Bioenergetics imbalance is a deleterious feature, which is present in the etiopathology of many human diseases, including in diabetes, cancer, and neurodegeneration. Therefore, targeting the components of mammalian bioenergetics, as well as the mechanisms that regulate the relationship between these components, could be a promising pharmacological strategy against a wide variety of pathologies. While for many years mammalian bioenergetics has been exclusively circumscribed to the mitochondrial oxidative phosphorylation (OXPHOS), which is the main mechanism to obtain adenosine triphosphate (ATP) in mammals, and to the cytoplasmic glycolysis and the closely related pentose phosphate pathway, in the last few decades many authors have advocated for expanding this term to include all the mechanisms that are involved in matching the cellular demands and production of energy to meet the needs of the cell under different states, including physiological and pathological conditions. This broader definition will include other key energy metabolites, such as the ubiquitous inorganic polyphosphate (polyP).
PolyP is an ancient polymer that is exceptionally well-conserved throughout evolution and present in all studied organisms. This highly negatively charged and multifunctional polymer is composed of multiple orthophosphates bond together by high-energy phosphoanhydride bonds, similar to those found in ATP (Figure 1). In mammalian cells, polyP is usually comprised of a few hundred orthophosphate units and its concentrations are within the micromolar range. While this polymer has been the object of study for more than a century (the first mention to polyP in the scientific literature dates back to 1890), it is still quite unknown, due to the complexity of the analytical tools to assay the levels and the subcellular location of polyP, as well as the fact that the enzymes involved in its metabolism in mammalian cells are still largely unknown. Thus, the exact role that polyP plays in the physiology of organisms, specifically in mammals, remains a mystery to this day.
Figure 1.
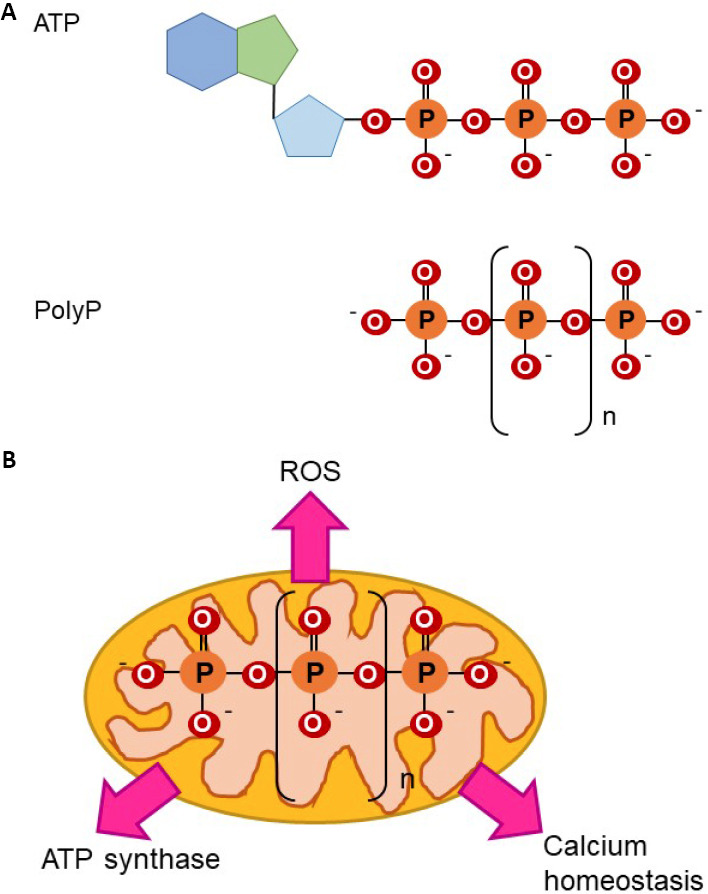
Molecular structure of polyP and role in bioenergetics.
(A) ATP and polyP have phosphoanhydride bonds. Note the presence of phosphoanhydride, isoenergetic bonds in both molecules. (B) Effects of polyP on mitochondrial bioenergetics are broad. Due to its phosphoanhydride bonds, which are isoenergetic to those found in ATP, and its mitochondrial location, the role of polyP as a component of mammalian bioenergetics has been proposed. There is evidence for this, such as data that prove the effects of mitochondrial polyP in the regulation of ROS production and in mitochondrial calcium homeostasis. Both processes are closely related to energy production. Moreover, recently it has been proposed that polyP can be produced and metabolized in mammalian, mitochondrial ATP synthase. ATP: Adenosine triphosphate; polyP: inorganic polyphosphate; ROS: reactive oxygen species.
