Abstract
Peripheral nerve injuries occur as the result of sudden trauma and lead to reduced quality of life. The peripheral nervous system has an inherent capability to regenerate axons. However, peripheral nerve regeneration following injury is generally slow and incomplete that results in poor functional outcomes such as muscle atrophy. Although conventional surgical procedures for peripheral nerve injuries present many benefits, there are still several limitations including scarring, difficult accessibility to donor nerve, neuroma formation and a need to sacrifice the autologous nerve. For many years, other therapeutic approaches for peripheral nerve injuries have been explored, the most notable being the replacement of Schwann cells, the glial cells responsible for clearing out debris from the site of injury. Introducing cultured Schwann cells to the injured sites showed great benefits in promoting axonal regeneration and functional recovery. However, there are limited sources of Schwann cells for extraction and difficulties in culturing Schwann cells in vitro. Therefore, novel therapeutic avenues that offer maximum benefits for the treatment of peripheral nerve injuries should be investigated. This review focused on strategies using mesenchymal stem cells to promote peripheral nerve regeneration including exosomes of mesenchymal stem cells, nerve engineering using the nerve guidance conduits containing mesenchymal stem cells, and genetically engineered mesenchymal stem cells. We present the current progress of mesenchymal stem cell treatment of peripheral nerve injuries.
Key Words: axonal regeneration, exosomes, genetic engineering, mesenchymal stem cells, neural conduit, peripheral nerve, peripheral nerve injury, peripheral nerve regeneration, Schwann cells, sudden trauma
Introduction
Peripheral nerve injury (PNI) is a common clinical neurological disorder that is characterized by sensory, motor, and autonomic dysfunction of the trunk or limbs. Unlike the central nervous system, peripheral nerves can regenerate spontaneously after injury because of the activation of the intrinsic growth capacity of neurons (Willand et al., 2016). Neurons are the basic structural and functional units of the nervous system and their axons are supported by neurolemmocytes, Schwann cells, which can be either unmyelinated or myelinated. In the latter, the medullary sheath, which comprises myelin and proteins forming the cell membrane of Schwann cells (SCs), is wrapped around axons repeatedly, acts as an insulator and accelerates the transmission of nerve signals. The Schwann cell has a nucleus and cytoplasm and its outer basement membrane wraps around the myelin sheath and has protective and regenerating functions (Muzio and Cascella, 2020). After peripheral nerve damage the nerve begins to degrade in an anterograde manner and involves mononuclear cell infiltration, neuroedema and even neurodegenerative demyelinating changes (El Soury and Gambarotta, 2019; Martin et al., 2019). PNI repairs occur using nerve chemotaxis and contact guidance (Faroni et al., 2015). SCs, which are derived from neural crest precursor cells and require the participation of fibroblast growth factor, bone morphogenetic protein and wingless/integrated signaling pathways, play an important role in the composition of the peripheral nerve structure and nerve regeneration (Stuhlmiller and Garcia-Castro, 2012). However, spontaneous repair of defective nerves in the peripheral nerve system is partial and leads to insufficient functionality.
Although microsurgical repair of damaged peripheral nerves is the current preferred treatment, surgery for PNI has entered a bottleneck stage. At present, there are two main methods for surgically repairing PNI when the nerve gap exceeds 1 cm (Bassilios Habre et al., 2018): autologous nerve transplantation and allogeneic nerve transplantation. Of these, autologous nerve transplantation has been considered the “gold standard” for PNI repair (Labroo et al., 2019). The common surgical approach is the direct suture of the two stumps (end-to-end suture) when a gap is not present (Tos et al., 2013). With the rapid development of medical technology, anastomosis of the nerve bundle and function-beam anastomosis have gradually become the focus of researchers as they enable better clinical outcomes than end-to-end anastomosis of the outer membrane of the nerve (Sakthivel et al., 2020). The primary problem currently facing allogeneic nerve transplantation is still immune rejection. Some researchers have used immunosuppressants to protect the main components, such as nerve membrane cells and interstitial cells, but certain adverse reactions remain in long-term use, including problems with liver and kidney functions (Nakayama et al., 2010; Loboda et al., 2018). Overcoming immune rejection is the primary requirement for the application of this technique in clinical treatment (Winfree, 2005; Campbell, 2008). Tissue engineering is an emerging discipline in the category of biological high technology. A suitable biological model is established by applying the principles of cell biology, biomaterials, tissue engineering, and growth factors to repair PNI. Over the years, the use of stem cells in tissue engineering has been examined. In addition to neural stem cells, mesenchymal stem cells (MSCs), adipose-derived MSCs, muscle-derived stem cells and induced pluripotent stem cells have been used in the treatment of PNI (Barton et al., 2017; Lv et al., 2018). However, the current approved therapies are still not satisfactory.
MSCs, a class of multipotent stem cells derived from the mesoderm, have been characterized as having high self-renewal ability, multi-directional differentiation potential and low immunogenicity. Because of their ubiquity, they can be easily isolated from various tissues such as bone marrow, umbilical cord and placenta (Pereira et al., 2018). Thus, they are often used as an ideal seed cell in cell therapy, with important clinical and research value. Great breakthroughs have been made using them in cardiovascular diseases, neurological diseases, autoimmune diseases and bone repair (Bartolucci et al., 2017; Chen et al., 2019). So far, MSCs have been combined with biological tissue engineering using various techniques to improve the effect of PNI regeneration and repair, but each application method has certain advantages and disadvantages. Therefore, the optimal method for MSCs to repair PNI needs further exploration. This review assesses the various techniques of MSCs for PNI (Figure 1) and provides a scientific basis for the direction of future research.
Figure 1.
