Abstract
Frailty is a critical intermediate status of the aging process with a multidimensional and multisystem nature and at higher risk for adverse health-related outcomes, including falls, disability, hospitalizations, institutionalization, mortality, dementia, and Alzheimer’s disease. Among different frailty phenotypes, oral frailty has been recently suggested as a novel construct defined as a decrease in oral function with a coexisting decline in cognitive and physical functions. We briefly reviewed existing evidence on operational definitions of oral frailty, assessment and screening tools, and possible relationships among oral frailty, oral microbiota, and Alzheimer’s disease neurodegeneration. Several underlying mechanism may explain the oral health-frailty links including undernutrition, sarcopenia linked to both poor nutrition and frailty, psychosocial factors, and the chronic inflammation typical of oral disease. Oral microbiota may influence Alzheimer’s disease risk through circulatory or neural access to the brain and the interplay with periodontal disease, often causing tooth loss also linked to an increased Alzheimer’s disease risk. On this bases, COR388, a bacterial protease inhibitor targeting Porphyromonas gingivalis implicated in periodontal disease, is now being tested in a double-blind, placebo-controlled Phase II/III study in mild-to-moderate Alzheimer’s disease. Therefore, oral status may be an important contributor to general health, including Alzheimer’s disease and late-life cognitive disorders, suggesting the central role of preventive strategies targeting the novel oral frailty phenotype and including maintenance and improvement of oral function and nutritional status to reduce the burden of both oral dysfunction and frailty.
Key Words: biomarkers, cognitive frailty, dementia, diet, mild cognitive impairment, nutritional frailty, oral health, oral microbiota, periodontal disease, tooth loss
Introduction
In older age, a series of age-associated and subclinical conditions together with the development of stressors (i.e., chronic diseases, psychosocial burden, injuries) may worsen functional physiological decline of several systems, resulting in a homeostatic imbalance or frailty (Hoogendijk et al., 2019). In the aging process, frailty appears to be a critical intermediate condition at higher risk for several adverse health-related outcomes, including, disability, falls, hospitalization, institutionalization, all-cause mortality, and dementia. Frailty may be a unidimensional entity defined within a physical/biological dimension, or a non-specific multidimensional construct defined within different and interrelated domains (Hoogendijk et al., 2019; Lozupone and Panza, 2020). Multidimensional frailty may be identified by a deficit accumulation model to better define the multidimensional and multisystem nature of this condition, incorporating several candidate factors ranging from chronic disease, symptoms, and signs to abnormal laboratory values. These factors can be combined and divided by the total number of deficits yielding the so-called frailty indexes. However, after two decades of clinical research on frailty, no universally accepted operational definition, assessment tool, reference standard, clinical criteria or biological markers have been established, and extensive international efforts are underway to identify the best approach to the measurement of this condition. Notwithstanding this lack of a universal consensus, given its largely recognized multidimensional and multisystem nature, frailty may involve a series of domains, i.e., physical, cognitive, social, psychological/depressive, sensorial, and nutritional phenotypes that should be considered in its definition, prevention, and management (Panza et al., 2018). Five easily measurable physical components (i.e. involuntary weight loss, exhaustion, low grip strength, slow gait speed, and low physical activity) constitute the operational definition of the physical frailty unidimensional model, often associated to an increased risk of cognitive impairment/decline in older age, mild cognitive impairment, Alzheimer’s disease (AD)/AD neuropathology, non-AD dementias, and vascular dementia (Panza et al., 2018). Furthermore, in hospital- and population-based studies, different frailty indexes were also associated with the increased risk of developing cognitive impairment/decline in older age, all-cause dementia, and AD (Panza et al., 2018). Among these different frailty phenotypes, a novel construct has recently been suggested, i.e., oral frailty, defined as a decrease in oral function with a coexisting decline in cognitive and physical functions, that should be considered as a geriatric syndrome and routinely screened for in older age (Morley, 2020). In the present article, we briefly reviewed existing evidence on operational definitions of oral frailty and suggested assessment and screening tools for this novel construct. We also described possible relationships among oral frailty, oral microbiota, and AD neurodegeneration with some implications for the prevention and management of the oral frailty-AD interplay.
Search Strategy and Selection Criteria
We performed separate searches in the US National Library of Medicine (PubMed), Medical Literature Analysis and Retrieval System Online (MEDLINE), EMBASE, Scopus, Ovid, and Google Scholar databases to find original studies from the inception to October 10, 2020, picking the following terms to identify the risk exposure (poor oral health indicator(s) OR oral microbiota OR periodontal disease OR oral healthcare AND frailty OR frailty phenotype) combined with terms to determine the outcomes of interest [cognitive AND (impairment OR decline OR disorders) OR neurodegeneration OR Alzheimer’s disease OR dementia OR mild cognitive impairment OR preclinical Alzheimer’s disease OR prodromal Alzheimer’s disease]. There were no language restrictions on the search.
