Abstract
Background:
Rhythm control strategies for atrial fibrillation (AF), including catheter ablation, are substantially underused in racial/ethnic minorities in North America.
Objective:
To describe outcomes in the CABANA trial as a function of race/ethnicity.
Methods:
CABANA randomized 2204 symptomatic participants with AF to ablation or drug therapy including rate and/or rhythm control drugs. Only participants in North America were included in this analysis, and participants were subgrouped as racial/ethnic minority or non-minority using National Institutes of Health definitions. The primary endpoint was a composite of death, disabling stroke, serious bleeding, or cardiac arrest.
Results:
Of 1280 participants enrolled in CABANA in North America, 127 (9.9%) were racial and ethnic minorities. Compared with non-minorities, racial and ethnic minorities were younger with median age 65.6 versus 68.5 years, had more symptomatic heart failure (37.0% versus 22.0%, respectively), hypertension (92.1% versus 76.8%, respectively), and an ejection fraction <40% (20.8% versus 7.1%). Racial/ethnic minorities treated with ablation had a 68% relative reduction in the primary endpoint (adjusted hazard ratio [aHR] 0.32, 95% confidence interval [CI] 0.13, 0.78), and a 72% relative reduction in all-cause mortality (aHR 0.28, 95% CI 0.10, 0.79). Primary event rates in racial/ethnic minority and non-minority participants were comparable in the ablation arm (4-year Kaplan-Meier event rates 12.3% versus 9.9%); however, racial and ethnic minorities randomized to drug therapy had a much higher event rate than non-minority participants (27.4% versus 9.4%).
Conclusion:
Among racial or ethnic minorities enrolled in the North American CABANA cohort, catheter ablation significantly improved major clinical outcomes compared with drug therapy. These benefits, which were not seen in non-minority participants, appear due to worse outcomes with drug therapy.
Condensed Abstract:
Catheter ablation to treat atrial fibrillation appears to be underused in racial/ethnic minorities in North America. Of 1280 North American patients in CABANA, 127 were racial/ethnic minorities. Ablation produced a 68% relative reduction in the primary composite endpoint of death, disabling stroke, serious bleeding, or cardiac arrest (adjusted hazard ratio [aHR] 0.32, 95% confidence interval [CI] 0.13, 0.78) in racial/ethnic minorities, and a 72% relative reduction in all-cause mortality (aHR 0.28, 95% CI 0.10, 0.79), compared with medical therapy alone. In this racial/ethnic minority cohort, ablation significantly improved major clinical outcomes compared with drug therapy due to worse outcomes with drug therapy.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT00911508
Keywords: Atrial fibrillation, minority race, outcomes, randomized trials, catheter ablation
INTRODUCTION
The 2014 American College of Cardiology, American Heart Association and Heart Rhythm Society updated guidelines support a more prominent role for catheter ablation in the treatment of atrial fibrillation (AF), including its use as first-line therapy for recurrent symptomatic paroxysmal or persistent AF (1). Historically, Blacks and other racial and ethnic minorities have been underrepresented in clinical trials focused on the management and treatment of AF (2,3). Observational data indicate lower use of rhythm control strategies including catheter ablation in the management of AF among racial and ethnic minorities, and there are no randomized trial data describing the safety and efficacy of catheter ablation therapy to treat AF in these patients (4–10).
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, the largest trial to date of catheter ablation versus drug therapy in AF, found that the strategy of catheter ablation did not significantly improve the composite primary clinical outcome (death, disabling stroke, serious bleeding, or cardiac arrest) compared with drug therapy when analyzed by intention-to-treat (11). Secondary endpoints of death or cardiovascular hospitalization, and AF recurrence were significantly reduced by ablation, and quality of life was improved out to 60 months (11–13). The objective of this analysis was to assess if the response to treatment assignment in CABANA varied according to minority race or ethnicity among patients enrolled in North America (NA).
