Abstract
The high metabolic demands of the brain require an efficient vascular system to be coupled with neural activity to supply adequate nutrients and oxygen. This supply is coordinated by the action of neurons, glial and vascular cells, known collectively as the neurovascular unit, which temporally and spatially regulate local cerebral blood flow through a process known as neurovascular coupling. In many neurodegenerative diseases, changes in functions of the neurovascular unit not only impair neurovascular coupling but also permeability of the blood-brain barrier, cerebral blood flow and clearance of waste from the brain. In order to study disease mechanisms, we need improved physiologically-relevant human models of the neurovascular unit. Advances towards modeling the cellular complexity of the neurovascular unit in vitro have been made using stem-cell derived organoids and more recently, vascularized organoids, enabling intricate studies of non-cell autonomous processes. Engineering and design innovations in microfluidic devices and tissue engineering are progressing our ability to interrogate the cerebrovasculature. These advanced models are being used to gain a better understanding of neurodegenerative disease processes and potential therapeutics. Continued innovation is required to build more physiologically-relevant models of the neurovascular unit encompassing both the cellular complexity and designed features to interrogate neurovascular unit functionality.
Key Words: Alzheimer's disease, cerebrovasculature, in vitro model, neurodegeneration, neurovascular unit
Introduction
The high metabolic demands of the brain, which only accounts for ~2% of total body mass but consumes ~20% of total body oxygen and glucose, requires an efficient vascular system to be coupled with neural activity (Rolfe and Brown 1997; Attwell and Laughlin 2001). The coordinated action of neurons, glial and vascular cells, known collectively as the neurovascular unit (NVU), temporally and spatially regulates local cerebral blood flow (CBF) through a process known as neurovascular coupling. This process links glutamate release from neurons to nitric oxide secretion by interneurons that modulates vascular relaxation of nearby smooth-muscle cells (SMC) in the arterioles, as well as ATP-triggered calcium entry in astrocytes that regulate the release of arachidonic acid modulating vascular tone of adjacent pericytes in the capillaries (Attwell et al., 2010; Hall et al., 2014; Biesecker et al., 2016; Mishra et al. 2016). Specialized endothelial cells (EC) within the NVU form the blood-brain barrier (BBB) that restricts blood-brain exchange and regulates brain waste excretion (Sweeney et al., 2018). The structure of the NVU is depicted in Figure 1.
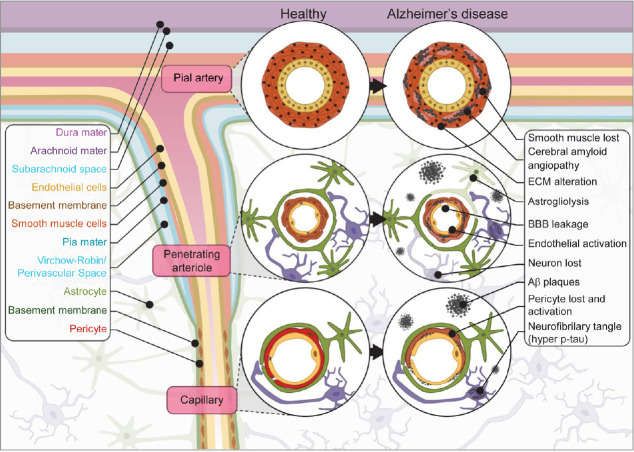
Figure 1.
NVU in health and AD.
Schematic representation of the NVU at the pial artery, penetrating arteriole and capillary levels in health and AD. Flowchart on the right depicts the changes associated with AD. AD: Alzheimer’s disease; Aβ: beta-amyloid; BBB: blood-brain barrier; ECM: extracellular matrix; NVU: neurovascular unit.
Neurodegenerative diseases such as Alzheimer’s disease (AD), affect million patients worldwide and cost billions of dollars every year (Gooch et al., 2017). Neuronal death and alteration in brain vascular health are pathological hallmarks common in all neurodegenerative diseases (Sweeney et al., 2018). Despite promising preclinical results, failure of drugs in recent clinical trials (Mehta et al., 2017), suggests that traditional monotypic cell culture and rodent models do not precisely replicate the human brain physiology. Among differences between rodent and human, there is differences between the white and gray matter and cortical volume ratio (Mota et al., 2019), mouse neural density is four times greater than humans and the length density m/mm3 about twice that of humans (Tsai et al., 2009). Moreover, mice are resistant to most neurodegenerative diseases (Lutz and Osborne, 2014). As such, there is tremendous interest in developing three-dimensional (3D) cell-based models to provide a more physiologically-relevant representation of the human BBB, brain parenchyma and NVU that can complement pre-clinical models. Furthermore, many of these new models can function as platforms for screening compounds to treat many neurological disorders (Choi et al., 2019). New models leveraging both knowledge of the NVU cellular complexity, and engineering solutions to assay NVU functions, would greatly improve our understanding of the interactions between neurons and the vasculature in physiological and pathophysiological conditions. In this concise review, we will first discuss the recent advances made to model in vitro the brain parenchyma, the cerebrovasculature or/and the NVU using either organoids, microfluidic devices or tissue engineering techniques. Then, we will discuss the advantages and disadvantages of models showing promise for studying neurodegenerative diseases. Finally, we will propose the cellular and engineering design features required of future, more physiologically-relevant human NVU models.
Search Strategy and Selection Criteria
Studies cited in this review were searched on PubMed, GoogleScholar or Mendeley databases using the following keywords: in vitro model, cerebrovasculature, blood-brain barrier, neurovascular unit, organoid, spheroid, microfluidic, neurodegenrative disease and Alzheimer’s disease.
Modeling the Brain Parenchyma Using Cerebral Organoids
Advances in pluripotent stem cell culture (Takahashi and Yamanaka, 2006), genome editing (Heidenreich and Zhang, 2016) and induced pluripotent stem cells (iPSC) differentiation protocols have provided important in vitro translational tools to improve our understanding of the biology of the brain parenchyma and vasculature in health and disease (Arber et al., 2017; Engle et al., 2018; Li et al., 2018; Mertens et al., 2018). Nevertheless, the limitations of 2D, monotypic cultures have been recognized when trying to model clinically relevant complexities of heterotypic cell-cell interactions and non-cell-autonomous disease processes (Engle et al., 2018). For example, the benefit of glial co-culture to neuronal maturity and activity (Burkhardt et al., 2013; Shi et al., 2013) and improved barrier function of EC when cultured with pericytes and astrocytes (Canfield et al., 2019b; Blanchard et al., 2020). In order to recapitulate the more complex cell-cell interactions found in the brain, 3D cultures of brain cells were developed to model more closely the architecture of the brain and are referred as organoids or spheroids.