Interestingly, as previously mentioned, the chemical bonds found in polyP are isoenergetic to those found in ATP. Moreover, polyP has been involved in the regulation of many energy-related cellular processes, which are key for organismal physiology. These processes range from protein homeostasis to apoptosis. Thus, the intriguing possibility here is that many of the effects of polyP at the cellular, and more specifically, at the mitochondrial level, could be a consequence of the action of this polymer as a key energy metabolite, especially in mammalian organisms.
This suggestion is supported by some interesting bibliography. For example, the late Nobel Laureate Dr. A. Kornberg, who devoted the final years of his career to the study of polyP, reported the key role of polyP in the stress response in bacteria. Specifically, his team demonstrated that bacteria with decreased levels of polyP show increased sensitivity to various stressors, including specific chemicals and heat shock. Moreover, a recent publication proved the broad effects that the increased presence of long-chain polyP has in mammalian cells (Bondy-Chorney et al., 2020). The authors of this comprehensive study, showed that polyP can accumulate to high concentrations in different cellular compartments, affecting the subcellular location of nuclear/cellular proteins and the phosphorylation status of the cells. Both protein phosphorylation and translocation are key processes in the stress response. Interestingly, stress response is a very energy-dependent processes in all organisms, ranging from bacteria to mammals. Further evidence comes from studies that show the rapid inter-conversion of phosphoryl groups between ATP and polyP (Saiardi, 2012). Additionally, the key role played by polyP in the regulation of the cellular levels and the production of reactive oxygen species (ROS) in bacteria has already been demonstrated (Gray and Jakob, 2015). ROS are a well-known byproduct of OXPHOS.
In various eukaryotic organisms, polyP can be found in different locations, including in the cytoplasm, membrane (associated with proteins), nucleus, and other organelles (Kumble and Kornberg, 1995), as well as in the extracellular space, where it can be released from different cell types, including platelets (Moreno-Sanchez et al., 2012; Suess et al., 2017). This variety of subcellular and cytoplasmic locations might suggest that polyP could play different roles in cellular metabolism depending on its location, as is the case with many other components of cell biology. Also in eukaryotic organisms, polyP has been proposed as a component of the mitochondrial permeability transition pore, a structure which is key for the proper functioning of bioenergetics within the organelle, among other physiological functions (Seidlmayer et al., 2012). In mammalian cells, polyP is highly co-localized to mitochondria (Solesio et al., 2016), the site of OXPHOS. This points toward a role for the polymer in maintaining cellular bioenergetics. Using various mammalian cells, including fibroblasts, kidney, and adrenal cells, a tight relationship between the cellular levels of ATP and polyP has been demonstrated (Kumble and Kornberg, 1995). Moreover, in an elegant article conducted in mammalian cellular models, the authors showed that exogenous polyP is able to reverse the dysfunctional bioenergetic status of cells, which was induced by treatment with the amyloid peptide (Muller et al., 2017), a well-known disturber of cellular bioenergetics. Interestingly, in mammalian mitochondria, it has been demonstrated that the levels of polyP are not static but highly dynamic and that they are closely related to the energetic metabolic state of mitochondria (Pavlov et al., 2010). The authors of this study hypothesized that the direct effect of polyP in the regulation of mitochondrial energy could be exerted either through the direct participation of the polymer in the production of ATP or through the regulation of the rates of enzymatic activity, serving as a reservoir of high energy phosphoryl groups readily available under cellular stress conditions. Some evidence in support of these findings is that another recent study has shown that the mitochondrial F0F1-ATP synthase is key in the synthesis and the hydrolysis of polyP (Bayev et al., 2020). Lastly, our recent findings also contributed to establish the crucial involvement of polyP in bioenergetics. Specifically, we described for the first time the key role played by polyP in the regulation of the concentrations of calcium within the organelle, in mammalian cells (Solesio et al., 2016, 2020). Intramitochondrial calcium is crucial in the production of ATP by activating the main dehydrogenase enzymes within the organelle, including those involved in the tricarboxylic cycle, which leads to increased levels of NADH and ATP generation.