METHODS
Trial Design and Setting
CABANA is an international, multicenter, unmasked randomized clinical trial that has been registered at ClinicalTrials.gov: NCT0091158. Complete details of the trial design and methods have been previously reported (11,14). Each site’s Institutional Review Board approved the CABANA study, and written informed consent was obtained from all participants.
Study Population
Participants ≥18 years old with electrocardiographic documentation of at least 2 episodes of paroxysmal AF or 1 episode of persistent AF in the 6 months prior to enrollment who were suitable candidates for either catheter ablation or drug therapies were eligible for enrollment (14). To ensure sufficient event rates to detect the hypothesized treatment effect, CABANA required participants to be either age ≥65, or age <65 and have at least one risk factor for stroke (14). For the purpose of this pre-specified racial and ethnic subgroup analysis, individuals were included if they were enrolled at a NA site. Race and ethnicity were self-identified through the enrolling site using the National Institutes of Health (NIH) categories that included: White, Black, Asian, American Indian (AI)/Alaskan Native (AN), Hawaiian or other Pacific Islander (PI), or multiracial. The ethnicity classification options included: Not Hispanic nor Latino or Latina origin; and Hispanic, Latino, or Latina origin. We chose to limit our analysis to North America given the challenges of racial and ethnic minority classification using US-based categories elsewhere. For the present report, individuals were categorized as: racial and ethnic minority (Black, AI/AN, Hawaiian or other PI, multiracial [excluding White and Asian combination], or of Hispanic, Latino or Latina origin), or non-minority (non-Hispanic Whites or Asians). Asian race was not included in the racial and ethnic minority grouping as currently this racial group is not considered a minority as defined by the NIH.
Outcomes
The primary endpoint in CABANA was a composite of all-cause mortality, disabling stroke, serious bleeding, or cardiac arrest (14). Secondary endpoints included all-cause mortality alone as well as the composite of all-cause mortality or cardiovascular hospitalization.
As previously reported, to assess AF recurrence in CABANA, patients were provided with a proprietary recording system (“CABANA Box”), and we prespecified that the primary AF recurrence endpoint outcomes would be limited to the subgroup of patients who used this recording system (11,13). Of the 1280 NA participants in this subgroup analysis, 72 (56.7%) of 127 racial/ethnic minority participants and 958 (83.1%) of 1153 non-minority participants used the CABANA Box. AF recurrence was defined as an episode of atrial fibrillation lasting ≥30 seconds after the blanking period. AF recurrences were adjudicated by the CABANA ECG Core Laboratory (13). The AF recurrence endpoint represents a combination of data from symptom-driven recordings, 24-hour auto-detect recordings, and every 6 month 96-hour continuous Holter monitor recordings. AF burden was assessed as the percentage of time spent in AF based on 96-hour Holter monitor recordings scheduled every six months (13).
Statistical Analysis
Descriptive summary statistics included counts (percentages) for categorical variables, and medians (25th and 75th percentiles) for continuous variables. The primary statistical comparisons were performed with treatment assigned as randomized (intention to treat [ITT]) (11). Kaplan-Meier cumulative event rates were estimated for each treatment group with time-to-event measured (in months) from the time of randomization (15). Treatment effect sizes for most clinical outcomes were expressed as hazard ratios (HRs) with associated 95% confidence intervals (CIs) and were estimated using a covariate-adjusted Cox proportional hazards model (16). The Cox model was stratified by racial and ethnic minority status and was adjusted for pre-specified baseline patient characteristics: age, sex, AF type, years since onset of AF, history of heart failure (HF), structural heart disease, CHA2DS2-VASc score, history of coronary artery disease, and hypertension. An interaction term, treatment group x racial and ethnic minority status, was included in the model. Statistical testing of treatment differences was performed with the Wald test from the Cox model. We examined the proportionality assumption in the Cox model for the primary composite endpoint and for all-cause mortality using the ASSESS statement in PROC PHREG (SAS 9.4), and the proportional hazards assumption was met.