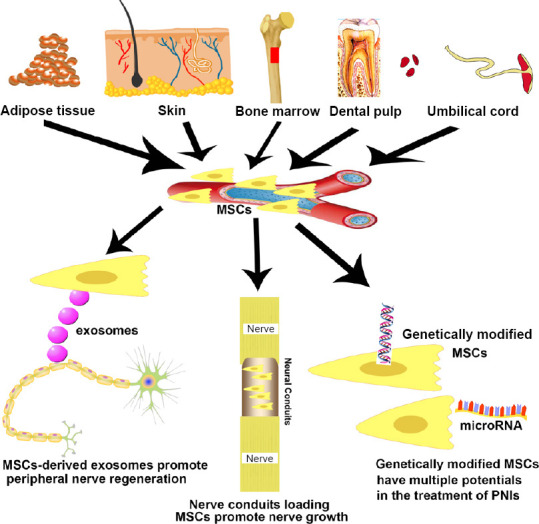
Novel strategies using mesenchymal stem cells (MSCs) for curing peripheral nerve injury (PNI).
MSCs can be isolated from adipose tissue, skin, bone marrow, dental pulp and umbilical cord.
Search Strategy and Selection Criteria
The initial literature search was performed on PubMed and the following keywords: “mesenchymal stem cells”, “peripheral nerve injuries”, “axonal regeneration”, “exosomes”, “neural conduits”, “genetic engineering” and combinations of those words. All fields were included in the search, which was limited to publications from 2000 to 2020. A second broad search was performed by PubMed using the above keywords with the aim to update information. No time limits were used.
Peripheral Nerve Injuries Causes and Endogenous Repair
Peripheral nerves injuries may be caused by excessive pressure, complex soft-tissue injury or limb ischemia with compartment syndrome (Maricevic and Erceg, 1997; Rosen et al., 2012; Wang et al., 2017). Nowadays, the most common cause of injury is road traffic accidents, particularly motorcycle crashes (Stanec et al., 1997). Approximately 5% of PNI cases were related to sports (Hirasawa and Sakakida, 1983). After suffering from PNI, a patient’s nerve injury prognosis can be divided into neurotmesis, axonotmesis and neurapraxia at the anatomic level (Lee and Wolfe, 2000; Table 1). Neurotmesis often occurs with nerve avulsions, massive trauma, and cutting injury. In neurotmesis, the prognosis is poor without surgical therapy. Recovery from this type of trauma, which involves in major axon loss, is usually delayed and deficient (De la Rosa et al., 2018).
Table 1.
Classification of nerve injuries
| Classification | Pathophysiologic features | Recovery |
|---|---|---|
| Neurapraxia | Temporary rupture of the nerve impulse delivery occurs with nerve demyelination at the site of injury without rupture of nerve continuity | Complete recovery is achieved upon remyelination which can take up to 12 wk |
| Axonotmesis | Loss of continuity of axons, with variable preservation of the connective tissue elements of the nerve | Regeneration of the axon proceeds from the site of injury to peripheral organs at a rate of 1 mm/d |
| Neurotmesis | Complete interruption of nerve continuity | No possibility of spontaneous function recovery |
Neurons in the peripheral nerve system can regenerate even after a severe PNI, but the potential for healing after accessible surgical therapy is dependent upon the brain as well as factors distal to the injury (Fex Svennigsen and Dahlin, 2013). Cortical plasticity has been identified as a contributary factor in the rehabilitation for patients with PNI (Baldassarre et al., 2020). Adult PNI patients may never achieve an encouraging outcome of functional recovery. This is because the brain is unable to accept the new afferent signaling patterns from the periphery caused by misdirection of the axonal regeneration after nerve injury. However, children show an admirable clinical recovery after PNIs, which may be because of the better regeneration and greater plasticity of the young brain (Chemnitz et al., 2013). At the time axons regenerate, the SCs have begun to proliferate, migrate and line up in nerves, which creates bands of Büngner and offers guidance substrates for the re-growth of axons (Bunn et al., 2019). SCs, specific type of cells that wrap around the axon of neurons in the peripheral nerve system, may play a vital role in the regeneration of axons (Gordon, 2020). The SCs in the bands of Büngner could produce an array of growth promoting factors, such as glial derived neurotrophic factor, ciliary neurotrophic factor, insulin like growth factor-1 and neuroregulin 1 (NRG1) (Springer et al., 1994; Tonazzini et al., 2017; Zhu et al., 2018; Godinho et al., 2020; Figure 2). PNI is followed by responses from the surrounding neural and non-neural cells, both proximally and distally to the damaged area. The endogenous repair process in neurons starts with an increase in its cell body size, the Nissl bodies dissolve and initiate a process of protein synthesis (Perrin et al., 2005). Then fragmentation of the axon is mediated by activated intrinsic proteases, including the ubiquitin-protease system and calpain (Zhai et al., 2003). Axonal and myelin debris is later phagocytosed by SCs and recruited macrophages that, attracted by specific chemokines, infiltrate through the myelin sheathing and broken blood-nerve-barrier (Coleman and Freeman, 2010; Figure 2). Clearance of myelin debris is a critical step for repair since myelin contains inhibitors of axonal regeneration (Berghoff et al., 2020). It is known that some adhesion proteins and growth factors affect axonal growth, Schwann cell proliferation and the formation of Büngner bands. Thus, understanding which proteins are critical in promoting the re-growth process will be useful for the improvements in peripheral nerve surgery and in the development to bridge nerve defects. It has been indicated that NRG1, probably along with laminin, is responsible for Schwann cell migration in nerve regeneration (Chang et al., 2013; Heermann and Schwab, 2013). The presence of laminin is also critical in the outgrowth of axons, and is now used clinically to reconstruct nerve defects (Kvist et al., 2011). Although axon regeneration and remyelination occur in the peripheral nerve system, the remyelinated axons often have a decreased internodal length and thinner myelin sheaths, which causes slower conduction (Sherman and Brophy, 2005). This is not only observed in the repair of acute nerve injury but is also observed after decompression of the median nerve in carpal tunnel syndrome and nerve repair in human median and ulnar nerves (Kim et al., 2000). These undesirable results might be due to either insufficient stimulation of redifferentiated SCs or inhibitory signals (Sherman and Brophy, 2005). Because of the unsatisfactory regaining of function, further improvements in PNI repair and regeneration have become an area of much interest.
