be a neuroregulatory target for the inhibition of tinnitus. DBS of the caudate nucleus interferes with tinnitus information integration in the central auditory system, thus suppressing tinnitus noise. Ahsan et al. (2018) were the first to confirm the therapeutic effects of DBS of the caudate nucleus on tinnitus suppression in animals with noise-induced tinnitus. They observed that electrical stimulation of the caudate nucleus reduced cluster discharge in neurons in the auditory cortex. Moreover, the assessment of startle reflex behavior indicated tinnitus suppression. Together, these studies provide strong evidence for the involvement of the CPu in tinnitus; however, it remains unclear how this nucleus regulates tinnitus.
Dopamine, an important monoamine neurotransmitter, is involved in somatic movement, psychological activity, psychological dependence, and other body regulation functions (Puopolo, 2019; Thomas Broome et al., 2020). Dopaminergic neurons are mainly located in the substantia nigra zona compacta and ventral tegmental area (Wu et al., 2017), and the CPu is dominated by long axial ascending neurons from the substantia nigra pars compacta. Moreover, previous clinical studies have indicated that dopaminergic agents may have a therapeutic effect on tinnitus (de Azevedo et al., 2009; Sziklai et al., 2011). We therefore hypothesized that the CPu might be involved in the underlying mechanisms of tinnitus through dopaminergic regulation of the indirect basal ganglia pathway.
Sodium salicylate (SS), the main ingredient in aspirin, is an effective anti-inflammatory drug for fever and chronic pain. Because large SS doses can cause tinnitus as a side effect, it is now used as a standard tool for establishing animal models of tinnitus (Yang et al., 2007; Lu et al., 2011). To investigate the potential role of the CPu in sensory gating, we first verified that SS induced tinnitus behavior in animals by measuring gap-prepulse inhibition of the acoustic startle reflex (GPIAS). Next, we explored the spontaneous firing rate (SFR) of neurons in the CPu in a rat model of SS-induced tinnitus. Given the association between neuronal electrical activity and neurotransmitter release, extracellular dopamine levels were also measured before and after SS treatment. Furthermore, to investigate regulatory mechanisms between the CPu and primary auditory cortex (Au1), we recorded SFR changes in the Au1 after electrical stimulation of the CPu.
Materials and Methods
Animals
Healthy adult male Sprague-Dawley rats (specific-pathogen-free level, 8 weeks old, 280–350 g) were obtained from the Department of Laboratory Animal Science, Peking University Health Science Center (PUHSC), Beijing, China (license No. SYXK (Jing) 2016-0041). All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All experimental procedures were approved by the Institutional Animal Care and Use Committee of PUHSC (approval No. A2010031) on December 6, 2017. Rats were individually housed under standard conditions with free access to food and water. A 12-hour light/dark cycle (lights on 7:00 to 19:00), room temperature of 24 ± 1°C, and air humidity of 50–60% was maintained in the housing environment. Eight rats underwent tinnitus evaluation using GPIAS, 12 rats were used to determine the effects of SS on neural SFRs in the CPu, 12 rats were used to detect SS-induced changes in extracellular dopamine levels in the CPu, and eight rats were used to determine SFRs in the Au1 by electrical stimulation of the CPu.
Behavioral assessment of tinnitus
Eight rats underwent behavioral testing for tinnitus assessment using GPIAS. This experiment was conducted in a noise-shielded box (ZS-ZJT, ZS Dichuang Co., Beijing, China). Each rat was restrained in a transparent polycarbonate breathable acoustic holder installed on a plexiglass base that contained a sensitive piezoelectric sensor with an output connected to an A/D converter on an RP2 real-time processor (ZS Dichuang Co., Beijing, China). Sound stimuli were generated using a custom RP2 software and presented by a loudspeaker placed approximately 20 cm above the startle platform (Figure 1). The background noise was centered at 6, 12, and 16 kHz, and broadband noise intensity of 65 dB sound pressure was used for each trial. A startle stimulus (115 dB sound pressure level, 20 ms duration) was embedded in the background noise of each trial. Intense startle reflexes were suppressed by inserting a 50-ms silent gap in a continuous background noise burst before the startle stimulus. The inhibitory effect of the silent gap was indicated by the GPIAS ratio and the absolute startle amplitude between the non-gap and gap protocols. We defined a GPIAS ratio of less than 30% as an indicator of tinnitus behavior (Kraus et al., 2010; Longenecker and Galazyuk, 2011; Longenecker et al., 2014). We measured gap detection deficits using a specific acoustic startle reflex hardware and software (ZS-ZJT, ZS Dichuang Co.). Similarity between the background noise and the tinnitus frequency indicated impaired gap inhibition in rats with tinnitus. The GPIAS values were recorded before and 2 hours after the SS injection.
Figure 1.
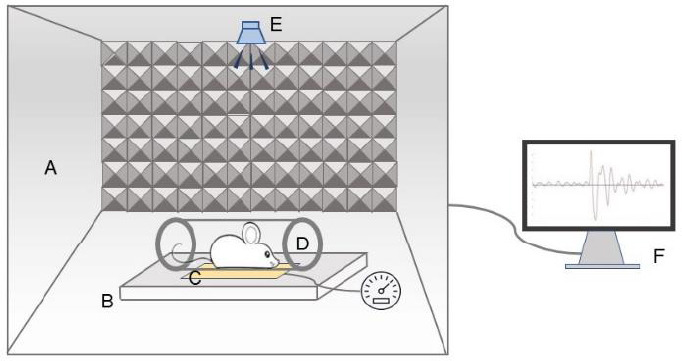
Schematic diagram of the behavioral experimental setup.