To examine whether clinical outcomes differed in patients with versus without CABANA Box AF recurrence data, we fitted a Cox model for the primary composite endpoint as well as all-cause mortality, including all pre-specified covariates and an interaction term between CABANA Box (use/non-use) and randomized treatment assignment. There were no significant interactions (p=0.87 for primary endpoint, p=0.67 for all-cause mortality). Recurrent atrial fibrillation cumulative incidences were estimated using a Fine-Gray model (17) adjusted for baseline covariates listed above, with death treated as a competing risk.
P values, where provided, are intended as adjunctive interpretive aids reflecting the level of unexpectedness of observed effects under assumption that the relevant null hypothesis is correct (18). No adjustments were made for multiple comparisons. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
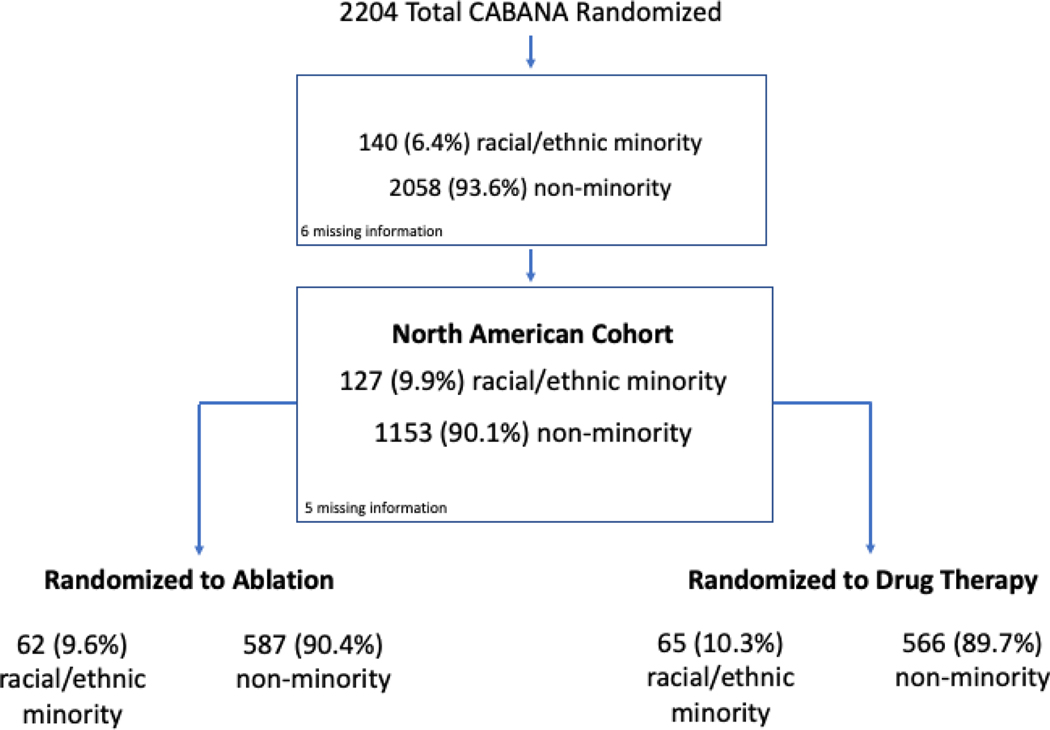
Of the 1285 participants randomized in CABANA in NA, 127 were racial and ethnic minorities (62 ablation, 65 drug therapy) (Figure 1), 1153 were non-minority (587 ablation, 566 drug therapy), and 5 did not identify as belonging to a racial or ethnic category. Of the 127 racial and ethnic minority participants, 66 were non-Hispanic Black (33 ablation, 33 drug), 4 AI/AN (3 ablation, 1 drug), 1 Hawaiian/other PI (drug), 6 non-Hispanic multiracial (3 ablation, 3 drug), 36 White Hispanic (17 ablation, 19 drug), 10 Black Hispanic (5 ablation, 5 drug), 2 unknown race and Hispanic (0 ablation, 2 drug), 1 Hispanic Hawaiian/PI (ablation), and 1 multiracial Hispanic (drug).