In the presence of minimal media, cerebral organoids mimic the tissue architecture and physiological functions of the developing human brain in the micro- to millimeter range (Lancaster et al., 2013). Provision of extracellular matrix (ECM) proteins was a critical step in the development of 3D cortical forebrain cultures (Nasu et al., 2012; Kadoshima et al., 2013) and embedding neural spheroids in extracellular matrix such as Matrigel® matrix rapidly promoted the formation of polarized neural tube-like buds facilitating outgrowth of neuroepithelial cells (Lancaster et al., 2013). Similar to the developing brain, these organoids display increasing regional specification with distinct areas exhibiting dorsal cortical morphology, choroid plexus, and ventral forebrain identity. Additional specification occurs within dorsal cortical areas AUTS2+ cells marking prefrontal cortex areas and TSHZ2+ cells marking occipital lobe. Functional cortical neurons develop within the cerebral organoids capable of spontaneous calcium surges, and glutamatergic receptor activity in response to glutamate (Lancaster et al., 2013). Cerebral organoids develop stochastically giving rise to a range of neuronal cell types including dorsal and ventral forebrain, midbrain, hindbrain, and retina (Lancaster et al., 2013; Lancaster and Knoblich, 2014; Camp et al., 2015; Quadrato et al., 2017). The stochastic nature of cerebral organoid formation can produce variable cellular compositions between batches, lines and protocols. This cellular heterogeneity in brain organoids can be constrained by altering media composition to guide cells to a specific fate. For example, neuroectodermal fate can be patterned with dual SMAD inhibition yielding dorsal forebrain identities (Kadoshima et al., 2013; Qian et al., 2016) and the further addition of sonic hedgehog agonists gives rise to ventral forebrain organoids (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017). Similarly, dopaminergic organoids are generated with dual SMAD inhibition and sonic hedgehog agonists, with the addition of fibroblast growth factor 8, and a Wnt activator to generate Forkhead Box A2 positive floorplate precursors, which develop into tyrosine hydroxylase positive dopaminergic neurons (Qian et al., 2016).
An advantage of 3D brain organoids for in vitro modelling is the contribution of non-neuronal lineages that support neuronal function. Glia, including astrocytes and oligodendrocyte precursor cells, have been observed in organoids (Dezonne et al., 2017; Kim et al., 2019). Transcriptional analyses of astrocytes isolated from brain organoids show astrocytes transition from a fetal to more mature state over time, accompanied by an increased peak amplitude of intracellular calcium concentration in immature neurons from human cerebral cortical spheroids (Sloan et al., 2017). Oligodendrocyte maturity also transitions over time, developing the ability to interact with neurons and myelinated neuronal axons (Madhavan et al., 2018; Kim et al., 2019; Marton et al., 2019). Oligodendrocyte-containing spheroids display oligodendrocytes with extended processes interacting with neurofilament positive axons. Furthermore, myelin is observed ensheathing axons with varying levels of compaction (Madhavan et al., 2018; Kim et al., 2019; Marton et al., 2019). As yet, this myelination does not display a mature in vivo structural organization, notably lacking nodes of Ranvier (Madhavan et al., 2018). Unlike astrocytes and oligodendrocytes, microglia derive form the mesoderm lineage (Ginhoux et al., 2010). Microglia are the innate immune cells in the central nervous system however in addition to immune functions, they have roles in synapse formation, and synapse elimination (Miyamoto et al., 2013, 2016), making them key players for mature neuronal function studies. Recently Ormel et al. reported that microglia innately occur in iPSC-derived cerebral organoids after 66 days in culture (Ormel et al., 2018). In addition to the expression of the microglial marker Iba1, the organoid-derived microglia display characteristic ramified morphology and are able to mediate phagocytosis, with evidence of postsynaptic material within the organoid microglia.
Modeling the Cerebrovasculature Using Cerebral Organoids
One of the major limitations of organoid models is the presence of a necrotic core due to limited diffusion of oxygen and nutrients through the organoid (Lancaster et al., 2013, 2017; Qian et al., 2016). A way to overcome this challenge is to provide a microvascular component in 3D organoid cultures to deliver nutrients and oxygen, with the added advantage of expanding the applicability of organoids to study the BBB and NVU.
One approach to vascularize cerebral organoids is to engraft the organoids in animal models. Mansour and colleagues developed an in vivo model where human cerebral organoids made from human embryonic stem cells (hESC) were engrafted in immune-deficient NOD-SCID mouse brains (Mansour et al., 2018). The engrafted organoids developed a vasculature that is perfused with host blood and had reduced apoptosis. Further, once engrafted, organoids showed progressive neuronal differentiation patterns and maturation with growth of axons to multiples host brain regions, gliogenesis and presence of microglia. Together this suggests that incorporation of vasculature within in vitro 3D cerebral organoids may not only eliminate the necrotic core but also aid in the development of a mature system. Similarly, Pham et al. implanted cerebral organoids coated with EC derived from the same iPSC donor into immune-deficient mice and observed increased organoid vascularization (Pham et al., 2018). Overall, cerebral organoid engraftment in vivo overcomes the limitation of the necrotic core. However, in these models, the blood vessels are of partial murine origin reducing their relevance and translatability to the human BBB, and the protocols provided limited high-throughput production for drug testing.
Another approach to introduce a human vasculature system into in vitro 3D cerebral organoid models is through co-differentiation of EC with neural cell types. Upon treatment of hESC self-aggregated neural organoids with VEGF, enhanced vascularization is observed with cells expressing CD31 and the BBB tight junction marker claudin-5 without a significant reduction in neuronal markers (Ham et al., 2020). Further treatment with VEGF (25 ng/mL) and Wnt7a (10 ng/mL), which are involved in brain angiogenesis and pericyte mesenchymal differentiation, promoted tube-like structures surrounded by α-smooth muscle actin positive pericyte-like cells by 4 months in culture (Ham et al., 2020). Another co-differentiation method used ectopically expressed human ETS variant 2 (ETV2) under Doxycycline (dox)-induced promoter in a subset of hESC using lentivirus transduction. When 20% of the cells express ETV2, in the presence of dox a complex vascular network is formed in the cerebral organoids (Cakir et al., 2019). ETV2-induced EC demonstrated increased expression of the tight junction proteins zonula occludens-1, claudin-5 and occludin, increased expression of the nutrient transporters glucose transporter 1 and higher trans-endothelial electrical resistance (TEER). Moreover, the vasculature is functionally perfused when engrafted into immunodeficient Rag2–/– GammaC−/− mice (Cakir et al., 2019).
Another approach to vascularize 3D organoids in vitro is to co-culture cerebral and EC spheroids. Song et al. generated hybrid NVU spheroids by combining neural progenitor cell spheroids and EC spheroids with the additional supporting iPSC-derived mesenchymal stem cells (Song et al., 2019). This tri-spheroid culture shows markers of MAP2+ neurons, GFAP+ astrocytes and CD31+ EC as well as increased expression of the BBB marker glucose transporter 1, efflux transporter breast cancer resistant protein, and tight junction marker occludens-1. Moreover, these vascularized organoids expressed greater ECM proteins such as collagen IV, laminin, chondroitin sulfate proteoglycan and matrix remodelling proteins matrix metalloproteinase 2 and matrix metalloproteinase 3 (Song et al., 2019). Recently, Shi et al. (2020) generated vascularized organoids by co-culture of human umbilical vein EC with hESC. Over time, human umbilical vein EC displayed more BBB endothelial phenotypes with increased expression of p-glycoprotein efflux transporter. Furthermore, vascular organoids display greater chemical and electrical synaptic activity observed by spontaneous excitatory postsynaptic currents, spontaneous inhibitory postsynaptic currents and bidirectional electrical transmission compared to organoid lacking EC.