Even though all the bibliography demonstrates a key role for polyP in bioenergetics, further studies should be conducted to clarify the mechanisms of the interaction of polyP with the rest of the components of mammalian bioenergetics, as well as the processes in charge of the regulation of these mechanisms. To do this, understanding the exact metabolism of polyP in mammalian cells, as well as developing reproducible analytical tools, is crucial. The polyP scientific community, which is small but highly supportive, is tirelessly working to accomplish this. Elucidating the role of polyP in mammalian bioenergetics could pave the road to targeting the metabolism of this polymer, which could constitute an innovative and valid pharmacological strategy against unbalanced bioenergetics, a dysfunction broadly observed in a wide variety of pathologies. In our opinion, this strategy will be especially useful in the field of neurodegenerative disorders, including Parkinson’s disease and Alzheimer’s disease, as many authors have already shown dysregulated bioenergetics. Interestingly, the protective role of polyP against some of the elements involved in the etiopathology of these diseases has already been proposed (Borden et al., 2020).
The authors would like to thank Mr. Mitch Maleki (Esq.) for editing the manuscript and to Dr. Pedro Urquiza, Dr. Mariona Guitart-Mampel, Vedangi Hambardikar and Ernest Scoma for reading it and providing significant insights. We apologize to colleagues whose work has not been cited, due to space constraints.
This present work was supported by startup funds from Rutgers University and by NIH (4R00AG055701-03) to MES.
Footnotes
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Jose A. Vega, Universidad de Oviedo, Spain.
P-Reviewer: Vega JA; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Bayev AY, Angelova PR, Abramov AY. Inorganic polyphosphate is produced and hydrolysed in F0F1-ATP synthase of mammalian mitochondria. Biochem J. 2020;477:1515–1524. doi: 10.1042/BCJ20200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondy-Chorney E, Abramchuk I, Nasser R, Holinier C, Denoncourt A, Baijal K, McCarthy L, Khacho M, Lavallee-Adam M, Downey M. A broad response to intracellular long-chain polyphosphate in human cells. Cell Rep. 2020;33:108318. doi: 10.1016/j.celrep.2020.108318. [DOI] [PubMed] [Google Scholar]
- 3.Borden EA, Furey M, Gattone NJ, Hambardikar VD, Liang XH, Scoma ER, Abou Samra A, D-Gary LR, Dennis DJ, Fricker D, Garcia C, Jiang Z, Khan SA, Kumarasamy D, Kuppala H, Ringrose S, Rosenheim EJ, Van Exel K, Vudhayagiri HS, Zhang J, et al. Is there a link between inorganic polyphosphate (polyP), mitochondria, and neurodegeneration. Pharmacol Res. 2020:105211. doi: 10.1016/j.phrs.2020.105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray MJ, Jakob U. Oxidative stress protection by polyphosphate--new roles for an old player. Curr Opin Microbiol. 2015;24:1–6. doi: 10.1016/j.mib.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 6.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller WEG, Wang S, Ackermann M, Neufurth M, Steffen R, Mecja E, Munoz-Espi R, Feng Q, Schroder HC, Wang X. Rebalancing beta-amyloid-induced decrease of ATP level by amorphous Nano/Micro polyphosphate: suppression of the neurotoxic effect of amyloid beta-protein fragment 25-35. Int J Mol Sci. 2017;18:2154. doi: 10.3390/ijms18102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gomez-Garcia MR, Abramov AY. Inorganic polyphosphate and energy metabolism in mammalian cells. J Biol Chem. 2010;285:9420–9428. doi: 10.1074/jbc.M109.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidlmayer LK, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate--an unusual suspect of the mitochondrial permeability transition mystery. Channels (Austin) 2012;6:463–467. doi: 10.4161/chan.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solesio ME, Demirkhanyan L, Zakharian E, Pavlov EV. Contribution of inorganic polyphosphate towards regulation of mitochondrial free calcium. Biochim Biophys Acta. 2016;1860:1317–1325. doi: 10.1016/j.bbagen.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solesio ME, Garcia Del Molino LC, Elustondo PA, Diao C, Chang JC, Pavlov EV. Inorganic polyphosphate is required for sustained free mitochondrial calcium elevation, following calcium uptake. Cell Calcium. 2020;86:102127. doi: 10.1016/j.ceca.2019.102127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suess PM, Watson J, Chen W, Gomer RH. Extracellular polyphosphate signals through Ras and Akt to prime Dictyostelium discoideum cells for development. J Cell Sci. 2017;130:2394–2404. doi: 10.1242/jcs.203372. [DOI] [PMC free article] [PubMed] [Google Scholar]