Figure 1: CONSORT Diagram for the Racial/Ethnic Minority Subgroup Analysis in CABANA.
This diagram shows the derivation of the cohort for the CABANA subgroup analysis examining outcomes by racial and ethnic minority status in patients enrolled in North America.
Overall median follow-up in the 1280 participants with non-missing racial and ethnic minority status was 54.9 months. Median follow-up was 48.0 months in the 127 racial and ethnic minority participants and 55.5 months in the 1153 non-minority participants.
Baseline Characteristics
Baseline characteristics were largely balanced between the treatment groups for non-minority participants but were less well balanced in the racial and ethnic minority group (Table 1). Among racial and ethnic minority participants, 45.2% of ablation and 52.3% of drug participants were age <65, while 14.5% and 6.2%, respectively, were age ≥75. Female sex was present in 27.4% of ablation participants and 35.4% of drug therapy participants. HF prevalence (New York Heart Association [NYHA] class ≥II) was the same in both arms. Diabetes was more common in the ablation arm (35.5% versus 20.0% for drug arm). Paroxysmal AF was more common in the drug arm (49.2% versus 38.7% in the ablation arm).
Table 1.
Baseline Demographics and Clinical Characteristics by Treatment Among Racial and Ethnic Minority and Nonminority Groups
| Racial/Ethnic Minority | Non-Minority | |||||
|---|---|---|---|---|---|---|
| Characteristic | Total | Ablation | Drug | Total | Ablation | Drug |
| N=127 | N=62 | N=65 | N=1153 | N=587 | N=566 | |
| Age, Median (25th, 75th) | 65.6 (56.8, 70.1) | 66.4 (56.1, 71.7) | 64.5 (57.2, 69.3) | 68.5 (63.9, 72.8) | 68.4 (63.4, 72.9) | 68.5 (64.3, 72.6) |
| Age group | ||||||
| < 65 years | 62/127 (48.8%) | 28/62 (45.2%) | 34/65 (52.3%) | 331/1153 (28.7%) | 171/587 (29.1%) | 160/566 (28.3%) |
| ≥ 65 to <75 years | 52/127 (40.9%) | 25/62 (40.3%) | 27/65 (41.5%) | 646/1153 (56.0%) | 323/587 (55.0%) | 323/566 (57.1%) |
| ≥ 75 years | 13/127 (10.2%) | 9/62 (14.5%) | 4/65 (6.2%) | 176/1153 (15.3%) | 93/587 (15.8%) | 83/566 (14.7%) |
| Female sex | 40/127 (31.5%) | 17/62 (27.4%) | 23/65 (35.4%) | 376/1153 (32.6%) | 194/587 (33.0%) | 182/566 (32.2%) |
| Body Mass Index | N=126 | N=62 | N=64 | N=1139 | N=577 | N=562 |
| Median (25th, 75th) | 31.5 (28.2, 37.5) | 31.8 (28.6, 35.8) | 31.4 (28.1, 37.8) | 31.3 (27.2, 36.1) | 30.9 (27.1, 35.4) | 31.6 (27.4, 36.6) |
| AF severity CCS Median (25th, 75th) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.5 (1.5, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) |
| CCS class | ||||||
| Class 0 | 7/126 (5.6%) | 4/62 (6.5%) | 3/64 (4.7%) | 81/1147 (7.1%) | 43/584 (7.4%) | 38/563 (6.7%) |
| Class 1 | 23/126 (18.3%) | 10/62 (16.1%) | 13/64 (20.3%) | 145/1147 (12.6%) | 72/584 (12.3%) | 73/563 (13.0%) |