Oral Frailty: from Sarcopenia to Nutritional Frailty
The causes of frailty as a general concept are multifactorial and the changes in health status in older age may lead to increased dependency. In the present section, we examined postulated mechanisms through which poor oral health may lead to frailty. In older age, poor oral health is a condition highly prevalent (Murray Thomson, 2014). Furthermore, oral health problems have been associated with chronic diseases, multimorbidity (> 1 chronic disease), and physical dysfunction in older age (Tôrres et al., 2015). A positive association between frailty and poor oral health, in particular with a low number of natural teeth and impaired oral function, has been suggested by recent systematic reviews (Tôrres et al., 2015; Hakeem et al., 2019). On these epidemiological bases, in 2013, the new concept of oral frailty was introduced in Japan as a decrease in oral function (Watanabe et al., 2020). In particular, in 2020, the Japan Dental Association defined oral frailty as a series of phenomena and processes that lead to age-associated changes in various oral conditions (number of remaining teeth, oral hygiene, oral dysfunction, etc.) and coexisting decreased interest in oral health and reduced physical and mental reserve capacity (Watanabe et al., 2020). Therefore, an oral frailty increase may lead to eating dysfunction with consequently a deterioration of physical and cognitive functions (Watanabe et al., 2020).
Another possible definition of oral frailty was that encompassing difficulty in chewing associated with age-related changes in swallowing (presbyphagia) (Wakabayashi, 2014; Morley, 2020). In fact, in older age, presbyphagia refers to age-related changes in swallowing associated with a frailty status (Wakabayashi, 2014), with other physical frailty biomarkers, in particular sarcopenia, predicting early cognitive decline and oral frailty. Sarcopenia is a progressive, generalized disorder of skeletal muscles involving the combination of loss of muscle mass and loss of muscle function as well as muscle performance (Cruz-Jentoft and Sayer, 2019). Older sarcopenic people commonly showed also swallowing disorders (Kuroda and Kuroda, 2012). Recently, a consensus paper on the diagnosis of sarcopenia-related dysphagia from four Japanese scientific associations proposed the definition of sarcopenic dysphagia as caused by sarcopenia of the whole body and swallowing-related muscles (Fujishima et al., 2019). In a person with dysphagia, these criteria required generalized sarcopenia and a decline in oropharyngeal muscle mass, excluding other causes for sarcopenia. However, in the sarcopenic dysphagia definition, aging and secondary sarcopenia after inactivity, malnutrition, and disease (wasting disorder and cachexia) may be included (Fujishima et al., 2019). Moreover, decreased tongue pressure, a common condition in older age, was also associated with poor nutrition and sarcopenia-related dysphagia (Maeda and Akagi, 2015).
Frailty and different oral health conditions could be linked through a series of underlying mechanisms, including nutritional (dentition impact on the nutritional status), biological (association with chronic inflammation), and psychological (oral health impact on self-esteem and depression) pathways. Nutrition appears to be a central element in most frailty concepts, with total energy and protein intakes as major determinants of nutritional status. In older age, undernutrition, given its association with multimorbidity and all-cause mortality, is the main cause for concern rather than overnutrition. Moreover, a physiological decrease in appetite and food intake, leading to unintentional weight loss, is a frequent finding in older people (Zupo et al., 2020). A recent systematic review examining longitudinal studies that investigated the relationship between oral health and frailty development suggested that undernutrition was significantly related with frailty, associated to fewer functional teeth, decreased bite force, and dry mouth (Hakeem et al., 2019). Therefore, undernutrition and sarcopenia could be ascribed in a recently suggested frailty phenotype, i.e., nutritional frailty (Zupo et al., 2020). This frailty phenotype was firstly defined as a sudden and significant sarcopenia or an essential loss of physiologic reserves leading vulnerable older people to disability. However, at present, no operational definition has been proposed for the nutritional frailty phenotype. A consensus on phenotypic and etiologic criteria to be included in a possible operational definition of nutritional frailty could be reached exploring the nutritional domains/items of different frailty tools (Zupo et al., 2020). Poor oral health and oral frailty may be both predictors and markers for the wider construct of nutritional frailty. However, in a very recent Japanese population-based study, self-assessed oral function and the number of teeth were not related with nutritional intakes, while two oral health items such as brushing teeth at least twice a day and regular attendance of dental clinic were significantly and positively associated with nutritional intakes (Nomura et al., 2020). These findings may suggest that a recovery of oral functions by prosthodontic treatment could not modify the nutritional status, while nutritional instructions plus prosthodontic treatment may be effective in improving nutritional status in older age. Furthermore, sarcopenia could negatively modify the prognosis of many chronic diseases, including AD, given the existing link between sarcopenia-related declines and cognition (Panza et al., 2018). Sarcopenia could share a bidirectional relationship with cognition producing muscle dysfunction, slow gait, and cognitive dysfunction. In older age, these links suggested the coexistence of both cognitive and motor dysfunctions characterizing the proposed conditions of motoric cognitive risk syndrome, defined by slow gait plus cognitive complaints, and cognitive frailty, characterized by coexisting physical frailty and mild cognitive impairment (Panza et al., 2018). In short, several underlying mechanism may explain the oral health-frailty links including undernutrition due to an inability to consume a healthy diet, sarcopenia linked to both poor nutrition and frailty, psychosocial factors such as lack of self esteem and decreased quality of life, and the chronic inflammation typical of oral disease.