The device included a noise-shielded box (A), startle platform (B), sensitive piezoelectric sensor (C), holder (D), loudspeaker (E), and workstation (F).
Single-neuron recordings in the CPu
All animals (N = 12) were anesthetized using isoflurane (RWD Life Science, Shenzhen, China) (anesthesia induction: 3–5% for 3 minutes; anesthesia maintenance: 1–2%; flow rate 0.2–0.3 L/min) and placed in a stereotaxic head frame on a heating blanket. Anesthesia adequacy was confirmed by the absence of a hind-paw withdrawal reflex. A craniotomy was performed over the right CPu (anteroposterior (AP) = 0 mm, mediolateral (ML) = 3 mm, dorsoventral (DV) = 3 mm), conforming to rat brain stereotaxic coordinates (Paxinos and Watson, 2007). Three stainless steel screws were drilled into the skull as reference electrodes, with the tip making slight contact with the dura. Using tweezers, the dura mater was carefully removed under a surgical microscope (YZ20P5, Suzhou Liuliu Vision Technology, Suzhou, China) to expose the brain tissue above the CPu. Subsequently, recording electrodes (Institute of Semiconductors, the Chinese Academy of Sciences, Beijing, China) were implanted along the dorsoventral axis of the micromanipulator. Next, multiple single neurons were recorded using a 16-channel silicon electrode (4 × 4 array; Plexon Inc., Dallas, TX, USA), as previously described (Song et al., 2016; Du et al., 2017; Xiong et al., 2019). The baseline SFR was recorded 5 minutes before the SS (350 mg/kg, 10%, intraperitoneal; Sigma-Aldrich, St. Louis, MO, USA) or equivalent saline injection. Subsequently, SFR recordings were performed for at least 5 minutes at the following post-injection time points: 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 hours. At the end of the experiment, the rats were euthanized with a lethal dose of urethane solution (Sigma-Aldrich).
Measurement of dopamine levels using high-performance liquid chromatography
Given the association between neuronal electrical activity and neurotransmitter release, we hypothesized that SS treatment might change neurotransmitter levels in the CPu. The CPu is rich in dopamine, which is an important neurotransmitter that modulates various physiological responses in the central nervous system (Grillner et al., 2020). To assess the effects of SS on dopamine levels, a microdialysis guide cannula was implanted in the CPu to measure the extracellular dopamine levels.
We randomly divided 12 rats into the SS (N = 6; 350 mg/kg, 10%, intraperitoneal) and saline (N = 6; equivalent dose of SS) groups. We performed microdialysis for the extracellular solution in the right CPu and analyzed the dopamine levels using a high-performance liquid chromatography system combined with electrochemical detection (Figure 2). Under isoflurane anesthesia, a microdialysis guide cannula (MAB.6.14.2ss, MBA, Stockholm, Sweden) was implanted into the vertical dural surface of the right CPu (AP = 0 mm, ML = 3 mm, DV = 3 mm) and permanently secured by supporting screws (Misumi, Shanghai, China) and dental cement (Tianjin Ruierdeyuan Medical Biomaterials, Tianjin, China). The rats were allowed at least 2 postoperative days to recover prior to the subsequent experiments. Next, the stylet was replaced by a concentric microdialysis probe with a 4-mm semipermeable membrane (Bioanalytical Systems Inc., West Lafayette, IN, USA) and inserted through the guide cannula into the CPu. The concentric microdialysis probe was connected to a perfusion pump (CMA100, CMA, Stockholm, Sweden) that maintained a flow rate of 2 μL/min with artificial cerebrospinal fluid (Beijing Chemical Works, Beijing, China). After a 90-minute stabilization period, three sequential dialysate samples were obtained at 20-minute intervals to establish pre-treatment baseline values. Next, dialysate samples were collected for the extracellular dopamine measurements at 30-minute intervals until 4 hours after the SS or saline injection. All dialysate samples were then analyzed using a reverse-phase high-performance liquid chromatography system (Shimadzu Corporation, Kyoto, Japan) with an analytical C18 column (150 mm × 4.6 mm, 5 mm particle size). A 20 μL injection of methanol/acetonitrile (98:2, v/v) was used as the mobile phase at a flow rate of 1.0 mL/min and a column temperature of 25°C. The mobile phase was filtered through a 0.45-mm nylon filter and degassed for 30 minutes by ultrasonication. The obtained brain dialysates were delivered into the thin-layer electrochemical flow cell through tubing. The electrochemical system had three components: a glassy carbon working electrode (6 mm in diameter), an Ag/AgCl reference electrode, and a stainless steel counter electrode.
Figure 2.
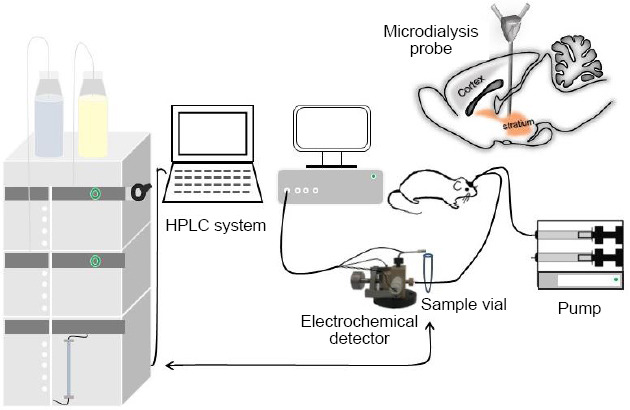
Schematic diagram of the experimental apparatus for in vivo dopamine analysis by microdialysis and high-performance liquid chromatography (HPLC) with electrochemical detection.