Figure 2.
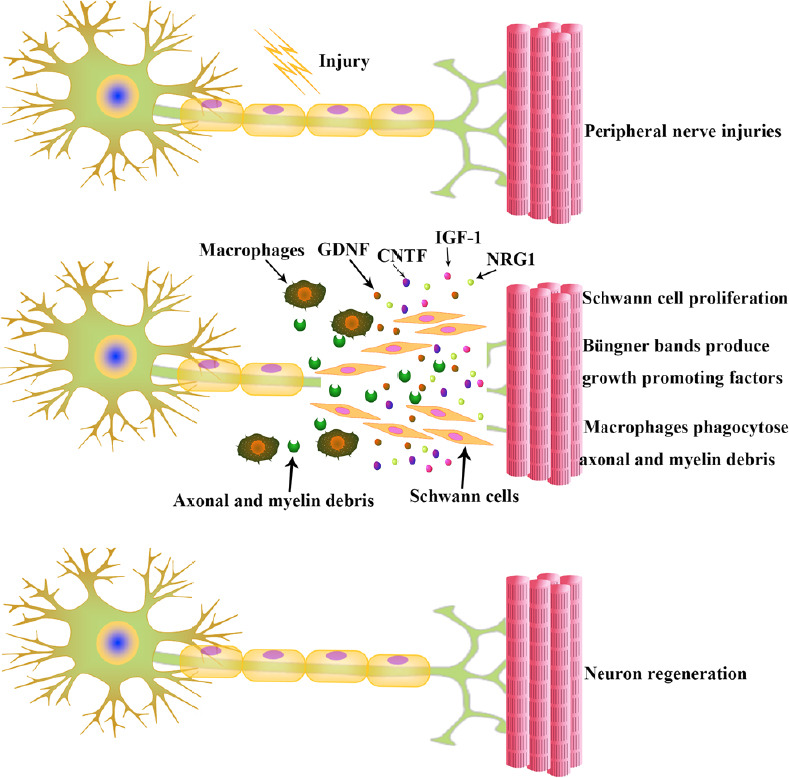
Neurons regenerate after peripheral nerve injuries.
Following injury, SCs start proliferating and help the recruited macrophages to clear the axonal and myelin debris. The SCs in the bands of Büngner produce an array of growth promoting factors. CNTF: Ciliary neurotrophic factor; GDNF: glial derived neurotrophic factor; IGF-1: insulin like growth factor-1; NRG1: neuroregulin 1; SCs: Schwann cells.
Mesenchymal Stem Cells Exosomes Based Therapy for Peripheral Nerve Injury Repair
Although some types of nerve damage can recuperate spontaneously, augmentation methods can accelerate the self-regeneration process after PNI. MSCs have been found to be a promising trigger for the regeneration following both axonal and nerve damage (Kizilay et al., 2017). MSCs are self-renewing, multipotent progenitors that have the capability to promote neuroprotection and tissue-repair. MSCs can be isolated from the stromal vascular fraction of several tissues that are extrinsic to blood vessels, such as adipose tissue, skin, bone marrow and dental pulp (Lopez-Verrilli et al., 2016; Figure 1).
Exosomes, cell-derived small (~100 nm in diameter) vesicles, are one of several groups of secreted vesicles, including ectosomes (large membranous vesicles that are shed directly from the plasma membrane) and apoptotic blebs that are released by dying cells. Exosomes carry a cargo of lipids and proteins, as well as nucleic acid material in the form of DNA, mRNA, microRNA (miRNA), and noncoding RNA. These components could provide essential information associated with the physiological and pathological status. Exosomes can also convey these components to neighboring or even distant cells through circulation and participate in many biological processes and cell-to-cell communication and exchange proteins and nucleic acids between the donor cells and receptor cells (Kourembanas, 2015). Exosomes derived from scratch-wounding cells inhibit wound healing (Zhou et al., 2017). This important subclass of extracellular vesicles, which exhibit diverse intercellular communication biofunctions including antigen presentation, tumor progression and tissue regeneration regulation, have gained much attention in biomedical research (Malda et al., 2016; Lindenbergh and Stoorvogel, 2018; Duan et al., 2020). Cumulated evidence has shown that exosomes derived from MSCs exert beneficial effects on various disease models, including models of myocardial infarction (Zhao et al., 2015; Zhang et al., 2016; Liu et al., 2017; Ma et al., 2018), hepatic fibrosis (Li et al., 2013; Jiang et al., 2018) and even cancer (Reza et al., 2016; Phinney and Pittenger, 2017; Qi et al., 2017; Kim et al., 2018b).