Oral Frailty: Operational Definitions and Screenings Tools
In the present section, we briefly reviewed existing evidence on operational definitions of oral frailty and suggested assessment and screening tools for this novel construct. At present, no universally accepted indexes or tools designed to evaluate the presence and severity of oral and maxillofacial frailty exist. Screening tools for oral frailty may include the D-E-N-T-A-L Questionnaire (Bush et al., 1996), based on a list of oral frailty components (dry mouth, tooth or mouth pain, eating difficulty, etc.). Recently, a definition of oral and maxillofacial frailty syndrome included five items of oral and maxillofacial dysfunctions, i.e., oral and maxillofacial movement disorders, chronic oral mucosal pain disorders, salivary gland hypofunction, taste disorders, and swallowing disorders (Nam et al., 2018). The severity of these dysfunctions constituted the evaluation of the oral and maxillofacial frailty construct (Nam et al., 2018). Another seminal study defined oral frailty as accumulated poor oral status suggesting six items as oral frailty components, i.e., three oral function tests (articulatory oral motor skill, chewing ability, and tongue pressure), dental status (the number of natural teeth), and two subjective assessments of oral functions (subjective difficulty in swallowing and subjective difficulty in eating tough foods) (Tanaka et al., 2018). Oral hypofunction was defined by the Japanese Society of Gerodontology as a presentation of seven oral signs and symptoms, i.e., five types of oral function declines such as motor function of tongue and lips, tongue pressure, occlusal force, chewing and swallowing and oral uncleanness and oral dryness. In this model, oral frailty was defined as a pre-stage of oral hypofunction (Minakuchi et al., 2018). However, the methods of evaluation used in this model require objective measures probably difficult to be used as a screening tool. Very recently, starting from the deficit accumulation model of frailty, the Oral and Maxillofacial Frailty Index has been proposed as a screening tool with good psychometric properties focusing on “functional limitation” and “discomfort or pain” in the oral and maxillofacial regions (Choi et al., 2020). The five items finally selected for the Oral and Maxillofacial Frailty Index were the necessity of water when eating dry food, difficulties in jaw or tongue movements, difficulties in chewing, difficulties in facial expression, and difficulties in speaking or pronunciation (Choi et al., 2020).
Very recently, both multidimensional frailty indexes (Bassim et al., 2020; Hakeem et al., 2020a, b; Noetzel et al., 2020) and the unidimensional physical frailty model (Hakeem et al., 2020c; Hironaka et al., 2020; Zhang et al., 2020) have been associated to a series of oral health items (decreased tongue pressure or maximum tongue pressure, low number of remaining teeth, decreased chewing ability, reduced articulatory oral motor skill, periodontal disease, presbyphagia, etc.) to propose possible oral frailty constructs in population- and hospital-based studies. In short, although, at present, there are no universally accepted indices or tools for evaluating the oral and maxillofacial frailty, in the last decade, some screening instruments and/or operational definitions including lists of oral frailty components have been suggested aiming to create a diagnostic framework for this novel frailty phenotype.