In order to get the dopamine standard solution electrochemical detection linear equation, 1000 nM dopamine solution was prepared by dissolving 0.95 mg dopamine (Sigma-Aldrich Chemmical Co., St. Louis, MO, USA) in 5 mL ultra-pure water then the dopamine concentration was diluted to five standard dopamine concentrations (1, 10, 25, 50 and 100 nM). From low to high solution, the standard was successively passed through the high performance liquid liquid-online electrochemical detection system to obtain the concentration-voltage dopamine standard linear equation.
CPu electrical stimulation
Eight rats were anesthetized using isoflurane (anesthesia induction: 3–5% for 3 minutes; anesthesia maintenance: 1–2%, flow rate 0.2–0.3 L/min) and were each mounted onto a stereotaxic head frame with hollow ear bars on a heating blanket. The right CPu was exposed as described as for the CPu single-neuron recordings, and a stimulating electrode was inserted along the dorsoventral axis for electrical stimulation. We obtained recordings from the right Au1 region (AP = –5.2 mm, ML = 6.8 mm, DV = –2.5 mm) (Paxinos and Watson, 2007), which was exposed from the bregma as previously described (Song et al., 2016; Du et al., 2017; Ding et al., 2018; Xiong et al., 2019). The skull and relevant dura mater were removed, and the recording electrode was implanted into the Au1 along the dorsoventral axis of the micromanipulator. A stainless steel ground electrode was placed on the skull surface. An electrical stimulator (Marster-9, A.M.P.I., Jerusalem, Israel) delivered shock trains (pulse duration, 2 ms; train duration, 16.7 ms; rate, 60 Hz; and intensity, 250 μA) to the CPu through a custom-made bipolar tungsten electrode. The SFR in the Au1 was recorded for 3 minutes before and after CPu stimulation (Figure 3).
Figure 3.
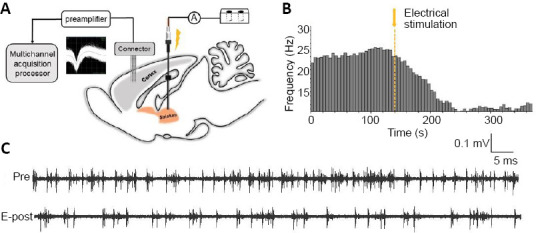
Effects of caudate-putamen nucleus (CPu) electrical stimulation on primary auditory cortex (Au1) neurons.
(A) Schematic diagram of the electrical stimulation system and multi-electrode array recording system. (B) Representative firing rate histogram of Au1 neurons showing an inhibitory response to CPu electrical stimulation. The arrow and dashed line denote the shock artifact. (C) Neuron spike discharges recorded in the Au1 before and after CPu electrical stimulation. E-post: After CPu electrical stimulation; Pre: before CPu electrical stimulation.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (IBM, Armonk, NY, USA) and the results are presented as the mean ± standard error of the mean (SEM). The paired-samples t-test was used to analyze GPIAS suppression and absolute startle amplitude with different background noises and groups, as well as the pre- and post-stimulation mean SFR in the Au1. Between-group comparisons of neuronal SFR and extracellular dopamine levels in the CPu were performed using two-way analysis of variance (ANOVA) with the least significant difference (LSD) post hoc test. One-way ANOVA with the LSD post hoc test was used to compare changes in the SFR and dopamine levels from baseline in different groups and at different time periods. A linear regression model was used to explore the linear response to the dopamine standard solution. All graphical presentations were obtained using GraphPad Prism 6.01 (GraphPad Prism, San Diego, CA, USA) and Origin 2017 (OriginLab Corporation, Northampton, MA, USA). We considered statistical significance at P < 0.05. N and n represent the number of animals and number of samples (neurons), respectively.
Results
treatment to induce tinnitus in rats
Eight rats underwent GPIAS testing before and after SS treatment to obtain behavioral evidence of tinnitus. Before the SS injection, the animals showed strong GPIAS suppression at background frequencies of 6 kHz (46.5 ± 5.6%), 12 kHz (54.1 ± 3.4%), and 16 kHz (55.6 ± 20.3%). However, 2 hours after the SS treatment, the GPIAS decreased to 38.7 ± 5.3%, 32.1 ± 6.1%, and 20.3 ± 6.3% at 6, 12, and 16 kHz, respectively. This decrease in the GPIAS was significant at 12 kHz (t = 3.649, P = 0.008) and 16 kHz (t = 5.214, P = 0.001), but not at 6 kHz (t = 1.467, P = 0.186).
The absolute amplitude of startle responses was also measured pre- and post-SS treatment in both gap and no-gap conditions. The startle response amplitude in both gap and no-gap conditions was significantly different at 6 kHz (t = –3.258, P = 0.014), 12 kHz (t = –3.569, P = 0.009), and 16 kHz (t = –4.119, P = 0.004) before SS treatment; however, after SS treatment, a significant difference was only observed at 16 kHz (t = –2.953, P = 0.022), but not at 6 kHz (t = –2.285, P = 0.056) or 12 kHz (t = –2.280, P = 0.057; Figures 4–6).
Figure 4.
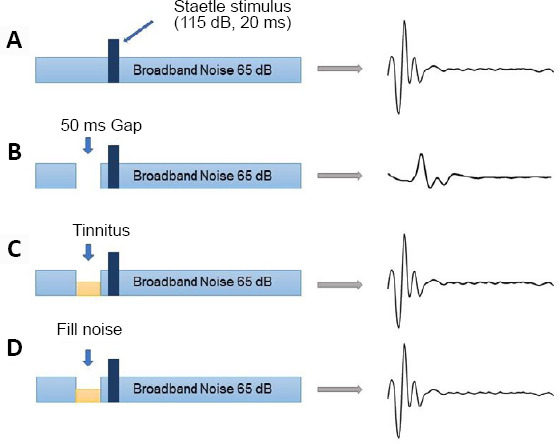
Schematic view of gap-prepulse inhibition of the acoustic startle reflex.