In the peripheral nerve system, SCs efficiently regulate many functions of axons and ongoing regenerative processes after nerve injury (Willand et al., 2016). Conversely, the regenerative capability in the central nervous system is poor because of glial responses that restrict regeneration and the intrinsic limitations of neurons (Filbin, 2003). After nerve damage, SCs dedifferentiate to a progenitor-like state and increase conduction velocities by myelinating axons as a source of trophic factors to neurons (Lopez-Verrilli et al., 2013). Importantly, the maintenance and development of peripheral nerves are dependent on local signaling between axons and SCs (Nocera and Jacob, 2020). The dedifferentiated SCs proliferate and provide a mechanical substrate and growth factors to regenerate axons. During this process, SCs secreted factors may be involved in processes activated after nerve injury. However, whether these effector molecules are soluble or delivered to axons using vesicular vectors has not been evaluated. Webber et al. (2011) demonstrated that transfer of SC-derived exosomes to axons constitutes a novel mechanism to facilitate the regeneration of nervous system. The clinical use of SC-derived exosomes is also emerging, as in reports that exploit the endogenous pro-regenerative activity of SC-derived exosomes by electroporating exosomes in vitro to load specific siRNAs (Alvarez-Erviti et al., 2011; Guo et al., 2019). These studies have indicated that MSC-derived extracellular vesicles exert functions similar to those of SCs by beneficially promoting peripheral nerve regeneration. It has been indicated that MSC-derived exosomes, as cellular paracrine products, may play a major role in recovery after PNI (Zhang et al., 2015a). Exosomes secreted from MSCs have a protective effect against glutamate-induced neuronal damage that may be mediated through the PI3-Akt signaling pathway (Wei et al., 2016). There is also increasing evidence to suggest that exosome miRNAs are ideal candidates for disease-related biomarkers with various applications in disease management and prevention programs (Bu et al., 2019). Although the exact biological functions of MSC exosomes have not been completely elucidated, it is widely accepted that they play a key role in the amelioration of disease (Huo et al., 2020; Li et al., 2020). Consequently, they are now subject to much research interest.
Neural Conduits of Mesenchymal Stem Cells for Peripheral Nerve Injury Repair
Neural conduit tissue engineering uses a supporting tubular type of scaffold to complement therapeutic technology for an autologous nerve graft and has been widely applied for peripheral nerve regeneration. At its core is the establishment of three-dimensional neural conduits composed of biological materials and seed cells (Kim et al., 2018a). Nerve conduits can prevent the ingrowth of surrounding tissue and reduce the incidence of neuromas (Ma et al., 2017). Although MSCs are transplanted to the stump of nerve injury by local injection to promote nerve repair and have achieved good repair results, some researchers have noticed that uncontrollable infiltration of surrounding tissues seriously affects the reconstruction of normal neural structures and there is a high probability of nerve scars (Cooney et al., 2016). However, nerve conduits loaded with MSCs could both promote nerve growth and regulate the microenvironment of the injured area. Therefore, the emergence of nerve conduit technology may solve the related problems of scarring and penetration by adjacent tissues.
To date, MSCs have been applied to biological tissue engineering through various techniques to improve the regeneration and repair effect after PNI. Adipose-derived MSCs come from a wide range of sources and are one of the most easily available types of MSCs. After Zuk et al. (2001) first reported the method of obtaining adipose-derived MSCs, they became popular among researchers. Adipose-derived MSCs have multi-directional differentiation potential and later studies confirmed that they can differentiate into neuron-like cells and nerve cells. Research on the application of adipose-derived MSCs in the repair of PNI has been conducted in various animal models (Mathot et al., 2020; Zhou et al., 2020). Ghoreishian et al. (2013) extracted undifferentiated autologous adipose-derived MSCs from canine adipose tissue to repair canine facial nerve injury. They encapsulated undifferentiated adipose-derived MSCs in alginate hydrogels and successfully repaired a 7-mm nerve defect gap (Ghoreishian et al., 2013). Allbright et al. (2018) also used adipose-derived MSCs loaded in hydrogels for the repair of sciatic nerve defects in rats and showed an effective repair outcome. Sowa et al. (2016) loaded adipose-derived MSCs and SCs into gelatin hydrogel catheters and transplanted them to the sciatic nerve injury of mice. All these results showed that adipose-derived MSCs transplantation can promote axon regeneration, myelin formation and recovery from denervated muscle atrophy, results that are equivalent to the repair effect of Schwann cell transplantation (Sowa et al., 2016). However, the undifferentiated characteristics of adipose-derived MSCs inevitably change after multiple generations of proliferation, and the risk of tumor formation in vivo needs to be assessed in further studies.
Bone marrow MSCs are mainly derived from bone marrow tissue and have strong differentiation potentials towards osteocytes, cartilage and fat. Studies have shown that bone marrow MSCs can differentiate into neuron-like and glial-like cells in the presence of specific growth factors, which provides a theoretical basis for bone marrow MSCs for nerve injury repair (Guan et al., 2014). Bone marrow MSCs have the advantage that they do not stimulate the significant proliferation of T cells and are not targeted by CD8+ T cells (Kim et al., 2018c). Therefore, when applied to autologous or allogeneic transplants, bone marrow MSCs could avoid the killing and elimination of immune cells in the body, thereby playing a repair role in PNIs. A previous study showed bone marrow MSCs in a polyglycolic acid nerve conduit could repair facial nerve defects in rats (Costa et al., 2013). The results demonstrated that bone marrow MSCs can be successfully integrated into the conduits, and survive up to 6 weeks in nerve tissue (Costa et al., 2013). In another study, researchers loaded bone marrow MSCs into a chitosan nerve duct and observed that the cells survived and proliferated within the duct for a period of 8–16 weeks, successfully promoting the repair of 8 mm nerve defects (Zheng and Cui, 2010). In a subsequent study, chitosan nerve conduits loaded with bone marrow MSCs not only accelerated the efficiency of nerve repair but also improved the quantity and quality of regenerated nerve fibers, obtaining a therapeutic effect comparable to autologous nerve transplantation (Zheng and Cui, 2012). Unfortunately, degradation products of nerve conduit materials often cause local immune responses, leaving the injury site in an inflammatory state, which affects the repair effect (Duffy et al., 2019). Thus, Hsu et al. (2013) modified chitosan neural conduits with laminin, which enhanced the adhesion of bone marrow MSCs to the conduits and observed successful inhibition of local inflammatory responses caused by the degradation of chitosan. Umbilical cord MSCs, which are more convenient to obtain compared with bone marrow MSCs and are less likely to be contaminated than adipose-derived MSCs, are highly proliferative cells. Cui et al. (2018) showed that the collagen catheters combined with umbilical cord MSCs promoted regeneration and repair after sciatic nerve transaction. The results demonstrated that collagen catheters could provide mechanical support for neural regeneration and, combined with umbilical cord MSCs, effectively promoted neural regeneration and functional recovery. In summary, MSCs tissue engineered nerve conduits could provide support for MSCs as a substitute for nerve transplantation and prevent the infiltration of tissues from around the injury site to the nerve stump, presenting satisfactory repairs for PNIs.