Oral Frailty, Oral Microbiota, and Alzheimer’s Disease Neurodegeneration
In this section, we examined the interplay between periodontal disease, generally due to oral bacteria and often causing tooth loss associated to AD risk, and oral microbiota, with a recognized role in the microbiota-gut-brain axis. Oral frailty components, including oral function, number of teeth, dentures, and occlusions have been associated with adverse health-related outcomes, in particular mortality risk (Watanabe et al., 2020) and AD/late-life cognitive disorders. In Figure 1, we depicted the bidirectional association between AD/late-life cognitive disorders and oral frailty components. Moreover, both tooth loss due to periodontal disease and irregular tooth brushing were associated with doubled AD risk (Fang et al., 2018) and higher dementia risk (Paganini-Hill et al., 2012). However, tooth loss may be associated to an increased risk of dementia also without the presence of periodontal disease. In fact, masticatory disorder due to tooth loss can lead to poor nutrition, so reducing cerebral blood flow, often linked to memory deficits (Fang et al., 2018). Moreover, a series of animal studies demonstrated that tooth loss may induce decreased acetylcholine levels due to masticatory dysfunction, leading to reductions in the number of pyramidal cells in the hippocampus, provoking cognitive dysfunction (Fang et al., 2018). There was also scarce evidence that periodontal disease may be associated with frailty development (Hakeem et al., 2020b). Frailty status may be influenced by periodontal disease through its association with inflammatory biomarkers, although there was only a weak association of frailty with the inflammatory pathway (Hakeem et al., 2019). Tooth loss due to periodontitis and its impact on food selection and nutritional status might also mediate the association between periodontal disease and frailty status. Periodontal disease is generally due to mouth bacteria infecting the tissue around the teeth and a growing interest has been recently placed in the role that oral microbiota may play in the microbiota-gut-brain axis (Panza et al., 2019; Goyal et al., 2021). Between dental plaque bacteria and the innate host defense system also exists a dynamic equilibrium and its perturbation may lead to dental caries and periodontal disease (Lamont et al., 2018). Oral bacteria accumulate in humans on both hard and soft oral tissues in biofilms (Kilian et al, 2016) and a series of clinical and preclinical studies showed an association between poor oral health and increased ability of oral microbiota to reach the brain, so affecting late-life cognitive function and increasing AD risk (Panza et al., 2019). In cognitively normal older subjects, clinical attachment loss (> 3 mm), representing a history of periodontitis, was associated with increased load of amyloid-β (Aβ), the principal neuropathological AD hallmark, in vulnerable brain regions (Kamer et al., 2015). Daily and transient bacteraemias due to periodontal disease can challenge the brain with intact bacteria and inflammatory mediators, while chronic periodontitis is also reported to double the AD risk (Panza et al., 2019). Leptomeningeal cells may transmit systemic inflammatory signals from macrophages to brain-resident microglia during chronic periodontitis (Olsen and Singhrao, 2015). Brain structures may be affected by oral pathogens through two mechanisms. In fact, proinflammatory cytokines may travel via the systemic circulation, or the central nervous system may be penetrated by periodontal bacteria or their products via the glossopharyngeal and/or trigeminal nerves (Kamer et al., 2008).
Figure 1.
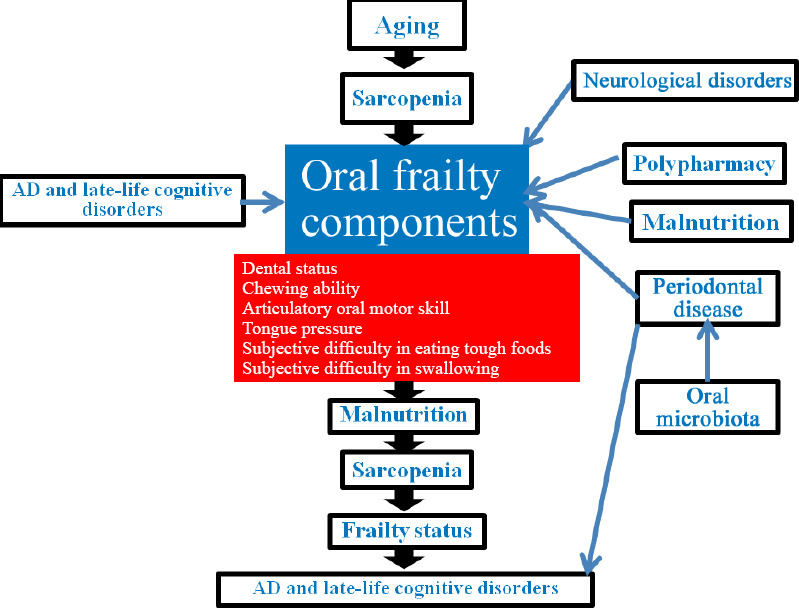
Bidirectional association between Alzheimer’s disease (AD)/late-life cognitive disorders and oral frailty components.
An increase in serum IgG antibodies due to periodontal pathogens has been associated with established or incident AD (Panza et al., 2019). In fact, in one study, 73% of plasma samples of AD patients were serum-positive for at least one of the pathogens tested [Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis (P. gingivalis), and Tannerella forsythia] compared to only 38% of control samples (Kamer et al., 2009). P. gingivalis is the main pathogen in chronic periodontitis, and toxic proteases from this bacterium (gingipains) were identified in AD brains, with levels correlating with protein tau, one of the neuropathological hallmarks of the disease, and ubiquitin pathology (Dominy et al., 2019). Furthermore, P. gingivalis has been associated also with increased systemic inflammation (Panza et al., 2019), and misregulated genes in infected macrophages matched those in the hippocampus of AD patients (Carter et al., 2017), suggesting a role for this pathogen in AD neurodegeneration.