(A) Normal rats can detect the acoustic startle stimulus in continuous background noise. (B) The acoustic startle reflex in normal rats is forcefully inhibited when a silent gap of 50 ms is inserted into continuous background noise. (C) The tinnitus noise frequency fills the silent gap and there is no inhibition of the acoustic startle reflex. (D) The background noise frequency is near or as high as the putative tinnitus frequency.
Figure 6.
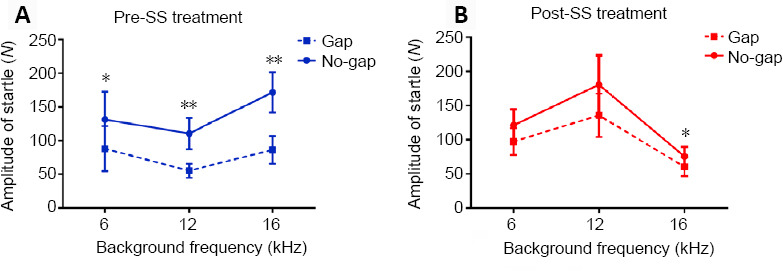
Effects of sodium salicylate (SS) on the startle response amplitude at different frequencies.
(A) The startle response amplitudes in both gap and no-gap conditions were significantly different at 6 kHz (t = −3.258, P = 0.014), 12 kHz (t = −3.569, P = 0.009), and 16 kHz (t = −4.119, P = 0.004) before the SS treatment. (B) After the SS treatment, there was a significant difference at 16 kHz (t = −2.953, P = 0.022) only. Data are presented as the mean ± SEM (N = 8 rats). *P < 0.05, **P < 0.01, vs. gap condition (paired-samples t-test).
Figure 5.
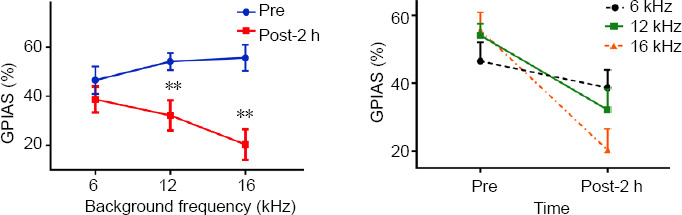
Gap detection performance at different frequencies measured before and after sodium salicylate (SS) treatment.
Injection of 350 mg/kg SS decreased the gap-prepulse inhibition of the acoustic startle reflex (GPIAS) at 12 kHz (t = 3.649, P = 0.008) and 16 kHz (t = 5.214, P = 0.001). Data are presented as the mean ± SEM (N = 8 rats). **P < 0.01, vs. pre-SS treatment (paired-samples t-test).
Effects of SS on the neuronal SFR of the CPu
To determine the effects of SS on the neuronal SFR in the rat CPu, the rats were injected with 350 mg/kg SS or an equivalent volume of saline. We recorded the neural firing activity of single units in the CPu both before and at 0.5-hour intervals after SS or saline treatment. We recorded the spontaneous activity of 49 and 57 CPu neurons in the SS and saline groups, respectively. Two-way ANOVA with LSD post hoc tests revealed significant between-group differences (F = 2.300, P = 0.019). One-way ANOVA with LSD post hoc tests demonstrated a significant increase in the mean SFR of the CPu at 2 hours (P = 0.012) and 2.5 hours (P = 0.016) after the SS treatment; the SFR increased from 4.8 ± 0.5 Hz to 7.4 ± 0.8 Hz and 7.2 ± 0.9 Hz, respectively. Conversely, saline treatment did not significantly affect the mean SFR of CPu neurons (F = 0.682, P = 0.707; Figure 7).
Figure 7.
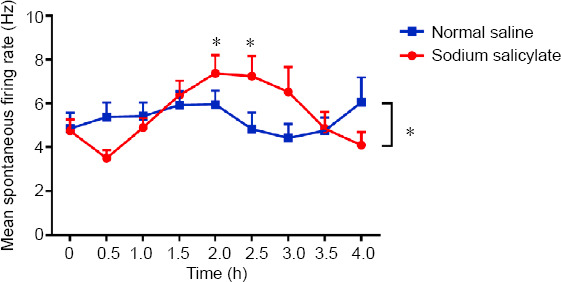
Effects of sodium salicylate (SS) on the neuronal firing rate of caudate-putamen neurons.
There was a significant between-group difference in the mean spontaneous firing rate (SFR; SS group: N = 6, n = 49; saline group: N = 6 rats, n = 57 neurons; *P < 0.05, two-way analysis of variance followed by the least significant difference post hoc test). SS treatment, but not saline treatment, significantly affected the mean SFR at 2 and 2.5 hours (*P < 0.05, one-way analysis of variance followed by the least significant difference post hoc test). Data are presented as the mean ± SEM.
Dopamine levels in the CPu
High-performance liquid chromatography analysis demonstrated a good linear response to the dopamine standard solution from 1 to 100 nM. The linear equation was U (V) = 0.5054 CDA (nM) + 0.8588 (U: the mathematical symbol for voltago; CDA: dopamine concentration) with a linear coefficient of 0.9934 (Figure 8A). Two-way ANOVA revealed a significant difference in dopamine levels between the SS and saline groups (F = 6.358, P = 0.013). In the SS group, there was a gradual decrease in extracellular dopamine levels in the CPu, reaching 78.9 ± 2.3% of the baseline level after 4 hours (t = 6.298, P = 0.000). Conversely, extracellular dopamine levels remained stable in the saline group (F = 1.090, P = 0.387; Figure 8B).