| Class 2 | 36/126 (28.6%) | 20/62 (32.3%) | 16/64 (25.0%) | 328/1147 (28.6%) | 158/584 (27.1%) | 170/563 (30.2%) |
| Class 3 | 45/126 | 21/62 | 24/64 | 498/1147 | 259/584 | 239/563 |
| (35.7%) | (33.9%) | (37.5%) | (43.4%) | (44.3%) | (42.5%) | |
| Class 4 | 15/126 (11.9%) | 7/62 (11.3%) | 8/64 (12.5%) | 95/1147 (8.3%) | 52/584 (8.9%) | 43/563 (7.6%) |
| NYHA class II or greater | 47/127 (37.0%) | 23/62 (37.1%) | 24/65 (36.9%) | 252/1147 (22.0%) | 123/584 (21.1%) | 129/563 (22.9%) |
| Medical History | ||||||
| Hypertension | 117/127 (92.1%) | 57/62 (91.9%) | 60/65 (92.3%) | 886/1153 (76.8%) | 448/587 (76.3%) | 438/566 (77.4%) |
| Left ventricular hypertrophy | 35/83 (42.2%) | 19/43 (44.2%) | 16/40 (40.0%) | 271/799 (33.9%) | 148/449 (33.0%) | 123/350 (35.1%) |
| Diabetes | 35/127 (27.6%) | 22/62 (35.5%) | 13/65 (20.0%) | 328/1153 (28.4%) | 156/587 (26.6%) | 172/566 (30.4%) |
| Sleep apnea | 38/127 (29.9%) | 16/62 (25.8%) | 22/65 (33.8%) | 389/1153 (33.7%) | 199/587 (33.9%) | 190/566 (33.6%) |
| Coronary artery disease | 31/127 (24.4%) | 17/62 (27.4%) | 14/65 (21.5%) | 267/1153 (23.2%) | 132/587 (22.5%) | 135/566 (23.9%) |
| History of heart failure | 46/127 (36.2%) | 26/62 (41.9%) | 20/65 (30.8%) | 206/1153 (17.9%) | 107/587 (18.2%) | 99/566 (17.5%) |
| Family history of atrial fibrillation | 13/126 (10.3%) | 5/61 (8.2%) | 8/65 (12.3%) | 186/1150 (16.2%) | 98/586 (16.7%) | 88/564 (15.6%) |
| History of cerebrovascular accident or transient ischemic attack | 16/127 (12.6%) | 10/62 (16.1%) | 6/65 (9.2%) | 124/1153 (10.8%) | 64/587 (10.9%) | 60/566 (10.6%) |
| Prior cerebrovascular accident | 11/127 (8.7%) | 7/62 (11.3%) | 4/65 (6.2%) | 64/1153 (5.6%) | 33/587 (5.6%) | 31/566 (5.5%) |
| Thromboembolic events | 6/127 (4.7%) | 1/62 (1.6%) | 5/65 (7.7%) | 52/1153 (4.5%) | 23/587 (3.9%) | 29/566 (5.1%) |
| Ejection Fraction | ||||||
| Median | 55.0 (40.0, 60.0) | 55.0 (44.0, 60.0) | 55.0 (40.0, 60.0) | 55.0 (52.0, 60.0) | 57.0 (53.0, 60.0) | 55.0 (50.0, 60.0) |
| <40% | 15/72 (20.8%) | 7/37 (18.9%) | 8/35 (22.9%) | 53/743 (7.1%) | 31/405 (7.7%) | 22/338 (6.5%) |
| ≥ 40% - 49% | 13/72 (18.1%) | 7/37 (18.9%) | 6/35 (17.1%) | 83/743 (11.2%) | 37/405 (9.1%) | 46/338 (13.6%) |
| ≥ 50% | 44/72 (61.1%) | 23/37 (62.2%) | 21/35 (60.0%) | 607/743 (81.7%) | 337/405 (83.2%) | 270/338 (79.9%) |
| Creatinine mg/dL, Median | 1.0 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.3) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.1) |
| eGFR mL/min/1.73m2, Median | 75.8 (62.2, 89.6) | 75.0 (62.1, 90.0) | 76.8 (63.4, 88.9) | 73.5 (61.6, 86.5) | 73.0 (61.2, 86.8) | 74.1 (61.8, 85.9) |
| CHA2DS2-VASc score | ||||||
| Median | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) |