In AD mouse models, periodontitis due to P. gingivalis caused an increase in brain Aβ deposition, higher inflammatory markers (inteleukin-1β and tumor necrosis factor-α), and an impairment of cognitive function in affected mice compared to controls (Singhrao et al., 2019). Of note, 10 wild-type 8-week-old C57BL/6 mice with an induced chronic periodontitis due to repeated oral application of P. gingivalis/gingipain for 22 weeks showed an AD-type neurodegeneration and the development of extracellular Aβ1–42 (Ilievski et al., 2018). Moreover, in apolipoprotein E gene knockout mice administered P. gingivalis was found diffuse punctuate staining suggesting tissue damage and appearance of age-related granules (Singhrao et al., 2017). In normal mice, brain exposure to lipopolysaccharide from P. gingivalis induced neuronal Aβ accumulation and learning/memory deficits (Wu et al., 2017), but continuous brain exposure of P. gingivalis lipopolysaccharide failed to cause cognitive impairment in another transgenic AD mouse model (Hayashi et al., 2019). On the other hand, COR388, a bacterial protease inhibitor targeting P. gingivalis (Arastu-Kapur et al., 2020), is now being tested in a double-blind, placebo-controlled Phase II/III study in 573 mild-to-moderate AD patients (NCT03823404, GAIN Trial). However, while P. gingivalis and other oral bacteria may represent a co-factor in AD pathogenesis, older demented subjects are more sensitive to oral pathogens and more prone to develop dental infections and therefore this putative causal association could be reversed (Panza et al., 2019). In short, oral microbiota may influence AD risk through circulatory or neural access to the brain and the interplay with periodontal disease, often causing tooth loss also linked to an increased AD risk.
Preventive Strategies for the Oral Frailty-Alzheimer’s Disease Interplay
In these conclusive remarks, given the recognized role of oral status as an important contributor to general health including AD and late-life cognitive disorders, we examined the potential impact of preventive strategies targeting the novel oral frailty phenotype and encompassing maintenance and improvement of oral function and nutritional status to possibly reduce the burden of both oral dysfunction and frailty. In fact, oral frailty, defined as a mild decline in oral function, could have the potential to be reversible in the early stage. Oral frailty prevention may also reduce medical and nursing care costs. Therefore, oral frailty can be identified as a pre-clinical construct that could be an important target for the prevention of adverse health-related outcomes such as falls, disability, institutionalization, hospitalization, increased all-cause mortality, dementia, and other neurodegenerative conditions in older people. In particular, there are strong links among dementia and AD and oral frailty components, i.e., dental status (the number of natural teeth) and oral function. Therefore, early detection and treatment of oral frailty may contribute to dementia prevention. Preventive strategies targeting the novel oral frailty phenotype should include the maintenance and improvement of oral function and nutritional status to reduce the burden of both frailty and oral dysfunction. At the community level, primary preventive intervention to delay frailty onset, as a general target, may include healthy diet promotion, increased physical activity and exercise, higher engagement in an active and socially integrated lifestyle, and body weight, metabolic, and vascular risk control (Zupo et al., 2020). In different settings, we have a lack of convincing evidence from high-quality studies on the best clinical management strategies targeting frailty, but nutrition and physical activity are the pillars for frailty prevention and management. Systematic reviews supported the role of increased physical activity and exercise interventions in improving gait and decreasing falls (Jadczak et al., 2018), while only preliminary evidence coming from randomized clinical trials or observational studies suggested that nutritional intervention could postpone frailty development in older people (Hernández Morante et al., 2019). In fact, a protective role of protein supplementation has been suggested against frailty development (daily 30 g protein), although excess protein can also be harmful and specific individual characteristics should be considered (Hernández Morante et al., 2019). Metanalytic evidence also suggested that protein supplementation plus resistance exercise training as well as multicomponent exercise training may be effective in improving frailty status, lean mass, muscle strength, and physical mobility in frail older individuals (Liao et al., 2018). This meta-analysis confirmed systematic review findings suggesting that multidomain interventions (i.e., two or more domains among physical exercise, nutritional, pharmacological, psychological, or social interventions) tended to be more effective than monodomain interventions on frailty status or score, muscle mass and strength, and physical functioning (Dedeyne et al., 2017).