Figure 8.
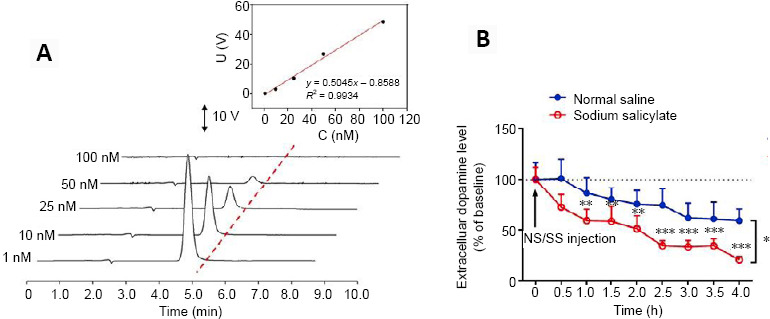
Effects of sodium salicylate (SS) on extracellular dopamine levels in the caudate-putamen nucleus (CPu).
(A) Typical linear response to the dopamine standard solution. The high-performance liquid chromatography analysis showed a good linear response to the dopamine standard solution from 1 to 100 nM. A linear regression model was used to explore correlations and the linear equation was U (V) = 0.5054 CDA (nM) + 0.8588 with a linear coefficient of 0.9934. (B) Between-group comparison of dopamine levels in the CPu at different time points. Statistical results of extracellular dopamine levels based on their baseline levels in the CPu in both groups as a function of time, with each point representing the mean dopamine percentage of the basal level. There was a significant between-group difference in the dopamine levels (SS group: N = 6 rats; saline group: N = 6 rats; *P < 0.05, two-way analysis of variance followed by the least significant difference post hoc test). SS treatment significantly decreased the dopamine levels in the CPu between 1 and 4 hours after treatment (**P < 0.01, ***P < 0.001, vs. normal saline group, one-way analysis of variance followed by the least significant difference post hoc test). Data are presented as the mean ± SEM.
Effects of CPu stimulation on the SFR of Au1 neurons
We recorded the SFR of neurons in the Au1 after CPu electrical stimulation. Eighty-seven Au1 neurons were recorded from eight rats, and the mean basal SFR was 7.2 ± 0.8 spikes/s. The paired-samples t-test revealed a significant decrease in the SFR of Au1 neurons after CPu electrical stimulation, from 7.2 ± 0.8 spikes/s to 4.1 ± 0.5 spikes/s (drop rate of 43.5%; t = 6.541, P < 0.01; Figures 3 and 9).
Figure 9.
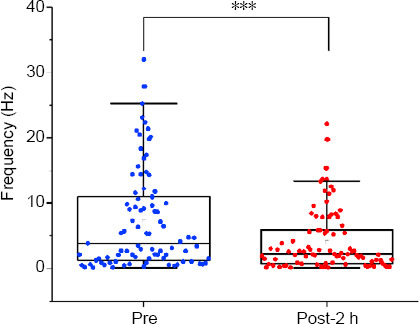
Effects of caudate-putamen nucleus (CPu) electrical stimulation on primary auditory cortex (Au1) neurons.
CPu electrical stimulation decreased the spontaneous firing rate of the Au1 from 7.2 ± 0.8 spikes/s to 4.1 ± 0.5 spikes/s. Data are presented as the mean ± SEM (N = 8 rats, n = 87 neurons). ***P < 0.001 (paired-samples t-test). E-post: After CPu electrical stimulation; Pre: before CPu electrical stimulation.
Discussion
This study investigated whether the CPu plays a potential regulatory role in tinnitus. To characterize the role of the CPu in SS-induced tinnitus in rats, we examined acoustic startle reflex behavior and performed electrophysiological and neurochemical tests. We obtained the following findings: (1) SS treatment significantly reduced the GPIAS with background noise at 12 and 16 kHz; (2) SS treatment significantly increased the SFR and decreased extracellular dopamine levels in the CPu, and (3) electrical stimulation of the CPu inhibited Au1 excitability. Overall, our findings indicate that the CPu plays an important role in tinnitus and may have a key role in sensory gating.
GPIAS is a behavioral screening method that can be used to validate the presence of tinnitus in SS-injected rats. In the present study, SS treatment significantly decreased the GPIAS at 12 and 16 kHz; this is consistent with previous findings (Yang et al., 2007; Wu et al., 2019). Conversely, there was no significant between-group difference in the GPIAS at 6 kHz. Moreover, the absolute startle amplitude did not differ significantly between the gap and no-gap conditions at 6 and 12 kHz after SS treatment. Tinnitus is a phantom auditory perception that can mask the gap in the acoustic startle reflex. Thus, the failure to detect a gap in background noise is considered evidence of tinnitus. We revealed that the tinnitus pitch in SS-treated rats was close to the frequency range of 12 to 16 kHz. Notably, in the GPIAS behavioral test, non-auditory systems (including the midbrain reticular formation, cuneiform nucleus, superior colliculus, striatum, and medial prefrontal cortex) as well as the auditory pathway are involved in startle inhibition (Azzopardi et al., 2018; Fulcher et al., 2020).