Increasing complexity of the 3D culture systems by the inclusion of vascular, neuronal and glia cell types brings greater relevance to the model systems, and enhances both vascular and neuronal physiology within the system (Song et al., 2019; Shi et al., 2020). For future studies, it remains to be seen if vascularized brain organoids are able to be perfused, independent of simple diffusion or animal implantation, and in a manner amenable to specifically assay the luminal/abluminal sides of the vessels. Furthermore, controlled perfusion will enable not only the possibility to perfuse the system with medium reducing the characteristic necrotic core in organoids but also permit the perfusion of blood cells, which play fundamental role both in healthy physiology and neurodegenerative disease pathophysiology (Zenaro et al., 2015; Baufeld et al., 2018). Other limitations of the cerebral organoid are their variability, however this could be extended to every complex 3D culture.
Modeling the Blood-Brain Barrier and Neurovascular Unit Using Microfluidic Platforms
While organoids predominantly focus on neuron-neuron or neuron-glial interaction with the aforementioned problems associated with the difficulties in perfusing potential vascular bed, many other groups have focused specifically on the vasculature and in particular the BBB. There is great interest in both academia and industry in developing experimental BBB models, with a particular focus on developing novel brain therapeutics that require BBB crossing. Historically, BBB models used static transwell systems where EC are cultured alone or with astrocytes and/or pericytes on a semipermeable membrane separating an upper and a lower chamber (e.g. (Canfield et al., 2019a; Jamieson et al., 2019)) Although this simple system is cost effective and user-friendly, the absence of luminal flow as well as the 2D structure do not recapitulate the complex architecture of the BBB and therefore limit the relevance of the results. Importantly, it has become clear that cells sense and respond to the dimensionality and rigidity of their environment, and such qualities cannot be modeled using 2D methods (Potjewyd et al., 2018). Multicellular spheroid systems consisting of human primary brain EC, primary pericytes and primary astrocytes spontaneously self-organize into a multicellular BBB-like structure with the glial cells in the middle and EC on the surface (Urich et al., 2013; Cho et al., 2017; Bergmann et al., 2018). In particular, Cho and colleagues generated BBB organoids expressing tight junctions, molecular transporters and exhibit drug efflux pump activity (Bergmann et al., 2018; Cho et al., 2017). Moreover, these organoids are amenable for drug penetration studies using confocal fluorescence microscopy and mass spectrometry imaging. However, these structures do not allow specific manipulations of the BBB to enable real-time monitoring of barrier integrity in drug delivery studies, nor are these models perfusable through a physical lumen. 3D dynamic in vitro BBB models, with the correct anatomical dimensions of the NVU, have distinct advantages over traditional, transwell and spheroid cultures due to the influence of perfusion conditions on cell behavior. EC and astrocytes were co-cultured in hollow fibers under luminal flow conditions (Cucullo et al., 2007). This model reproduces multiple functional properties of the BBB such as high TEER values and low penetrance of polarized molecules but the rigidity and the difficulties to image the cellular structure limits its relevance.
Recent advances in microfluidic technology have resulted in the development of novel BBB and NVU models (review in Oddo et al., 2019). A few studies developed 3D microfluidic BBB models consisting of EC with astrocytes and pericytes originating from different species (Griep et al., 2013; Prabhakarpandian et al., 2013; Campisi et al., 2018). While these studies better recapitulate the physiology of the brain capillary, they lack neurons. Adriani et al. (2017) engineered the first 3D neurovascular microfluidic model consisting of rat neurons and glial cells with human cerebral EC. In this model, the cells are physically organized in layers using a four-channel chip with direct contact between the channels. The neurons and astrocytes were seeded in specific collagen-I based hydrogels in the two middle channels, EC were seeded in the channel on the astrocyte side and the fourth channel served to provide nutriment to the neurons. Although the EC permeability is 2 × 10–5 cm/s using 10 kDA dextran and neuron have neurite outgrowth and activity upon KCl stimulation using the calcium dye X-Rhod-1, this study mixed species reducing the relevance of the model (Adriani et al., 2017). Human neurons were integrated in a sandwich microfluidic device where primary brain microvascular EC line a microfluidic channel with a polycarbonate filter membrane with 0.2 μm pores separating the brain chamber composed of primary astrocytes and pericytes and iPSC derived neurons cultured in collagen-I (Brown et al., 2015). Although authors reported EC barrier formation using both a four probes custom-built impedance system to measure TEER and 10 kDa FITC dextran, they did not provide physiological validation of neuronal function. Recently Maoz and colleagues developed a linked organ-on-chip NVU system consisting of a BBB chip (primary human brain EC, pericytes and astrocytes) linked via cerebrospinal fluid perfusion (0.0007 dyne/cm2 , 0.06 mL/h) to a brain chip composed of hippocampal stem cell derived neurons and primary astrocytes (Maoz et al., 2018). Under luminal flow conditions (0.02 dyne/cm2 , 0.06 mL/h), the BBB was impermeable to both BSA (66.5 kDa) and fluorescent Cascade blue (530 Da) and recovered after methamphetamines challenge. Although there is a metabolic coupling between BBB and the neurons, this model lacks anatomical fidelity as the BBB and neurons are separated in different chips with EC and astrocytes/pericytes further separated by a porous (0.4 µm) polyethylene terephthalate membrane. Vatine and colleagues used a sandwich microfluidic device where iPSC-derived EC were cultured in a perfusable channel with a polydimethylsiloxane porous membrane (7 μm pores) separating the brain chamber composed of iPSC-derived neurons and astrocytes (Vatine et al., 2019). The authors demonstrated BBB function using both luminal circulated 3 kDa FITC-dextran (0.03 mL/h), and modified chips with gold electrodes on both sides of the porous membrane. In this sandwich model, the TEER measures were as high as 1500 Ω/cm2 two days after seeding corresponding to the lower limit of reported in vivo BBB resistance (1500–8000 Ω/cm2) (Wolff et al., 2015). Further using the cell permeable calcium dye Fluo-4 and confocal microscopy, the authors showed that neurons in the chip exhibit spontaneous activity that was inhibited by the sodium channel blocker tetrodotoxin indicating that the chip environment supports neuronal synaptogenesis and network formation. Further, this model allows the use of patient derived iPSCs and the possibility to flow patient blood through the circulation channel opening avenues to study neurodegenerative diseases and personalized medicine.