Application of Genetic Engineering of Mesenchymal Stem Cells in Peripheral Nerve Injury
Gene modification mainly refers to the use of biochemical methods to either modify the DNA sequence or introduce the target gene fragment into the host cell or delete specific gene fragments from the genome to achieve the effect of changing the host cell genotype or strengthening the original genotype (Gupta and Shukla, 2017). Once a modified gene is introduced into a cell, it can over-express a specific gene or constructed gene, and continuously synthesize a specific protein, making it more sensitive to physiological stimuli, including high blood sugar, hormone concentrations, drugs or chemical factors (Liang et al., 2009; Barros and Offenbacher, 2014). Original or genetically modified MSCs have been used in the regeneration of liver, heart muscle, nerves, bones, tendons, and other connective tissues. Normal MSCs have the disadvantages of short survival time and low concentration at the injury site after implantation into the host, whereas genetically modified MSCs can overcome the above defects. By optimizing stem cell and vector selection, effectively introducing specific genes into MSCs and verifying the ability of the modified MSC to express proteins through in vitro experiments, the selected modified MSCs can be used in the clinic to treat acquired and hereditary diseases (Wakitani et al., 2007). In the field of neural repair tissue engineering, the main purpose of genetic modification is to design target cells so that they can release growth factors, migration molecules and adhesion molecules in excess and can inhibit the expression of defective genes.
Genetically modified MSCs are widely applied in nervous system research. Kim et al. (2008) demonstrated that transcription factor, neurogenin 1 (Ngn1), can induce MSCs to differentiate into neurons, which present voltage-gated L-type calcium channels and tetrodotoxin-sensitive voltage-gated sodium channels. Transplantation of Ngn1-modified MSCs into the skull of a rat model of stroke was shown to promote contact between neurons and improve conduction function. Human telomerase reverse transcriptase-modified MSCs have be used for functional recovery in rat cerebral ischemia models (Honma et al., 2006; Kim et al., 2008). Modified MSCs can also promote neuronal cell survival and neurogenesis through a type of neuroregulatory molecule brain-derived neurotrophic factor (Ko et al., 2018). Zhang et al. (2015b) employed bone marrow MSCs transfected with brain-derived neurotrophic factor and ciliary neurotrophic factor for the treatment of sciatic nerve injury in rats. These MSCs combined with nerve transplantation improved the sciatic nerve function and increased the thickness of regenerating nerve myelin sheath, which suggests that they effectively promoted the growth of axons and facilitated the repair of PNIs.
Studies have shown that microRNAs play an important role in neural development and the differentiation of stem cells into neural cells. Researchers aim to promote the differentiation of MSCs into nerve cells by manipulating the expression of specific microRNAs. For example, Hu et al. (2017) achieved a good outcome by regulating microRNA-218 of adipose-derived MSCs and using it to repair sciatic nerve injury in rats. Furthermore, Wang et al. (2014) illustrated that JMJD1C can maintain microRNA-302 expression through epigenetics, thereby inhibiting neural differentiation of stem cells. Therefore, knocking out JMJD1C can be beneficial to the neural differentiation ability of stem cells.
Genetically modified MSCs have multiple potentials in the treatment of PNIs. Transplanted, appropriately genetically modified MSCs can survive longer in the host environment and secrete specific proteins that are beneficial to the patient’s need. The urgent problem to be solved is how to accurately improve MSCs, translate specific proteins and effectively apply them to treat peripheral nerve system damage.
Conclusion and Perspectives
Although MSCs can differentiate into nerve cells and regulate the microenvironment of the injured area to accelerate the regeneration of peripheral nerves, the mechanism of MSCs differentiation and the ability to secrete neurotransmitters remain to be clarified. A large number of studies have reported the use of MSCs in treatment of neurological disease, such as multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer’s disease and spinal cord injury models (Squillaro et al., 2016). However, fewer clinical trials have utilized MSCs after PNI. MSCs must be cultivated in vitro to be used clinically because of the limited supply of MSCs in tissues. The cultivation in vitro is performed in an artificial environment, therefore, the cells may be biologically different from freshly extracted MSCs and may cause iatrogenic adverse reactions. In addition, aging is a common feature of all organisms, and stem cells are no exception. Thus, it is important to establish a system to detect the degree and proportion of aging and prevent or delay aging of MSCs. The clinical application of MSCs raises some ethical and safety concerns. In China, we attach great importance to the ethical issues, safety and effectiveness of stem cells in clinical application. However, with the increase of global clinical stem cell research projects and the accumulation of experience, the cost, safety, and effective consequences after transplantation are urgent problems to solve before MSC treatment is applied clinically.
Additional file: Open peer review report 1 (88.3KB, pdf) .
Footnotes
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was funded by Clinical Medicine Key Project from the Social Development and Collaborative Innovation Plans in Xuzhou City of China, No. KC14SX016 (to DQG). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Michele R Colonna, Universita degli Studi di Messina, Italy.
P-Reviewer: Colonna MR; C-Editor: Zhao M; S-Editors: Qiu Y, Li CH; T-Editor: Jia Y
Funding:This work was funded by Clinical Medicine Key Project from the Social Development and Collaborative Innovation Plans in Xuzhou City of China, No. KC14SX016 (to DQG).