Several studies have suggested that tinnitus is caused by altered central structural and functional plasticity, and many studies have reported that the CPu is involved in tinnitus (Cheung and Larson, 2010; Larson and Cheung, 2012, 2013; Chen et al., 2017; Ahsan et al., 2018; Perez et al., 2019). In the present study, SS treatment significantly increased the neuronal SFR and decreased extracellular dopamine levels in the CPu, which indicates the involvement of the CPu in tinnitus. SS can cross the blood-brain barrier after intraperitoneal injection and suppress GABAergic inhibition, leading neural hyperactivity. However, SS also had an effect on dopaminergic regulation of the indirect pathway of basal ganglia, which may make the neuronal SFR recover after 2.5 hours. Further studies should investigate whether the increased SFR of CPu neurons is related to the release of dopamine and other neurotransmitters, including the excitatory neurotransmitter glutamate, the inhibitory neurotransmitter gamma aminobutyric acid, and several neuromodulators (such as ascorbic acid), and ions (such as Ca2+ , Mg2+). These neurotransmitters may be involved in the relevant electrophysiological processes and should therefore be further assessed.
Dopamine is an important neurotransmitter that modulates various physiological responses in the central nervous system. We revealed that SS treatment significantly decreased extracellular dopamine levels in the CPu. This suggests that SS-induced acute tinnitus causes dopamine suppression in this nucleus. In keeping with these findings, previous clinical studies have shown that dopaminergic agents may have a therapeutic effect on tinnitus (de Azevedo et al., 2009; Sziklai et al., 2011). The dopamine agonists piribedil (de Azevedo et al., 2009) and pramipexole (Sziklai et al., 2011) may alleviate tinnitus by regulating neuronal activity in the auditory pathway. In addition, the extrapyramidal system is regulated by neural circuits of the basal ganglia, and the substantia nigra/striatum dopaminergic system is an important link, with the striatum playing a central regulatory role. Decreased dopamine levels in the CPu may therefore affect the indirect basal ganglia pathway and decrease inhibition in the globus pallidus externa. Consequently, this decreased inhibition may enhance baseline subthalamic nucleus inhibition, reducing the inhibitory effect of the globus pallidus internus on the thalamus, in turn leading to increased Au1 excitability (Figure 10). However, this potential, complex mechanism requires further validation.
Figure 10.
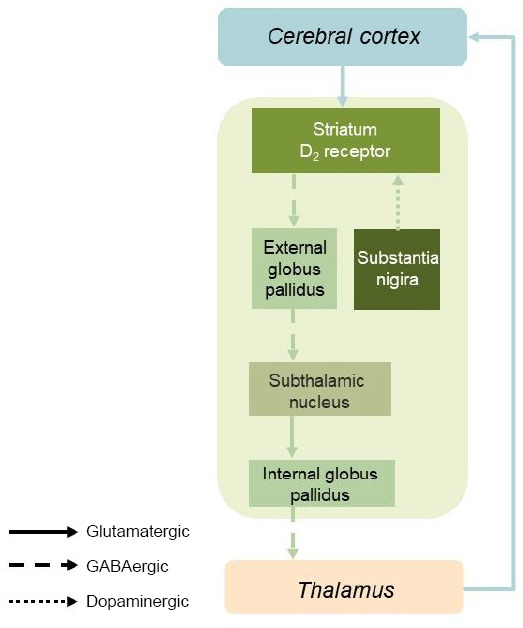
Neuroregulatory mechanism of the striatum–auditory cortex loop in the sodium salicylate-related tinnitus rat model.
GABA: γ-Aminobutyric acid.
The CPu receives comparable afferent fiber projections from the Au1 and anterior auditory field (Nakata et al., 2020). Increased SFR in the auditory cortex is a biomarker for tinnitus (Ochi and Eggermont, 1996; Kimura and Eggermont, 1999; Song et al., 2016; Ahsan et al., 2018). Furthermore, functional magnetic resonance imaging studies have shown that both auditory and non-auditory systems, including the CPu, are involved in tinnitus (Chen et al., 2015). We demonstrated that electrical stimulation of the CPu led to decreased SFR in Au1 neurons. This result is consistent with the findings of Ahsan et al. (2018), wherein DBS of non-auditory pathway structures (i.e., the anterior caudate) was able to reduce tinnitus through modulation of the auditory cortex. Therefore, tinnitus inhibition may be dependent on the mechanisms underlying the excitatory and inhibitory effects of DBS (McIntyre et al., 2004). Electrical stimulation of the CPu activates the indirect basal ganglia pathway, which increases the inhibitory effects on the globus pallidus externa. This, in turn, decreases suppression of the subthalamic nucleus, which in turn increases excitation of the globus pallidus internus. This leads to further inhibition of the thalamus and auditory cortex, which results in the observed decrease in the SFR of the Au1 neurons (Yamamoto et al., 2006; Graybiel, 2008; Ahsan et al., 2018; Figure 10).
To the best of our knowledge, this is the first study to report SFR and dopamine level changes in the CPu in a rat model of tinnitus. Our findings suggest the potential role of DBS of the CPu in the treatment of tinnitus. The major strength of the current study is that it provides accumulating evidence of the role of the basal ganglia in tinnitus and suggests a potential therapeutic target. Moreover, dopaminergic agents may be potential therapeutic agents for tinnitus. Given that a previous study suggested that improper DBS of the CPu increases the risk of tinnitus development and aggravation, it is crucial to determine the appropriate stimulus parameters and accurate stimulus location for this therapy (Larson and Cheung, 2012).
The present study has several limitations. First, the sample size was small; therefore, our findings are preliminary and must be confirmed by large-scale studies. Second, the electrophysiological and neurochemical experiments were performed under anesthesia. Although we tried to ensure a consistent anesthesia status across all animals, the anesthetic itself is likely to have affected the neuronal firing activity. Future studies should therefore be performed on neuronal firing activity in awake animals.