Due to the nature of microfluidic chip production using soft lithography, perfusion channels are rectangular resulting in non-uniform flow and a shear stress profile which alters EC behavior (van der Helm et al., 2016). In order to generate a circular channel, several groups have used micro-needles placed into a collagen gel. Once the gel polymerized the needle is removed to generate a hollow channel that is lined with EC (Chrobak et al., 2006; Kim et al., 2015a). Other groups have used viscous fingering to create a hollow channel in a collagen-I matrix containing glial cells and pericytes (Herland et al., 2016). Although these techniques generate a single circular vascular channel, there is as yet no study demonstrating the ability to culture functional human neurons in these systems. The use of collagen-I represents another limitation, as although collagen-I is present in the cerebrovasculature, the brain parenchyma ECM is composed of hyaluronan, chrondroitin sulphate proteoglycan and tenascin R (Lau et al., 2013). Finally, the group of Li Jeon used a parallel microfluidic chip design where EC grow via angiogenesis from a perfusion channel into a channel filled with ECM (e.g. fibrinogen, collagen or Matrigel® matrix) containing neurons and glia (Bang et al., 2017). Because this system relies on angiogenesis, flow dynamics in the vascular bed are not altered like in rectangular channels. Furthermore, this model promoted cell-cell interactions as there is no porous membrane separating the vascular and the brain chambers. Recently this system was further improved in a high throughput 96 well plate format (Lee et al., 2019). These systems hold great promise but so far have been generated using cells from different species. Osaki et al. (2018) generated a fully humanized model using a parallel chip design where iPSC-derived EC formed a perfusable vascular network in a porcine skin collagen-I matrix containing iPSC-derived neurons. They further demonstrated the formation of a low permeability BBB using 40 kDa fluorescent dextran and neuronal function via calcium imaging. Because these models rely on EC angiogenesis, the vascular network and the flow might be highly variable between chips and reproducibility remains to be demonstrated.
Development of In Vitro Neurodegenerative Models
In the last decade, several groups have made considerable efforts to develop human in vitro models intended to study neurodegenerative diseases in particular for AD (Slanzi et al., 2020). Among all these models only a handful recapitulate the 3D complexity of human tissues. Choi and colleagues first described a model made of the immortalized human neural progenitor cell line ReNcell VM that overexpressed the amyloid precursor protein and/or presenilin 1 (PSEN1) with amyloid precursor protein (K670N/M671L and V717I) and PSEN1 (ΔE9) commonly known as early onset familial AD (FAD) mutations (Choi et al., 2014; Kim et al., 2015b). ReNcell were grown in thick (1:1) or thin (1:10–20 cell:Matrigel® matrix) 3D culture on transwell or regular 24 or 96 well plates. After 10–14 weeks, ReNcell showed both extracellular beta-amyloid (Aβ) plaque accumulation and phosphorylated Tau (pTau) deposition in neuron soma and neurites, two pathological hallmarks of AD. ReNcell derived neurons were also used to generate neurospheroids within arrays of microwells, where they produced both Aβ plaques and hyperphosphorylated tau protein after 8 weeks in culture (Jorfi et al., 2018). This model presents undeniable progress for drug screening against amyloid plaques and pTau deposition but lacks non-neuronal cell types. In 2018, Park et al. used the ReNcell with the FAD mutations and the microglial human cell line SV40 to generate a 3D tri-culture model composed of neurons, astrocytes and microglia to study AD (Park et al., 2018). ReNcell were seeded and differentiated into neurons and astrocytes in Matrigel® matrix at a ratio of 1:1 or 1:5 in a homemade polydimethylsiloxane well with 50 μm side micro-channels for microglial recruitment from the outside of the well. Unlike previous ReNcell models, neuron functionality was confirmed using calcium imaging using microscopy. After 3-9 weeks of culture, microglia were seeded on the outside of the well and maintained in culture for up to 6 days to allow recruitment and migration via the micro-channels. In addition to Aβ plaque formation and pTau accumulation, the authors reported microglial recruitment, nitric oxide release and axonal cleavage as a result of neuroinflammation. The lack of vasculature and circulating monocyte in this model might under report the role of immune cells in AD as in addition of resident microglia, blood-circulating monocytes infiltrate the brain and participate in the immune response (Maleki and Rivest, 2019). Recently Shin and colleagues developed a five channels microfluidic AD model composed of neurons and astrocytes differentiated from ReNCell with FAD mutations and co-cultured with the BBB cell line hCMEC/D3 (Shin et al., 2019). Using this model, they showed several aspects of BBB dysfunction in AD including increased BBB permeability, matrix metalloproteinase 2 level, and reactive oxygen species (ROS), decreased tight junction expression, and deposition of Aβ in the vasculature. Although these studies represent undeniable progress toward novel human in vitro models, these studies required immortalized cells lines and the overexpression of FAD mutations which may both limit the relevance of the models as cells lines do not always recapitulate primary cells (Kaur and Dufour, 2012) and FAD mutations only account for 1% of total AD cases (Lane et al., 2018).
To circumvent the limitation associated with cell lines, Lee et al. generated iPSC from blood cells of five AD patients before differentiating them into neurospheroids composed of both neurons and astrocytes (Lee et al., 2016). More recently, a physiological 3D model of AD was described in which neural areas with a cortical-like organization are generated from fibroblast-reprogrammed iPSCs donated by an adult with FAD mutation (PSEN1 A246E) and Down syndrome patients using the protocol described by Lancaster and Knochblich (Gonzalez et al., 2018). After 110 days in culture aggregated Aβ plaques and hyperphosphorylated tau protein spontaneously accumulate in these highly complex cerebral organoids. The large-scale application of such patient derived organoid models might be limited in the future due to the laborious procedures, high costs and time associated with their generation.
Recently, Blanchard and colleagues developed a 3D, self-assembled BBB to study cerebral amyloid angiopathy (CAA) and AD (Blanchard et al., 2020). Briefly, iPSC-derived astrocytes, pericytes and endothelial cells were encapsulated in Matrigel® matrix and self-organized over four weeks into a capillary bed. In this model, ECs form vessel-like structures, mural cells migrate to positions proximal to the vessel, and aquaporin-4 positive astrocytes surround the vessels and extend GFAP+ projections into the perivascular space. Additionally, the authors demonstrated the BBB cultures remodel the ECM, acquiring laminin-4 found in the BBB basement membrane (BM) - the site of amyloid deposition in capillary CAA. The anatomy of this self-assembled 3D model recapitulates the BBB, however it is unclear if the vessels are perusable and therefore limits its usage with respect to vessel biology. In parallel to the development of spheroid, organoid and microfluidic models, bioengineering techniques were used to generate perfusable vessel models. We developed a 3D human perfusable model of large cerebral vessels to study CAA and Aβ-associated vascular inflammation. This model consists of primary human EC, SMC and astrocytes, seeded on a tubular biodegradable scaffold and cultured in a bioreactor with the vascular tissue separating a “brain” like chamber and a “blood” like chamber (Robert et al., 2017). Although this model was generated with umbilical EC and SMC, the endothelium developed a BBB like phenotype with Claudin-5 and glucose transporter 1 expression. To recapitulate the CAA conditions, recombinant Aβ40 and Aβ42 are added to the brain chamber medium, thus studying Aβ accumulation and transport through the vascular wall. Since Aβ activates endothelial cells increasing leukocyte binding and transmigration, this 3D biomimetic model was also used to study Aβ-induced peripheral blood monocyte adhesion to the human endothelium (Robert et al., 2017). This bioengineered model was recently used to mechanistically understand how blood-circulating high-density lipoprotein promotes cerebral vascular health in AD by reducing amyloid beta (Aβ) vascular accumulation and Aβ-induced monocyte binding to the endothelium (Robert et al. 2020). However, this model lacks neurons and relies solely on exogenous recombinant Aβ, and thus, is limited in its ability to study the role of neuronal biology and function. Recently, we successfully expanded this platform to generate a model of the arterial neurovascular unit composed of primary human EC, SMC, and astrocytes cultured in the presence of apical human iPSC-derived glutamatergic cortical neurons. In this novel model, Aβ is produced by iPSC neurons and transported through the vascular wall where they accumulated gradually with Aβ40 accumulating the strongest. In addition to Aβ, pTau also accumulated in the vascular wall further endorsing this system as a potential viable AD model (Robert et al., 2020).