References
- 1.Allbright KO, Bliley JM, Havis E, Kim DY, Dibernardo GA, Grybowski D, Waldner M, James IB, Sivak WN, Rubin JP, Marra KG. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve. 2018;58:251–260. doi: 10.1002/mus.26094. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 3.Baldassarre BM, Lavorato A, Titolo P, Colonna MR, Vincitorio F, Colzani G, Garbossa D, Battiston B. Principles of cortical plasticity in peripheral nerve surgery. Surg Technol Int. 2020;36:444–452. [PubMed] [Google Scholar]
- 4.Barros SP, Offenbacher S. Modifiable risk factors in periodontal disease: epigenetic regulation of gene expression in the inflammatory response. Periodontol 2000. 2014;64:95–110. doi: 10.1111/prd.12000. [DOI] [PubMed] [Google Scholar]
- 5.Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton MJ, John JS, Clarke M, Wright A, Ekberg J. The glia response after peripheral nerve injury: a comparison between schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int J Mol Sci. 2017;18:287. doi: 10.3390/ijms18020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassilios Habre S, Bond G, Jing XL, Kostopoulos E, Wallace RD, Konofaos P. The surgical management of nerve gaps: present and future. Ann Plast Surg. 2018;80:252–261. doi: 10.1097/SAP.0000000000001252. [DOI] [PubMed] [Google Scholar]
- 8.Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, Duking T, Winchenbach J, Neuber J, Ewers D, Scholz P, van der Meer F, Cantuti-Castelvetri L, Sasmita AO, Meschkat M, Ruhwedel T, Mobius W, Sankowski R, Prinz M, Huitinga I, et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat Neurosci. 2021;24:47–60. doi: 10.1038/s41593-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bu H, He D, He X, Wang K. Exosomes: isolation, analysis, and applications in cancer detection and therapy. Chembiochem. 2019;20:451–461. doi: 10.1002/cbic.201800470. [DOI] [PubMed] [Google Scholar]
- 10.Bunn SJ, Lai A, Li J. DC electric fields induce perpendicular alignment and enhanced migration in Schwann cell cultures. Ann Biomed Eng. 2019;47:1584–1595. doi: 10.1007/s10439-019-02259-4. [DOI] [PubMed] [Google Scholar]
- 11.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Chang HM, Shyu MK, Tseng GF, Liu CH, Chang HS, Lan CT, Hsu WM, Liao WC. Neuregulin facilitates nerve regeneration by speeding Schwann cell migration via ErbB2/3-dependent FAK pathway. PLoS One. 2013;8:e.53444. doi: 10.1371/journal.pone.0053444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemnitz A, Andersson G, Rosen B, Dahlin LB, Bjorkman A. Poor electroneurography but excellent hand function 31 years after nerve repair in childhood. Neuroreport. 2013;24:6–9. doi: 10.1097/WNR.0b013e32835b6efd. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Xu Y, Zhang T, Ma Y, Liu J, Yuan B, Chen X, Zhou P, Zhao X, Pang F, Liang W. Mesenchymal stem cell sheets: a new cell-based strategy for bone repair and regeneration. Biotechnol Lett. 2019;41:305–318. doi: 10.1007/s10529-019-02649-7. [DOI] [PubMed] [Google Scholar]
- 15.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmuller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci Rep. 2016;6:31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa HJ, Bento RF, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013;1510:10–21. doi: 10.1016/j.brainres.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Yao Y, Zhao Y, Xiao Z, Cao Z, Han S, Li X, Huan Y, Pan J, Dai J. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med. 2018;12:1285–1296. doi: 10.1002/term.2660. [DOI] [PubMed] [Google Scholar]
- 19.De la Rosa MB, Kozik EM, Sakaguchi DS. Adult stem cell-based strategies for peripheral nerve regeneration. Adv Exp Med Biol. 2018;1119:41–71. doi: 10.1007/5584_2018_254. [DOI] [PubMed] [Google Scholar]
- 20.Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, Liang Y, Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2020;13:1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- 21.Duffy P, McMahon S, Wang X, Keaveney S, O’Cearbhaill ED, Quintana I, Rodriguez FJ, Wang W. Synthetic bioresorbable poly-alpha-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater Sci. 2019;7:4912–4943. doi: 10.1039/c9bm00246d. [DOI] [PubMed] [Google Scholar]
- 22.El Soury M, Gambarotta G. Soluble neuregulin-1 (NRG1): a factor promoting peripheral nerve regeneration by affecting Schwann cell activity immediately after injury. Neural Regen Res. 2019;14:1374–1375. doi: 10.4103/1673-5374.253516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Fex Svennigsen A, Dahlin LB. Repair of the peripheral nerve-remyelination that works. Brain Sci. 2013;3:1182–1197. doi: 10.3390/brainsci3031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 26.Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013;71:577–587. doi: 10.1016/j.joms.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Godinho MJ, Staal JL, Krishnan VS, Hodgetts SI, Pollett MA, Goodman DP, Teh L, Verhaagen J, Plant GW, Harvey AR. Regeneration of adult rat sensory and motor neuron axons through chimeric peroneal nerve grafts containing donor Schwann cells engineered to express different neurotrophic factors. Exp Neurol. 2020;330:113355. doi: 10.1016/j.expneurol.2020.113355. [DOI] [PubMed] [Google Scholar]
- 28.Gordon T. Peripheral nerve regeneration and muscle reinnervation. Int J Mol Sci. 2020;21:8652. doi: 10.3390/ijms21228652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan M, Xu Y, Wang W, Lin S. Differentiation into neurons of rat bone marrow-derived mesenchymal stem cells. Eur Cytokine Netw. 2014;25:58–63. doi: 10.1684/ecn.2014.0357. [DOI] [PubMed] [Google Scholar]
- 30.Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SK, Shukla P. Gene editing for cell engineering: trends and applications. Crit Rev Biotechnol. 2017;37:672–684. doi: 10.1080/07388551.2016.1214557. [DOI] [PubMed] [Google Scholar]