| 0 – 1 | 20/127 (15.7%) | 8/62 (12.9%) | 12/65 (18.5%) | 181/1153 (15.7%) | 101/587 (17.2%) | 80/566 (14.1%) |
| 2 – 3 | 66/127 (52.0%) | 31/62 (50.0%) | 35/65 (53.8%) | 622/1153 (53.9%) | 297/587 (50.6%) | 325/566 (57.4%) |
| 4 – 5 | 29/127 (22.8%) | 16/62 (25.8%) | 13/65 (20.0%) | 284/1153 (24.6%) | 161/587 (27.4%) | 123/566 (21.7%) |
| 6 – 7 | 10/127 (7.9%) | 6/62 (9.7%) | 4/65 (6.2%) | 64/1153 (5.6%) | 28/587 (4.8%) | 36/566 (6.4%) |
| ≥ 8 | 2/127 (1.6%) | 1/62 (1.6%) | 1/65 (1.5%) | 2/1153 (0.2%) | 0/587 (0.0%) | 2/566 (0.4%) |
| Arrhythmia History | N=126 | N=61 | N=65 | N=1148 | N=584 | N=564 |
| Years since onset of atrial fibrillation, median | 0.8 (0.2, 2.6) | 1.0 (0.2, 3.8) | 0.8 (0.2, 2.0) | 0.8 (0.2, 3.3) | 0.8 (0.2, 3.7) | 0.8 (0.2, 2.9) |
| Type of atrial fibrillation at | ||||||
| enrollment | ||||||
| Paroxysmal | 56/127 (44.1%) | 24/62 (38.7%) | 32/65 (49.2%) | 510/1153 (44.2%) | 253/587 (43.1%) | 257/566 (45.4%) |
| Persistent or long standing persistent | 71/127 (55.9%) | 38/62 (61.3%) | 33/65 (50.8%) | 643/1153 (55.8%) | 334/587 (56.9%) | 309/566 (54.6%) |
| Prior hospitalization for atrial fibrillation | 43/127 (33.9%) | 21/62 (33.9%) | 22/65 (33.8%) | 375/1152 (32.6%) | 196/586 (33.4%) | 179/566 (31.6%) |
| Prior direct cardioversion for atrial fibrillation | 39/127 (30.7%) | 20/62 (32.3%) | 19/65 (29.2%) | 449/1152 (39.0%) | 224/586 (38.2%) | 225/566 (39.8%) |
| Current or past use of rate control drugs at baseline | 124/125 (99.2%) | 59/60 (98.3%) | 65/65 (100.0%) | 1065/1135 (93.8%) | 537/576 (93.2%) | 528/559 (94.5%) |
| Prior use of rhythm control drugs | ||||||
| 1 Rhythm control drug | 49/61 (80.3%) | 25/29 (86.2%) | 24/32 (75.0%) | 465/543 (85.6%) | 214/253 (84.6%) | 251/290 (86.6%) |
| ≥ 2 Rhythm control drugs | 12/61 (19.7%) | 4/29 (13.8%) | 8/32 (25.0%) | 78/543 (14.4%) | 39/253 (15.4%) | 39/290 (13.4%) |
CCS=Canadian Cardiovascular Society, eGFR=estimated glomular filtration rate, mg/dL=milligrams per deciliter, mL/min=milliliters per minute, NYHA=New York Heart Association
Racial and ethnic minority compared with non-minority participants (Table 1) were younger (median age 65.6 years versus 68.5 years), more likely to have NYHA class II or greater HF symptoms (37.0% versus 22.0%), and to have a history of hypertension (92.1% versus 76.8%). Additionally, racial and ethnic minority participants had higher rates of left ventricular hypertrophy (42.2% versus 33.9%), and among those with an ejection fraction (EF) assessment (72 racial and ethnic minority participants, 743 non-minority participant), more racial and ethnic minorities individuals had an EF <40% (20.8% versus 7.1% for non-minorities). There were no differences in years since AF diagnosis or type of AF at enrollment (44.1% and 44.2% paroxysmal for minority and non-minorities, respectively).