Therefore, nutrition appears to be a targetable intervention with the potential to modulate frailty risk. Oral health is also a modifiable risk factor strictly linked to nutrition, with a consequent central role of oral health evaluation in frailty assessment. A series of oral health components, including articulatory oral motor skill, weak tongue pressure, fewer than 20 natural teeth, difficulties in eating tough foods, and difficulties in swallowing tea or soup are risk factors for physical frailty and sarcopenia, and disability (Tanaka et al., 2018). Improving oral health and consequently nutritional intake should be included in interventions to prevent or delay frailty onset. Total energy intake as well as the intake of dietary protein, calcium, fiber, iron, niacin, vitamin C and vitamin D may decrease with masticatory dysfunction (Sheiham et al., 2001). Moreover, the associations between oral function and sarcopenia (Murakami et al., 2015), masticatory and exercise function (Yamaga et al., 2002), and walking speed and oral function (Takata et al., 2004) may be central for the prevention of the adverse health-related outcomes associated to oral frailty.
In conclusion, oral health is a part of an individual’s general health status, and a multidisciplinary approach is needed to assess the contribution of oral health measures to specific conditions, in particular, AD and late-life cognitive disorders. The number of teeth may serve as a good marker for general health, reflecting the net accumulation of several experiences over time, from poor hygiene habits to the occurrence of caries, periodontal diseases, and trauma. Furthermore, teeth count is a clinical-friendly information that can be easily retrieved during the comprehensive geriatric assessment of older people, providing useful insights for the design of the most appropriate intervention, i.e., maintenance and improvement of oral function and nutritional status. In this context, in the next future, the general dental practitioner will have a greater impact in the prevention and maintenance of oral and general health status and will be less active with treatment. Poor oral health is therefore a marker for frailty onset, and given that the majority of older people at risk of developing frailty are in the community, the role of the general dental practitioner in identifying those at risk appears to be crucial. Maintaining or increasing oral function and so decreasing the impact of oral frailty may be associated with the improvement of dietary and functional status in older people, and may be implicated in reducing the mortality risk and other adverse health-related outcomes, including dementia and AD.
Additional file: Open peer review report 1 (77.1KB, pdf) .
Acknowledgments:
The authors thank the “Salus in Apulia” Research Team for the workplace. This manuscript is the result of the research work on frailty undertaken by the “Research Network on Aging” team, supported by the resources of the Italian Ministry of Health—Research Networks of National Health Institutes.
Footnotes
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Juana María Morillas-Ruiz, Universidad católica San Antonio de Murcia, Spain.
P-Reviewer: Morillas-Ruiz JM; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Arastu-Kapur S, Nguyen M, Raha D, Ermini F, Haditsch U, Araujo J, De Lannoy IAM, Ryder MI, Dominy SS, Lynch C, Holsinger LJ. Treatment of porphyromonas gulae infection and downstream pathology in the aged dog by lysine-gingipain inhibitor COR388. Pharmacol Res Perspect. 2020;8:e00562. doi: 10.1002/prp2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassim C, Mayhew AJ, Ma J, Kanters D, Verschoor CP, Griffith LE, Raina P. Oral health, diet, and frailty at baseline of the Canadian longitudinal study on aging. J Am Geriatr Soc. 2020;68:959–966. doi: 10.1111/jgs.16377. [DOI] [PubMed] [Google Scholar]
- 3.Bush LA, Horenkamp, Morley JE, Spiro A., 3rd D-E-N-T-A-L: a rapid self-administered screening instrument to promote referrals for further evaluation in older adults. J Am Geriatr Soc. 1996;44:979–981. doi: 10.1111/j.1532-5415.1996.tb01872.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter CJ, France J, Crean S, Singhrao SK. The Porphyromonas gingivalis/Host Interactome shows enrichment in GWASdb genes related to Alzheimer’s disease, diabetes and cardiovascular diseases. Front Aging Neurosci. 2017;9:408. doi: 10.3389/fnagi.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Kang JH, Koh SB, Kim NH, Kho HS. Development of an oral and maxillofacial frailty index: a preliminary study. J Oral Rehabil. 2020;47:187–195. doi: 10.1111/joor.12890. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 7.Dedeyne L, Deschodt M, Verschueren S, Tournoy J, Gielen E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging. 2017;12:873–896. doi: 10.2147/CIA.S130794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang WL, Jiang MJ, Gu BB, Wei YM, Fan SN, Liao W, Zheng YQ, Liao SW, Xiong Y, Li Y, Xiao SH, Liu J. Tooth loss as a risk factor for dementia: systematic review and meta-analysis of 21 observational studies. BMC Psychiatry. 2018;18:345. doi: 10.1186/s12888-018-1927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujishima I, Fujiu-Kurachi M, Arai H, Hyodo M, Kagaya H, Maeda K, Mori T, Nishioka S, Oshima F, Ogawa S, Ueda K, Umezaki T, Wakabayashi H, Yamawaki M, Yoshimura Y. Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int. 2019;19:91–97. doi: 10.1111/ggi.13591. [DOI] [PubMed] [Google Scholar]
- 11.Goyal D, Ali SA, Singh RK. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2021 doi: 10.1016/j.pnpbp.2020.110112. doi: 101016/jpnpbp2020110112. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Hasegawa Y, Takemoto Y, Cao C, Takeya H, Komohara Y, Mukasa A, Kim-Mitsuyama S. Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer’s disease. Exp Gerontol. 2019;120:1–5. doi: 10.1016/j.exger.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Hakeem FF, Bernabé E, Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36:205–215. doi: 10.1111/ger.12406. [DOI] [PubMed] [Google Scholar]
- 14.Hakeem FF, Bernabé E, Sabbah W. Self-rated oral health and frailty index among older Americans. Gerodontology. 2020a doi: 10.1111/ger.12513. doi: 101111/ger12513. [DOI] [PubMed] [Google Scholar]
- 15.Hakeem FF, Bernabé E, Sabbah W. Association between oral health and frailty among American older adults. J Am Med Dir Assoc. 2020b doi: 10.1016/j.jamda.2020.07.023. doi: 101016/jjamda202007023. [DOI] [PubMed] [Google Scholar]
- 16.Hakeem FF, Bernabé E, Fadel HT, Sabbah W. Association between oral health and frailty among older adults in Madinah, Saudi Arabia: a cross-sectional study. J Nutr Health Aging. 2020c;24:975–980. doi: 10.1007/s12603-020-1419-z. [DOI] [PubMed] [Google Scholar]
- 17.Hernández Morante JJ, Gómez Martínez C, Morillas-Ruiz JM. Dietary factors associated with frailty in old adults: a review of nutritional interventions to prevent frailty development. Nutrients. 2019;11:102. doi: 10.3390/nu11010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hironaka S, Kugimiya Y, Watanabe Y, Motokawa K, Hirano H, Kawai H, Kera T, Kojima M, Fujiwara Y, Ihara K, Kim H, Obuchi S, Kakinoki Y. Association between oral, social, and physical frailty in community-dwelling older adults. Arch Gerontol Geriatr. 2020;89:104105. doi: 10.1016/j.archger.2020.104105. [DOI] [PubMed] [Google Scholar]
- 19.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 20.Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O’Brien-Simpson NM, Reynolds EC, Watanabe K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 2018;13:e0204941. doi: 10.1371/journal.pone.0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16:752–775. doi: 10.11124/JBISRIR-2017-003551. [DOI] [PubMed] [Google Scholar]
- 22.Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ. Alzheimer’s disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis. 2008;13:437–449. doi: 10.3233/jad-2008-13408. [DOI] [PubMed] [Google Scholar]
- 23.Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamer AR, Pirraglia E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, Yi L, McHugh P, Craig RG, Svetcov S, Linker R, Shi C, Glodzik L, Williams S, Corby P, Saxena D, de Leon MJ. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015;36:627–633. doi: 10.1016/j.neurobiolaging.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda Y, Kuroda R. Relationship between thinness and swallowing function in Japanese older adults: Implications for sarcopenic dysphagia. J am Geriatr Soc. 2012;60:1785–1786. doi: 10.1111/j.1532-5415.2012.04123.x. [DOI] [PubMed] [Google Scholar]
- 27.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao CD, Lee PH, Hsiao DJ, Huang SW, Tsauo JY, Chen HC, Liou TH. Effects of protein supplementation combined with exercise intervention on frailty indices, body composition, and physical function in frail older adults. Nutrients. 2018;10:1916. doi: 10.3390/nu10121916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozupone M, Panza F. A multidimensional frailty approach in predicting and preventing dementia. Lancet Healthy Longev. 2020;2:e.49–50. doi: 10.1016/S2666-7568(20)30009-X. [DOI] [PubMed] [Google Scholar]
- 30.Maeda K, Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenia dysphagia in the elderly. Dysphagia. 2015;30:80–87. doi: 10.1007/s00455-014-9577-y. [DOI] [PubMed] [Google Scholar]