- 32.Heermann S, Schwab MH. Molecular control of Schwann cell migration along peripheral axons: keep moving! Cell Adh Migr. 2013;7:18–22. doi: 10.4161/cam.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirasawa Y, Sakakida K. Sports and peripheral nerve injury. Am J Sports Med. 1983;11:420–426. doi: 10.1177/036354658301100607. [DOI] [PubMed] [Google Scholar]
- 34.Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu SH, Kuo WC, Chen YT, Yen CT, Chen YF, Chen KS, Huang WC, Cheng H. New nerve regeneration strategy combining laminin-coated chitosan conduits and stem cell therapy. Acta Biomater. 2013;9:6606–6615. doi: 10.1016/j.actbio.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Hu F, Sun B, Xu P, Zhu Y, Meng XH, Teng GJ, Xiao ZD. MiR-218 induces neuronal differentiation of ASCs in a temporally sequential manner with fibroblast growth factor by regulation of the Wnt signaling pathway. Sci Rep. 2017;7:39427. doi: 10.1038/srep39427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo W, Li Y, Zhang Y, Li H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. FASEB J. 2020;34:13345–13360. doi: 10.1096/fj.202000102RR. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. doi: 10.1155/2018/6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HA, Pomeroy SL, Whoriskey W, Pawlitzky I, Benowitz LI, Sicinski P, Stiles CD, Roberts TM. A developmentally regulated switch directs regenerative growth of Schwann cells through cyclin D1. Neuron. 2000;26:405–416. doi: 10.1016/s0896-6273(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 40.Kim JI, Kim CS, Park CH. Harnessing nanotopography of electrospun nanofibrous nerve guide conduits (NGCs) for neural tissue engineering. Adv Exp Med Biol. 2018;1078:395–408. doi: 10.1007/978-981-13-0950-2_20. [DOI] [PubMed] [Google Scholar]
- 41.Kim R, Lee S, Lee J, Kim M, Kim WJ, Lee HW, Lee MY, Kim J, Chang W. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018;51:406–411. doi: 10.5483/BMBRep.2018.51.8.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SH, Jung J, Cho KJ, Choi JH, Lee HS, Kim GJ, Lee SG. Immunomodulatory effects of placenta-derived mesenchymal stem cells on T cells by regulation of FoxP3 expression. Int J Stem Cells. 2018;11:196–204. doi: 10.15283/ijsc18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 44.Kizilay Z, Aktas S, Kahraman Cetin N, Bakay Ilhan D, Ersoy G, Erken HA. Effect of systemic application of bone marrow-derived mesenchymal stem cells on healing of peripheral nerve injury in an experimental sciatic nerve injury model. Turk Neurosurg. 2017 doi: 10.5137/1019-5149.JTN.20811-17.1. doi: 105137/1019-5149JTN20811-171. [DOI] [PubMed] [Google Scholar]
- 45.Ko HR, Ahn SY, Chang YS, Hwang I, Yun T, Sung DK, Sung SI, Park WS, Ahn JY. Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res Ther. 2018;9:326. doi: 10.1186/s13287-018-1052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 47.Kvist M, Sondell M, Kanje M, Dahlin LB. Regeneration in, and properties of, extracted peripheral nerve allografts and xenografts. J Plast Surg Hand Surg. 2011;45:122–128. doi: 10.3109/2000656X.2011.571847. [DOI] [PubMed] [Google Scholar]
- 48.Labroo P, Hilgart D, Davis B, Lambert C, Sant H, Gale B, Shea JE, Agarwal J. Drug-delivering nerve conduit improves regeneration in a critical-sized gap. Biotechnol Bioeng. 2019;116:143–154. doi: 10.1002/bit.26837. [DOI] [PubMed] [Google Scholar]
- 49.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Wang Y, Shi L, Li B, Li J, Wei Z, Lv H, Wu L, Zhang H, Yang B, Xu X, Jiang J. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology. 2020;18:113. doi: 10.1186/s12951-020-00670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang H, Balas B, Tantiwong P, Dube J, Goodpaster BH, O’Doherty RM, DeFronzo RA, Richardson A, Musi N, Ward WF. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab. 2009;296:E945–954. doi: 10.1152/ajpendo.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. 2018;36:435–459. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43:52–68. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- 55.Loboda A, Mucha O, Podkalicka P, Sobczak M, Miksza-Cybulska A, Kaczara P, Jozkowicz A, Dulak J. Kidney injury by cyclosporine A is aggravated in heme oxygenase-1 deficient mice and involves regulation of microRNAs. Acta Biochim Pol. 2018;65:613–620. doi: 10.18388/abp.2018_2658. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 58.Lv W, Deng B, Duan W, Li Y, Liu Y, Li Z, Xia W, Li C. Schwann cell plasticity is regulated by a weakened intrinsic antioxidant defense system in acute peripheral nerve injury. Neuroscience. 2018;382:1–13. doi: 10.1016/j.neuroscience.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Ma F, Zhu T, Xu F, Wang Z, Zheng Y, Tang Q, Chen L, Shen Y, Zhu J. Neural stem/progenitor cells on collagen with anchored basic fibroblast growth factor as potential natural nerve conduits for facial nerve regeneration. Acta Biomater. 2017;50:188–197. doi: 10.1016/j.actbio.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 60.Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, Yu Y, Huang H, Hu Y, Yang Z, Yang J, Shen Z. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018;2018:3290372. doi: 10.1155/2018/3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malda J, Boere J, van de Lest CH, van Weeren P, Wauben MH. Extracellular vesicles - new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12:243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maricevic A, Erceg M. War injuries to the extremities. Mil Med. 1997;162:808–811. [PubMed] [Google Scholar]
- 63.Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception. Neural Regen Res. 2019;14:939–947. doi: 10.4103/1673-5374.250566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathot F, Rbia N, Bishop AT, Hovius SER, Shin AY. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery. 2020;40:585–592. doi: 10.1002/micr.30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muzio MR, Cascella M. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Histology, Axon. [PubMed] [Google Scholar]