In conclusion, our findings support the premise that the CPu plays a key role in sensory gating in SS-induced tinnitus, and that dopamine receptor agonists may serve as a potential tinnitus treatment. This study is beneficial to explore the mechanism of tinnitus outside the classical auditory pathway. Abnormal changes in CPu electrophysiology and dopamine level provide animal experimental basis for the etiology diagnosis and treatment of tinnitus and indicate the need to explore the neural mechanistic role of the basal ganglia in tinnitus.
Additional file:
Additional file 1 (281.8KB, pdf) : Original data of the experiment.
Acknowledgments:
We would like to extend our thanks to Chinese Academy of Sciences for the excellent technique assistance and consistent support.
Footnotes
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 21790391 (to LQM) and 81870727 (to FRM). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Institutional Animal Care and Use Committee of PUHSC (approval No. A2010031) on December 6, 2017.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:This study was supported by the National Natural Science Foundation of China, Nos. 21790391 (to LQM) and 81870727 (to FRM).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Longworth-Mills E, GardnerB, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Ahsan SF, Luo H, Zhang J, Kim E, Xu Y. An animal model of deep brain stimulation for treating tinnitus: A proof of concept study. Laryngoscope. 2018;128:1213–1222. doi: 10.1002/lary.26876. [DOI] [PubMed] [Google Scholar]
- 2.Azzopardi E, Louttit AG, DeOliveira C, Laviolette SR, Schmid S. The role of cholinergic midbrain neurons in startle and prepulse inhibition. J Neurosci. 2018;38:8798–8808. doi: 10.1523/JNEUROSCI.0984-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142:959–965. doi: 10.1001/jamaoto.2016.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt JM, Bhattacharyya N, Lin HW. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope. 2017;127:466–469. doi: 10.1002/lary.26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai Z, Ma C, Jin X. Cortical stimulation for treatment of neurological disorders of hyperexcitability: a role of homeostatic plasticity. Neural Regen Res. 2019;14:34–38. doi: 10.4103/1673-5374.243696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YC, Chen GD, Auerbach BD, Manohar S, Radziwon K, Salvi R. Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress. Hear Res. 2017;349:208–222. doi: 10.1016/j.heares.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. 2015;4:e06576. doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC) Neuroscience. 2010;169:1768–1778. doi: 10.1016/j.neuroscience.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 9.de Azevedo AA, Langguth B, de Oliveira PM, Rodrigues Figueiredo R. Tinnitus treatment with piribedil guided by electrocochleography and acoustic otoemissions. Otol Neurotol. 2009;30:676–680. doi: 10.1097/MAO.0b013e3181ab8fd5. [DOI] [PubMed] [Google Scholar]
- 10.Ding YJ, Song Y, Liu JX, Du YL, Zhu L, Ma FR. Effect of neuronal excitability in hippocampal CA1 area on auditory pathway in a rat model of tinnitus. Chin Med J (Engl) 2018;131:1969–1974. doi: 10.4103/0366-6999.238148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Y, Liu J, Jiang Q, Duan Q, Mao L, Ma F. Paraflocculus plays a role in salicylate-induced tinnitus. Hear Res. 2017;353:176–184. doi: 10.1016/j.heares.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Eggermont JJ. Tinnitus: neurobiological substrates. Drug Discov Today. 2005;10:1283–1290. doi: 10.1016/S1359-6446(05)03542-7. [DOI] [PubMed] [Google Scholar]
- 13.Fulcher N, Azzopardi E, De Oliveira C, Hudson R, Schormans AL, Zaman T, Allman BL, Laviolette SR, Schmid S. Deciphering midbrain mechanisms underlying prepulse inhibition of startle. Prog Neurobiol. 2020;185:101734. doi: 10.1016/j.pneurobio.2019.101734. [DOI] [PubMed] [Google Scholar]
- 14.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 15.Grillner S, Robertson B, Kotaleski JH. Basal ganglia-A motion perspective. Compr Physiol. 2020;10:1241–1275. doi: 10.1002/cphy.c190045. [DOI] [PubMed] [Google Scholar]
- 16.Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204–1235. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- 17.Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife. 2016;5:e19103. doi: 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kameda K, Shono T, Hashiguchi K, Yoshida F, Sasaki T. Effect of tumor removal on tinnitus in patients with vestibular schwannoma. J Neurosurg. 2010;112:152–157. doi: 10.3171/2009.3.JNS081053. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Lee HJ, An SY, Sim S, Park B, Kim SW, Lee JS, Hong SK, Choi HG. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 2015;10:e0127578. doi: 10.1371/journal.pone.0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res. 1999;135:146–162. doi: 10.1016/s0378-5955(99)00104-5. [DOI] [PubMed] [Google Scholar]
- 21.Kraus KS, Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res. 2012;288:34–46. doi: 10.1016/j.heares.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Larson PS, Cheung SW. Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery. 2012;70:398–405. doi: 10.1227/NEU.0b013e3182320ab5. discussion 405-406. [DOI] [PubMed] [Google Scholar]
- 25.Larson PS, Cheung SW. A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J Neurosurg. 2013;118:192–194. doi: 10.3171/2012.9.JNS12594. [DOI] [PubMed] [Google Scholar]