Taken together, these studies summarized in Table 1 clearly demonstrate how human based 3D in vitro models might revolutionize the mechanistic investigation of neurodegenerative diseases, and facilitate the development of associated therapeutics.
Table 1.
AD model summary
| Structure | Advantages | Limitations | References |
|---|---|---|---|
| Cortical model based on 3D hydrogel gel (neurons and astrocytes) | • Deposition of amyloid plaques | • Absence of vascular cells | Choi et al. (2014); Kim et al. (2015b); Jorfi et al. (2018) |
| • Accumulation of pTau | • Absence of flow | ||
| • High-throughput possible | • Cell lines with FAD mutations | ||
| • Short time of culture | |||
| Cortical model based on 3D hydrogel gel (neurons, astrocyte and microglia) | • Deposition of amyloid plaques | • Absence of vascular cells | Park et al. (2018) |
| • Accumulation of pTau | • Absence of flow | ||
| • Microglial migration assay | • Cell lines | ||
| • Short time of culture | |||
| NVU capillary model based on microfluidic chip (EC, astrocytes and neurons) | • CAA and amyloid plaque deposition | • Cell lines | Shin et al. (2019) |
| • Live imaging | • Absence of mural cells | ||
| • Vascular flow | • Non-contractile | ||
| • EC permeability assays | |||
| • Short time of culture | |||
| Cortical model based on organoids (neurons and astrocytes) | • IPSC from AD patient | • Low through put | Lee et al. (2016); Gonzalez et al. (2018) |
| • Deposition of amyloid plaques | • Long time of culture | ||
| • Accumulation of pTau | • Absence of vascular cells | ||
| • Apoptosis assay | • Absence of flow | ||
| Capillary model based on 3D hydrogel (EC, pericytes and astrocytes) | • IPSC from patient | • No perfusion | Blanchard et al. (2020) |
| • CAA | • Exogenous Ab | ||
| • Deposition of BM | • Absence of neuron | ||
| • Self-organization of the vasculature | • Non-contractile? | ||
| • Short time of culture | |||
| Arterial model based on tissue engineering (EC, SMC and astrocytes) | • CAA | • Recombinant Ab | Robert et al. (2017) |
| • Vascular flow | • Absence of neuron | ||
| • Trans-endothelial transport | • Low throughput | ||
| • Primary cells | • Long time of culture | ||
| • Monocyte perfusion | • Non-contractile | ||
| • Live media sampling in lumen and ablumen | |||
| NVU arterial model based on tissue engineering (EC, SMC, astrocytes and neurons) | • CAA | • Low throughput | Robert et al. (2020) |
| • Accumulation of pTau | • Long time of culture | ||
| • Neuronal and glial biomarkers | • Non-contractile | ||
| • Trans-endothelial transport | • Umbilical origin of the vascular cells | ||
| • Vascular flow | |||
| • Live media sampling in lumen and ablumen |
3D: Three-dimensional; AD: Alzheimer’s disease; Aβ: amyloid beta; BM: basement membrane; CAA: cerebral amyloid angiopathy; EC: endothelial cells; FAD: early onset familial AD; IPSC: induced pluripotent stem cells; NVU: neurovascular unit; pTau: phosphorylated Tau; SMC: smooth-muscle cells.
Towards Improved Models of the Neurovascular Unit
The use of in vitro models to study in vivo physiology and disease processes comes with limitations. Acknowledging that not every model is suited to answer every biological question, has thus far demanded the use of complementary systems to build a complete picture of disease mechanisms (Blanchard et al., 2020). As we look to the future, we now envisage the biological and engineering components of new models as we progress towards an ideal NVU model.
Increased cellular complexity in future models will enhance the physiological relevance, and better recapitulate the anatomical and functional features of the NVU. In existing systems, inclusion of vascular and parenchymal cell types enhances both vascular and neuronal physiology (Song et al., 2019; Shi et al., 2020). To advance cerebrovascular models, vessels formed of EC need to be structurally supported by appropriate mural cells, such as pericytes in the capillary and SMC in larger vessels, which regulate EC gene expression, barrier permeability and CBF (Armulik et al., 2010; Vanlandewijck et al., 2018; Liu et al., 2020). In addition to mural cells, astrocytes should form endfeet structures to interact with the vessels as they regulate lumen dilation and constriction to control blood flow (Iadecola and Nedergaard, 2007; Masamoto et al., 2015). Support for the interactions of EC, mural cells and astrocytes is provided also by BM proteins collagen IV, laminin, nidogen and perlecan (Xu et al., 2019). Notably in AD, changes to the BM are observed including deposition of Aβ, BM thickening, and alteration in BM protein composition (Thomsen et al., 2017). Therefore, systems incorporating relevant BM will be able to investigate mechanisms of these disease related changes.
Brain EC show a gradual gene expression from the arteries, capillaries and veins (Vanlandewijck et al., 2018) and like other cells, EC gene expression changes while aging (Ximerakis et al., 2019). In addition to specific tight junction (Claudin 5, etc.), which are often used to define the specificity EC in in vitro models, brain EC express a wide array of polarized transporters reflecting the importance of trans-BBB transport (Barar et al., 2016). Further characterisation of EC will therefore improve the relevance of future models. In addition, EC are due to their anatomical localization, directly exposed to CBF and shear stress and neurodegenerative disease alter CBF. In particular, shear stress directly regulates the structure and functions of EC and pathological shear stress causes endothelial dysfunction (Colgan et al., 2007). For example, within capillaries blood flow rate typically range between 6 to 12 nL/min with a shear stress ranging from 10 to 20 s/cm2 for a capillary of 10 μm (Wong et al., 2013). Systems modeling native like blood flow and shear stress will further enhance the translatability to human.