- 66.Nakayama K, Kakinoki R, Ikeguchi R, Yamakawa T, Ohta S, Fujita S, Noguchi T, Duncan SF, Hyon SH, Nakamura T. Storage and allogeneic transplantation of peripheral nerve using a green tea polyphenol solution in a canine model. J Brachial Plex Peripher Nerve Inj. 2010;5:17. doi: 10.1186/1749-7221-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77:3977–3989. doi: 10.1007/s00018-020-03516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira MRJ, Pinhatti VR, Silveira MDD, Matzenbacher CA, Freitas TRO, Silva JD, Camassola M, Nardi NB. Isolation and characterization of mesenchymal stem/stromal cells from Ctenomys minutus. Genet Mol Biol. 2018;41:870–877. doi: 10.1590/1678-4685-GMB-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- 70.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 71.Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, Chen H, Yang L, Zhu H, Li Y. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through Hedgehog signaling pathway. Cell Physiol Biochem. 2017;42:2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 72.Reza A, Choi YJ, Yasuda H, Kim JH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016;6:38498. doi: 10.1038/srep38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen B, Chemnitz A, Weibull A, Andersson G, Dahlin LB, Bjorkman A. Cerebral changes after injury to the median nerve: a long-term follow up. J Plast Surg Hand Surg. 2012;46:106–112. doi: 10.3109/2000656X.2011.653257. [DOI] [PubMed] [Google Scholar]
- 74.Sakthivel P, Singh CA, Thakar A, Thirumeni G, Raveendran S, Sharma SC. Masseteric-facial nerve anastomosis: surgical techniques and outcomes-a pilot Indian study. Indian J Otolaryngol Head Neck Surg. 2020;72:92–97. doi: 10.1007/s12070-019-01758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 76.Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, Tabata Y, Mazda O. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plast Reconstr Surg. 2016;137:318.e–330.e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 77.Springer JE, Mu X, Bergmann LW, Trojanowski JQ. Expression of GDNF mRNA in rat and human nervous tissue. Exp Neurol. 1994;127:167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- 78.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 79.Stanec S, Tonkovic I, Stanec Z, Tonkovic D, Dzepina I. Treatment of upper limb nerve war injuries associated with vascular trauma. Injury. 1997;28:463–468. doi: 10.1016/s0020-1383(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 80.Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tonazzini I, Moffa M, Pisignano D, Cecchini M. Neuregulin 1 functionalization of organic fibers for Schwann cell guidance. Nanotechnology. 2017;28:155303. doi: 10.1088/1361-6528/aa6316. [DOI] [PubMed] [Google Scholar]
- 82.Tos P, Ronchi G, Geuna S, Battiston B. Future perspectives in nerve repair and regeneration. Int Rev Neurobiol. 2013;109:165–192. doi: 10.1016/B978-0-12-420045-6.00008-0. [DOI] [PubMed] [Google Scholar]
- 83.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 84.Wang E, Inaba K, Byerly S, Escamilla D, Cho J, Carey J, Stevanovic M, Ghiassi A, Demetriades D. Optimal timing for repair of peripheral nerve injuries. J Trauma Acute Care Surg. 2017;83:875–881. doi: 10.1097/TA.0000000000001570. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Park JW, Drissi H, Wang X, Xu RH. Epigenetic regulation of miR-302 by JMJD1C inhibits neural differentiation of human embryonic stem cells. J Biol Chem. 2014;289:2384–2395. doi: 10.1074/jbc.M113.535799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webber CA, Christie KJ, Cheng C, Martinez JA, Singh B, Singh V, Thomas D, Zochodne DW. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5H2. Glia. 2011;59:1503–1517. doi: 10.1002/glia.21194. [DOI] [PubMed] [Google Scholar]
- 87.Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH. Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:33–36. doi: 10.3881/j.issn.1000-503X.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Willand MP, Nguyen MA, Borschel GH, Gordon T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair. 2016;30:490–496. doi: 10.1177/1545968315604399. [DOI] [PubMed] [Google Scholar]
- 89.Winfree CJ. Peripheral nerve injury evaluation and management. Curr Surg. 2005;62:469–476. doi: 10.1016/j.cursur.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015a;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang YR, Ka K, Zhang GC, Zhang H, Shang Y, Zhao GQ, Huang WH. Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells. Neural Regen Res. 2015b;10:1498–1506. doi: 10.4103/1673-5374.165523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc. 2016;5:e002856. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:761643. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng L, Cui HF. Use of chitosan conduit combined with bone marrow mesenchymal stem cells for promoting peripheral nerve regeneration. J Mater Sci Mater Med. 2010;21:1713–1720. doi: 10.1007/s10856-010-4003-y. [DOI] [PubMed] [Google Scholar]
- 96.Zheng L, Cui HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med. 2012;23:2291–2302. doi: 10.1007/s10856-012-4694-3. [DOI] [PubMed] [Google Scholar]
- 97.Zhou LN, Wang JC, Zilundu PLM, Wang YQ, Guo WP, Zhang SX, Luo H, Zhou JH, Deng RD, Chen DF. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res Ther. 2020;11:153. doi: 10.1186/s13287-020-01661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou X, Zhang W, Yao Q, Zhang H, Dong G, Zhang M, Liu Y, Chen JK, Dong Z. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Physiol Renal Physiol. 2017;312:F963–970. doi: 10.1152/ajprenal.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu H, Xue C, Yao M, Wang H, Zhang P, Qian T, Zhou S, Li S, Yu B, Wang Y, Gu X. miR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 2018;9:720. doi: 10.1038/s41419-018-0760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