- 26.Leaver AM, Seydell-Greenwald A, Rauschecker JP. Auditory-limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear Res. 2016;334:49–57. doi: 10.1016/j.heares.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longenecker RJ, Chonko KT, Maricich SM, Galazyuk AV. Age effects on tinnitus and hearing loss in CBA/CaJ mice following sound exposure. Springerplus. 2014;3:542. doi: 10.1186/2193-1801-3-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry LD, Eisenman LM, Saunders JC. An absence of tinnitus. Otol Neurotol. 2004;25:474–478. doi: 10.1097/00129492-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, Sun W. GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience. 2011;189:187–198. doi: 10.1016/j.neuroscience.2011.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manche SK, Madhavi J, Meganadh KR, Jyothy A. Association of tinnitus and hearing loss in otological disorders: a decade-long epidemiological study in a South Indian population. Braz J Otorhinolaryngol. 2016;82:643–649. doi: 10.1016/j.bjorl.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martz E, Jelleberg C, Dougherty DD, Wolters C, Schneiderman A. Tinnitus, depression, anxiety, and suicide in recent veterans: A retrospective analysis. Ear Hear. 2018;39:1046–1056. doi: 10.1097/AUD.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 33.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto Y, Katayama S, Shigematsu N, Nishi A, Fukuda T. Striosome-based map of the mouse striatum that is conformable to both cortical afferent topography and uneven distributions of dopamine D1 and D2 receptor-expressing cells. Brain Struct Funct. 2018;223:4275–4291. doi: 10.1007/s00429-018-1749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakata S, Takemoto M, Song WJ. Differential cortical and subcortical projection targets of subfields in the core region of mouse auditory cortex. Hear Res. 2020;386:107876. doi: 10.1016/j.heares.2019.107876. [DOI] [PubMed] [Google Scholar]
- 36.Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol. 2002;13:323–331. [PubMed] [Google Scholar]
- 37.Ochi K, Eggermont JJ. Effects of salicylate on neural activity in cat primary auditory cortex. Hear Res. 1996;95:63–76. doi: 10.1016/0378-5955(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press/Elsevier; 2007. [Google Scholar]
- 39.Perez PL, Wang SS, Heath S, Henderson-Sabes J, Mizuiri D, Hinkley LB, Nagarajan SS, Larson PS, Cheung SW. Human caudate nucleus subdivisions in tinnitus modulation. J Neurosurg. 2019 doi: 10.3171/2018.10.JNS181659. doi: 103171/201810JNS181659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puopolo M. The hypothalamic-spinal dopaminergic system: a target for pain modulation. Neural Regen Res. 2019;14:925–930. doi: 10.4103/1673-5374.250567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts LE. Neural plasticity and its initiating conditions in tinnitus. HNO. 2018;66:172–178. doi: 10.1007/s00106-017-0449-2. [DOI] [PubMed] [Google Scholar]
- 43.Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, Radziwon K, Auerbach BD. Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front Neurosci. 2016;10:621. doi: 10.3389/fnins.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedley W, Parikh J, Edden RA, Tait V, Blamire A, Griffiths TD. Human auditory cortex neurochemistry reflects the presence and severity of tinnitus. J Neurosci. 2015;35:14822–14828. doi: 10.1523/JNEUROSCI.2695-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12:150–160. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol. 2003;42:289–294. doi: 10.3109/14992020309078348. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Liu J, Ma F, Mao L. Diazepam reduces excitability of amygdala and further influences auditory cortex following sodium salicylate treatment in rats. Acta Otolaryngol. 2016;136:1220–1224. doi: 10.1080/00016489.2016.1204664. [DOI] [PubMed] [Google Scholar]
- 49.Sziklai I, Szilvássy J, Szilvássy Z. Tinnitus control by dopamine agonist pramipexole in presbycusis patients: a randomized, placebo-controlled, double-blind study. Laryngoscope. 2011;121:888–893. doi: 10.1002/lary.21461. [DOI] [PubMed] [Google Scholar]
- 50.Thomas Broome S, Louangaphay K, Keay KA, Leggio GM, Musumeci G, Castorina A. Dopamine: an immune transmitter. Neural Regen Res. 2020;15:2173–2185. doi: 10.4103/1673-5374.284976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trevis KJ, McLachlan NM, Wilson SJ. Psychological mediators of chronic tinnitus: The critical role of depression. J Affect Disord. 2016;204:234–240. doi: 10.1016/j.jad.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 52.Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62–86. doi: 10.1016/j.cpr.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Wu C, Stefanescu RA, Martel DT, Shore SE. Tinnitus: Maladaptive auditory-somatosensory plasticity. Hear Res. 2016;334:20–29. doi: 10.1016/j.heares.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, Bao W, Yi B, Wang Q, Wu X, Qian M, Zuo C, Huang Z. Increased metabolic activity and hysteretic enhanced GABA(A) receptor binding in a rat model of salicylate-induced tinnitus. Behav Brain Res. 2019;364:348–355. doi: 10.1016/j.bbr.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 55.Wu YN, Shen KZ, Johnson SW. Differential actions of AMP kinase on ATP-sensitive K(+) currents in ventral tegmental area and substantia nigra zona compacta neurons. Eur J Neurosci. 2017;46:2746–2753. doi: 10.1111/ejn.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong S, Song Y, Liu J, Du Y, Ding Y, Wei H, Bryan K, Ma F, Mao L. Neuroprotective effects of MK-801 on auditory cortex in salicylate-induced tinnitus: Involvement of neural activity, glutamate and ascorbate. Hear Res. 2019;375:44–52. doi: 10.1016/j.heares.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto K, Shishido T, Masaoka T, Katori Y, Tanaka S. Postoperative clinical results in cubital tunnel syndrome. Orthopedics. 2006;29:347–353. doi: 10.3928/01477447-20060401-14. [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