A highly important aspect for these complex cellular models is to ensure reproducibility. The origin of primary cells and the differentiation protocols for stem cells will need to be standardized. Higher reproducibility might also be achieved in the upcoming years with companies offering differentiation kits for the generation of organoids (e.g. StemCell, Vancouver Canada), selling premade organoids (e.g. Neurix, Geneva, Switzerland) or producing ready to seed microfluidic devices for low (e.g. Emulate Boston, USA or Aim Biotech, Singapore) and high throughput (e.g. Mimetas, Leiden Netherland) research.
With the increasing cellular complexity and therefore relevant physiology of future models, engineering and design solutions to interrogate enhanced functionality of models will be key to maximizing biological readouts. Devices to support the use of imaging allows leveraging of the numerous fluorescent based assays for function, from basic protein expression using immunocytochemistry, to fluorescent calcium indicators for signal transduction (e.g. Fura-2), and organelle trackers (e.g. Mitotracker, Lysotracker). Tractable perfusion of vessels is of high importance and the addition of sensor devices opens up models to in line measurements of flow, pressure, sheer stress, compliance and vascular integrity (Webb et al., 2007; Booth and Kim, 2012; Laterreur et al., 2014; Tsai et al., 2015; Varma and Voldman, 2015). Designs that include ports will permit sampling of analytes from perfused vessels, or the parenchyma (Robert et al., 2017b). Finally, thoughtfully designed electrodes are useful for live measurements of TEER, chemical and biological molecules quantification at the nanoscale or electrophysiological studies of neuronal activity (Schaffhauser et al., 2011; Rackus et al., 2015; Mahshid et al., 2017; Liu et al., 2018; Vatine et al., 2019). A schematic of the propose features is depicted in Figure 2. We anticipate as more commercial devices become available, these models will become user-friendly and cost-effective for use in most research facilities.
Figure 2.
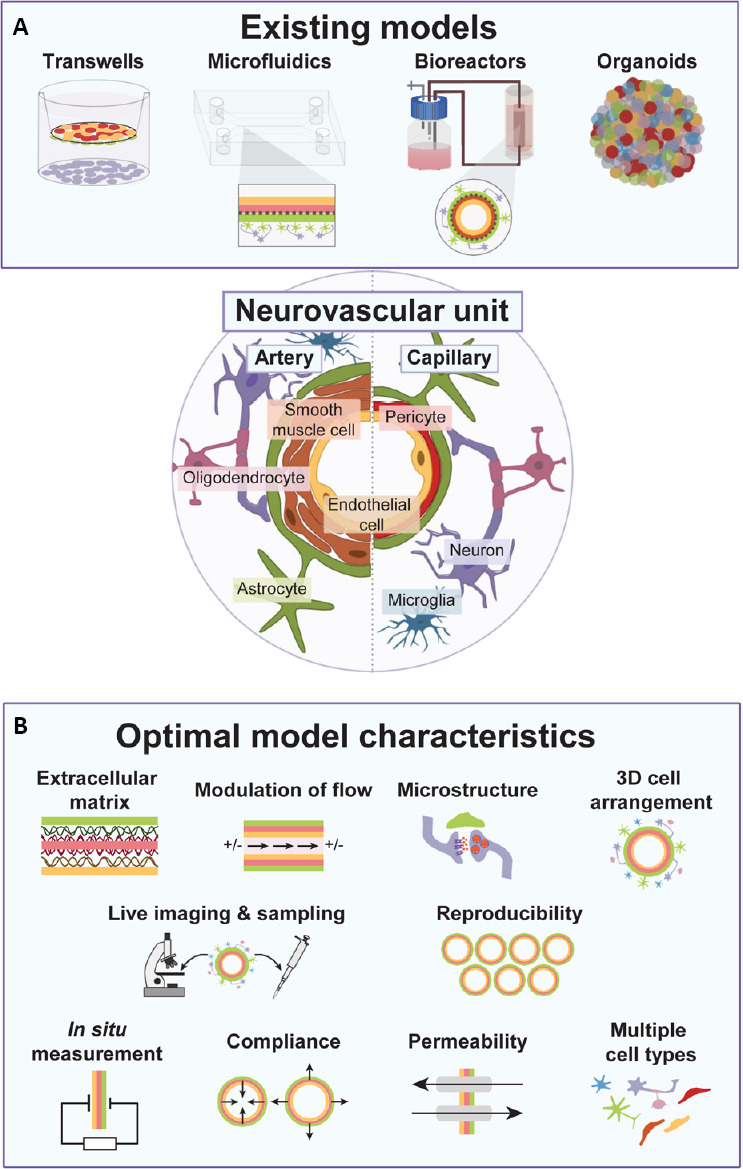
In vitro models of the NVU.
(A) Current NVU model types. (B) Proposed features for future in vitro models of the NVU. NVU: Neurovascular unit.
Summary
As we progress towards more physiologically-relevant models of the NVU, innovations drawn from advanced cell culture systems, and novel engineering designs will drive our understanding of the biology and pathophysiology of the NVU. With increased fidelity, it is hoped model systems will be better positioned to identify disease mechanisms and treatment targets, as well as inform which therapeutics will be effective in vivo.
Additional file:Open peer review reports 1 (103.2KB, pdf) , 2 (97KB, pdf) , and 3 (96KB, pdf) .
Footnotes
Conflicts of interest:JR has a patent (USA 10451637) named “synthetic blood vessels and uses thereof” for three models described in the “Development of in vitro neurodegenerative models” part. The authors declare no other competing interest.
Financial support:This work was supported by the Weston Brain Institute Rapid Response Grant, No. RR182093 (to JR).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewers:Baruh Polis, Bar-Ilan University, Israel; Alexey Petrov, Kazanskij institut biohimii i biofiziki, Russian Federation; Roberto Piacentini, Università Cattolica del Sacro, Italy.
Funding:This work was supported by the Weston Brain Institute Rapid Response Grant, No. RR182093 (to JR).