- 31.Minakuchi S, Tsuga K, Ikebe K, Ueda T, Tamura F, Nagao K, Furuya J, Matsuo K, Yamamoto K, Kanazawa M, Watanabe Y, Hirano H, Kikutani T, Sakurai K. Oral hypofunction in the older population: position paper of the Japanese Society of Gerodontology in 2016. Gerodontology. 2018;35:317–324. doi: 10.1111/ger.12347. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE. Editorial: oral frailty. J Nutr Health Aging. 2020;24:683–684. doi: 10.1007/s12603-020-1438-9. [DOI] [PubMed] [Google Scholar]
- 33.Murray Thomson W. Epidemiology of oral health conditions in older people. Gerodontology. 2014;31:9–16. doi: 10.1111/ger.12085. [DOI] [PubMed] [Google Scholar]
- 34.Nam Y, Kim NH, Kho HS. Geriatric oral and maxillofacial dysfunctions in the context of geriatric syndrome. Oral Dis. 2018;24:317–324. doi: 10.1111/odi.12647. [DOI] [PubMed] [Google Scholar]
- 35.Noetzel N, Meyer AM, Siri G, Pickert L, Heeß A, Verleysdonk J, Benzing T, Pilotto A, Barbe AG, Polidori MC. The impact of oral health on prognosis of older multimorbid inpatients: the 6-month follow up MPI oral health study (MPIOH) Eur Geriatr Med. 2020 doi: 10.1007/s41999-020-00427-7. doi: 101007/s41999-020-00427-7. [DOI] [PubMed] [Google Scholar]
- 36.Nomura Y, Ishii Y, Suzuki S, Morita K, Suzuki A, Suzuki S, Tanabe J, Ishiwata Y, Yamakawa K, Chiba Y, Ishikawa M, Sogabe K, Kakuta E, Okada A, Otsuka R, Hanada N. Nutritional status and oral frailty: a community based study. Nutrients. 2020;12:E2886. doi: 10.3390/nu12092886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease. J Oral Microbiol. 2015;7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paganini-Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: the Leisure World Cohort Study. J Am Geriatr Soc. 2012;60:1556–1563. doi: 10.1111/j.1532-5415.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 39.Panza F, Lozupone M, Solfrizzi V, Sardone R, Dibello V, Di Lena L, D’Urso F, Stallone R, Petruzzi M, Giannelli G, Quaranta N, Bellomo A, Greco A, Daniele A, Seripa D, Logroscino G. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis. 2018;62:993–1012. doi: 10.3233/JAD-170963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer’s disease. Brain. 2019;142:2905–2929. doi: 10.1093/brain/awz244. [DOI] [PubMed] [Google Scholar]
- 41.Singhrao SK, Chukkapalli S, Poole S, Velsko I, Crean SJ, Kesavalu L. Chronic Porphyromonas gingivalis infection accelerates the occurrence of age-related granules in ApoE-/- mice brains. J Oral Microbiol. 2017;9:1270602. doi: 10.1080/20002297.2016.1270602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol. 2019;11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka T, Takahashi K, Hirano H, Kikutani T, Watanabe Y, Ohara Y, Furuya H, Tetsuo T, Akishita M, Iijima K. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73:1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 44.Tôrres LH, Tellez M, Hilgert JB, Hugo FN, de Sousa MD, Ismail AI. Frailty, frailty components, and oral health: A systematic review. J Am Geriatr Soc. 2015;63:2555–2562. doi: 10.1111/jgs.13826. [DOI] [PubMed] [Google Scholar]
- 45.Wakabayashi H. Presbyphagia and sarcopenic dysphagia: association between aging, sarcopenia, and deglutition disorders. J Frailty Aging. 2014;3:97–103. doi: 10.14283/jfa.2014.8. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe Y, Okada K, Kondo M, Matsushita T, Nakazawa S, Yamazaki Y. Oral health for achieving longevity. Geriatr Gerontol Int. 2020;20:526–538. doi: 10.1111/ggi.13921. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Ni J, Liu Y, Teeling JL, Takayama F, Collcutt A, Ibbett P, Nakanishi H. Cathepsin B plays a critical role in inducing Alzheimer’s diseaselike phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun. 2017;65:350–361. doi: 10.1016/j.bbi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Yamaga T, Yoshihara A, Ando Y, Yoshitake Y, Kimura Y, Shimada M, Nishimuta M, Miyazaki H. Relationship between dental occlusion and physical fitness in an elderly population. J Gerontol A Biol Sci Med Sci. 2002;57:M616–620. doi: 10.1093/gerona/57.9.m616. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Ge M, Zhao W, Hou L, Xia X, Liu X, Zuo Z, Zhao Y, Yue J, Dong B. Association between number of teeth, denture use and frailty: findings from the west China health and aging trend study. J Nutr Health Aging. 2020;24:423–428. doi: 10.1007/s12603-020-1346-z. [DOI] [PubMed] [Google Scholar]
- 50.Zupo R, Castellana F, Bortone I, Griseta C, Sardone R, Lampignano L, Lozupone M, Solfrizzi V, Castellana M, Giannelli G, De Pergola G, Boeing H, Panza F. Nutritional domains in frailty tools: working towards an operational definition of nutritional frailty. Ageing Res Rev. 2020 doi: 10.1016/j.arr.2020.101148. doi: 10.1016/j.arr.2020.101148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