P-Reviewers: Polis B, Petrov A, Piacentini R; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Adriani G, Ma D, Pavesi A, Kamm RD, Goh ELK. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip. 2017;17:448–459. doi: 10.1039/c6lc00638h. [DOI] [PubMed] [Google Scholar]
- 2.Arber C, Lovejoy C, Wray S. Stem cell models of Alzheimer’s disease: progress and challenges. Alzheimers Res Ther. 2017;9:42. doi: 10.1186/s13195-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 4.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang S, Lee SR, Ko J, Son K, Tahk D, Ahn J, Im C, Jeon NL. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-07416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barar J, Rafi MA, Pourseif MM, Omidi Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts. 2016;6:225–248. doi: 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baufeld C, O’Loughlin E, Calcagno N, Madore C, Butovsky O. Differential contribution of microglia and monocytes in neurodegenerative diseases. J Neural Transm (Vienna) 2018;125:809–826. doi: 10.1007/s00702-017-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann S, Lawler SE, Qu Y, Fadzen CM, Wolfe JM, Regan MS, Pentelute BL, Agar NYR, Cho CF. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc. 2018;13:2827–2843. doi: 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biesecker KR, Srienc AI, Shimoda AM, Agarwal A, Bergles DE, Kofuji P, Newman EA. Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J Neurosci. 2016;36:9435–9445. doi: 10.1523/JNEUROSCI.1782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard JW, Bula M, Davila-Velderrain J, Akay LA, Zhu L, Frank A, Victor MB, Bonner JM, Mathys H, Lin YT, Ko T, Bennett DA, Cam HP, Kellis M, Tsai LH. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med. 2020;26:952–963. doi: 10.1038/s41591-020-0886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 15.Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:054124. doi: 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhardt MF, Martinez FJ, Wright S, Ramos C, Volfson D, Mason M, Garnes J, Dang V, Lievers J, Shoukat-Mumtaz U, Martinez R, Gai H, Blake R, Vaisberg E, Grskovic M, Johnson C, Irion S, Bright J, Cooper B, Nguyen L, et al. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2013;56:355–364. doi: 10.1016/j.mcn.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, Knoblich JA, Lachmann R, Pääbo S, Huttner WB, Treutlein B. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A. 2015;112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canfield SG, Stebbins MJ, Faubion MG, Gastfriend BD, Palecek SP, Shusta EV. An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS. 2019a;16:25. doi: 10.1186/s12987-019-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canfield SG, Stebbins MJ, Faubion MG, Gastfriend BD, Palecek SP, Shusta EV. Correction to: An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS. 2019b;16:31. doi: 10.1186/s12987-019-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho CF, Wolfe JM, Fadzen CM, Calligaris D, Hornburg K, Chiocca EA, Agar NYR, Pentelute BL, Lawler SE. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun. 2017;8:1–14. doi: 10.1038/ncomms15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JH, Santhosh M, Choi JW. In vitro blood-brain barrier-integrated neurological disorder models using a microfluidic device. Micromachines. 2019;11:21. doi: 10.3390/mi11010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190–3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- 27.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 28.Dezonne RS, Sartore RC, Nascimento JM, Saia-Cereda VM, Romão LF, Alves-Leon SV, de Souza JM, Martins-de-Souza D, Rehen SK, Gomes FC. Derivation of functional human astrocytes from cerebral organoids. Sci Rep. 2017;7:45091. doi: 10.1038/srep45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engle SJ, Blaha L, Kleiman RJ. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron. 2018;100:783–797. doi: 10.1016/j.neuron.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez C, Armijo E, Bravo-Alegria J, Becerra-Calixto A, Mays CE, Soto C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry. 2018;23:2363–2374. doi: 10.1038/s41380-018-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479–484. doi: 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- 33.Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, Couraud PO, Vermes I, van der Meer AD, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 34.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ham O, Jin YB, Kim J, Lee MO. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun. 2020;521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 36.Heidenreich M, Zhang F. Applications of CRISPR-Cas systems in neuroscience. Nat Rev Neurosci. 2016;17:36–44. doi: 10.1038/nrn.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herland A, van der Meer AD, FitzGerald EA, Park TE, Sleeboom JJF, Ingber DE. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PloS One. 2016;11:e0150360. doi: 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson JJ, Linville RM, Ding YY, Gerecht S, Searson PC. Role of iPSC-derived pericytes on barrier function of iPSC-derived brain microvascular endothelial cells in 2D and 3D. Fluids Barriers CNS. 2019;16:15. doi: 10.1186/s12987-019-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorfi M, D’Avanzo C, Tanzi RE, Kim DY, Irimia D. Human neurospheroid arrays for in vitro studies of Alzheimer’s disease. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur G, Dufour JM. Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Xu R, Padmashri R, Dunaevsky A, Liu Y, Dreyfus CF, Jiang P. Pluripotent stem cell-derived cerebral organoids reveal human oligodendrogenesis with dorsal and ventral origins. Stem Cell Reports. 2019;12:890–905. doi: 10.1016/j.stemcr.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JA, Kim HN, Im SKK, Chung S, Kang JY, Choi N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics. 2015a;9:024115. doi: 10.1063/1.4917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YH, Choi SH, D’Avanzo C, Hebisch M, Sliwinski C, Bylykbashi E, Washicosky KJ, Klee JB, Brüstle O, Tanzi RE, Kim DY. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat Protoc. 2015b;10:985–1006. doi: 10.1038/nprot.2015.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 50.Laterreur V, Ruel J, Auger FA, Vallières K, Tremblay C, Lacroix D, Tondreau M, Bourget JM, Germain L. Comparison of the direct burst pressure and the ring tensile test methods for mechanical characterization of tissue-engineered vascular substitutes. J Mech Behav Biomed Mater. 2014;34:253–263. doi: 10.1016/j.jmbbm.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 52.Lee HK, Velazquez Sanchez C, Chen M, Morin PJ, Wells JM, Hanlon EB, Xia W. Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS One. 2016;11:e0163072. doi: 10.1371/journal.pone.0163072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SR, Hyung S, Bang S, Lee Y, Ko J, Lee S, Kim HJ, Jeon NL. Modeling neural circuit, blood-brain barrier, and myelination on a microfluidic 96 well plate. Biofabrication. 2019;11:035013. doi: 10.1088/1758-5090/ab1402. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Chao J, Shi Y. Modeling neurological diseases using iPSC-derived neural cells: iPSC modeling of neurological diseases. Cell Tissue Res. 2018;371:143–151. doi: 10.1007/s00441-017-2713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Q, Yang Y, Fan X. Microvascular pericytes in brain-associated vascular disease. Biomed Pharmacother. 2020;121:109633. doi: 10.1016/j.biopha.2019.109633. [DOI] [PubMed] [Google Scholar]
- 56.Liu W, Das J, Mepham AH, Nemr CR, Sargent EH, Kelley SO. A fully-integrated and automated testing device for PCR-free viral nucleic acid detection in whole blood. Lab Chip. 2018;18:1928–1935. doi: 10.1039/c8lc00371h. [DOI] [PubMed] [Google Scholar]
- 57.Lutz CM, Osborne MA. Optimizing mouse models of neurodegenerative disorders: are therapeutics in sight. Future Neurol. 2014;9:67–75. doi: 10.2217/fnl.13.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahshid SS, Ricci F, Kelley SO, Vallée-Bélisle A. Electrochemical DNA-based immunoassay that employs steric hindrance to detect small molecules directly in whole blood. ACS Sens. 2017;2:718–723. doi: 10.1021/acssensors.7b00176. [DOI] [PubMed] [Google Scholar]
- 60.Maleki FA, Rivest S. Innate immune cells: monocytes, monocyte-derived macrophages and microglia as therapeutic targets for Alzheimer’s disease and multiple sclerosis. Front Cell Neurosci. 2019;13:355. doi: 10.3389/fncel.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segrè D, Budnik B, Ingber DE, Parker KK. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol. 2018;36:865–874. doi: 10.1038/nbt.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Paşca SP. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masamoto K, Unekawa M, Watanabe T, Toriumi H, Takuwa H, Kawaguchi H, Kanno I, Matsui K, Tanaka KF, Tomita Y, Suzuki N. Unveiling astrocytic control of cerebral blood flow with optogenetics. Sci Rep. 2015;5:11455. doi: 10.1038/srep11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing. A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26:735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mertens J, Reid D, Lau S, Kim Y, Gage FH. Aging in a dish: iPSC-derived and directly induced neurons for studying brain aging and age-related neurodegenerative diseases. Annu Rev Genet. 2018;52:271–293. doi: 10.1146/annurev-genet-120417-031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19:1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyamoto A, Wake H, Moorhouse AJ, Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front Cell Neurosci. 2013;7:70. doi: 10.3389/fncel.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mota B, Dos Santos SE, Ventura-Antunes L, Jardim-Messeder D, Neves K, Kazu RS, Noctor S, Lambert K, Bertelsen MF, Manger PR, Sherwood CC, Kaas JH, Herculano-Houzel S. White matter volume and white/gray matter ratio in mammalian species as a consequence of the universal scaling of cortical folding. Proc Natl Acad Sci U S A. 2019;116:15253–15261. doi: 10.1073/pnas.1716956116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasu M, Takata N, Danjo T, Sakaguchi H, Kadoshima T, Futaki S, Sekiguchi K, Eiraku M, Sasai Y. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS One. 2012;7:e53024. doi: 10.1371/journal.pone.0053024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oddo A, Peng B, Tong Z, Wei Y, Tong WY, Thissen H, Voelcker NH. Advances in microfluidic blood-brain barrier (BBB) models. Trends Biotechnol. 2019;37:1295–1314. doi: 10.1016/j.tibtech.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9:4167. doi: 10.1038/s41467-018-06684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osaki T, Sivathanu V, Kamm RD. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-23512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, Tanzi RE, Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941–951. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potjewyd G, Moxon S, Wang T, Domingos M, Hooper NM. Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends Biotechnol. 2018;36:457–472. doi: 10.1016/j.tibtech.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rackus DG, Dryden MD, Lamanna J, Zaragoza A, Lam B, Kelley SO, Wheeler AR. A digital microfluidic device with integrated nanostructured microelectrodes for electrochemical immunoassays. Lab Chip. 2015;15:3776–3784. doi: 10.1039/c5lc00660k. [DOI] [PubMed] [Google Scholar]
- 82.Robert J, Button EB, Martin EM, McAlary L, Gidden Z, Gilmour M, Boyce G, Caffrey TM, Agbay A, Clark A, Silverman JM, Cashman NR, Wellington CL. Cerebrovascular amyloid angiopathy in bioengineered vessels is reduced by high-density lipoprotein particles enriched in Apolipoprotein E. Mol Neurodegener. 2020a;15:23. doi: 10.1186/s13024-020-00366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robert J, Button EB, Stukas S, Boyce GK, Gibbs E, Cowan CM, Gilmour M, Cheng WH, Soo SK, Yuen B, Bahrabadi A, Kang K, Kulic I, Francis G, Cashman N, Wellington CL. High-density lipoproteins suppress Aβ-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol Neurodegener. 2017a;12:60. doi: 10.1186/s13024-017-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A, Stukas S, Zhao W, Kulic I, Wellington CL. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. Elife. 2017b;6:e29595. doi: 10.7554/eLife.29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robert J, Weilinger NL, Cao LP, Cataldi S, Button EB, Stukas S, Martin EM, Seibler P, Gilmour M, Caffrey TM, Rowe EM, Fan J, MacVicar B, Farrer MJ, Wellington CL. An in vitro bioengineered model of the human arterial neurovascular unit to study neurodegenerative diseases. Mol Neurodegener. 2020b;15:70. doi: 10.1186/s13024-020-00418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 87.Schaffhauser DF, Andrini O, Ghezzi C, Forster IC, Franco-Obregón A, Egli M, Dittrich PS. Microfluidic platform for electrophysiological studies on Xenopus laevis oocytes under varying gravity levels. Lab Chip. 2011;11:3471–3478. doi: 10.1039/c0lc00729c. [DOI] [PubMed] [Google Scholar]
- 88.Shi M, Majumdar D, Gao Y, Brewer BM, Goodwin CR, McLean JA, Li D, Webb DJ. Glia co-culture with neurons in microfluidic platforms promotes the formation and stabilization of synaptic contacts. Lab Chip. 2013;13:3008–3021. doi: 10.1039/c3lc50249j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18:e3000705. doi: 10.1371/journal.pbio.3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE. Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer ’s disease. Adv Sci (Weinh) 2019;6:1900962. doi: 10.1002/advs.201900962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slanzi A, Iannoto G, Rossi B, Zenaro E, Constantin G. In vitro models of neurodegenerative diseases. Front Cell Dev Biol. 2020;8:328. doi: 10.3389/fcell.2020.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95:779–790. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song L, Yuan X, Jones Z, Griffin K, Zhou Y, Ma T, Li Y. Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues. Sci Rep. 2019;9:5977. doi: 10.1038/s41598-019-42439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37:3300–3317. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai CD, Nakamura T, Kaneko M. An on-chip, electricity-free and single-layer pressure sensor for microfluidic applications. 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) Hamburg: Germany. 2015 doi:101109/IROS20157353369. [Google Scholar]
- 98.Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urich E, Patsch C, Aigner S, Graf M, Iacone R, Freskgård PO. Multicellular self-assembled spheroidal model of the blood brain barrier. Sci Rep. 2013;3:1500. doi: 10.1038/srep01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Helm MW, van der Meer AD, Eijkel JCT, van den Berg A, Segerink LI. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers. 2016;4:e1142493. doi: 10.1080/21688370.2016.1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 102.Varma S, Voldman J. A cell-based sensor of fluid shear stress for microfluidics. Lab Chip. 2015;15:1563–1573. doi: 10.1039/c4lc01369g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, Wen N, Spivia WR, Chen Z, Van Eyk J, Svendsen CN. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995–1005. doi: 10.1016/j.stem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Webb AR, Macrie BD, Ray AS, Russo JE, Siegel AM, Glucksberg MR, Ameer GA. In vitro characterization of a compliant biodegradable scaffold with a novel bioreactor system. Ann Biomed Eng. 2007;35:1357–1367. doi: 10.1007/s10439-007-9304-z. [DOI] [PubMed] [Google Scholar]
- 105.Wolff A, Antfolk M, Brodin B, Tenje M. In vitro blood-brain barrier models-an overview of established models and new microfluidic approaches. J Pharm Sci. 2015;104:2727–2746. doi: 10.1002/jps.24329. [DOI] [PubMed] [Google Scholar]
- 106.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21:383–398. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, Mayweather BA, Nguyen L, Niziolek Z, Ozek C, Butty VL, Isserlin R, Buchanan SM, Levine SS, Regev A, Bader GD, Levin JZ, Rubin LL. Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci. 2019;22:1696–1708. doi: 10.1038/s41593-019-0491-3. [DOI] [PubMed] [Google Scholar]
- 109.Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4:78–82. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.