Treatment Data
Ablation
Among the 62 racial and ethnic minority individuals randomized to catheter ablation, 9 (14.5%) never received catheter ablation. In the non-minority individuals randomized to catheter ablation 39/587 (6.6%) did not receive catheter ablation. The most common reasons for no ablation were: principal investigator decision due to medical condition (33% racial and ethnic minority patients, 13% non-minority patients), patient decision (44% racial/ethnic minority, 67% non-minority), and other personal reasons (22% racial and ethnic minority, 13% non-minority).
In the racial and ethnic minority group randomized to and receiving catheter ablation, 21/52 (40.4%) received a rhythm-control drug in the post blanking period compared with 251/542 (46.3%) non-minorities. Among racial and ethnic minorities randomized to drug therapy, 27.7% also received catheter ablation versus 32.5% of non-minorities.
Medication Use
Following the blanking period, rate control medication use was well balanced by treatment group in both racial and ethnic minority and non-minority subgroups (Table 2). Beta blockers were the predominant rate control medications used and digoxin the least used for both groups.
Table 2.
Rate and Rhythm Control Therapies at the End of Blanking Period* Among Racial and Ethnic Minority and Non-minority Groups
| Racial/Ethnic Minority Participants | Non-minority Participants | |||
|---|---|---|---|---|
| Medication | Drug | Ablation | Drug | Ablation |
| Rate Control | 59/62 (95.2%) | 49/52 (94.2%) | 484/557 (86.9%) | 459/542 (84.7%) |
| Beta Blocker | 52/62 (83.9%) | 47/52 (90.4%) | 402/557 (72.2%) | 403/542 (74.4%) |
| Calcium Blocker | 29/62 (46.8%) | 25/52 (48.1%) | 237/557 (42.5%) | 195/542 (36.0%) |
| Digoxin | 8/62 (12.9%) | 6/52 (11.5%) | 63/557 (11.3%) | 37/542 (6.8%) |
| Rhythm Control | 50/62 (80.6%) | 21/52 (40.4%) | 500/557 (89.8%) | 251/542 (46.3%) |
| Dronedarone | 4/62 (6.5%) | 4/52 (7.7%) | 82/557 (14.7%) | 43/542 (7.9%) |
| Quinidine | 0 | 0 | 0 | 0 |
| Flecainide | 5/62 (8.1%) | 2/52 (3.8%) | 133/557 (23.9%) | 58/542 (10.7%) |
| Propafenone | 3/62 (4.8%) | 0 | 50/557 (9.0%) | 18/542 (3.3%) |
| Sotalol | 12/62 (19.4%) | 7/52 (13.5%) | 123/557 (22.1%) | 68/542 (12.5%) |
| Dofetilide | 17/62 (27.4%) | 3/52 (5.8%) | 157/557 (28.2%) | 60/542 (11.1%) |
| Amiodarone | 26/62 (41.9%) | 9/52 (17.3%) | 168/557 (30.2%) | 88/542 (16.2%) |
The blanking period was defined as the 90 days after receiving randomized treatment. For patients randomized to ablation, however, a 3-month window was added after the blanking period in order to allow time to resolve whether medications that may have been used during the blanking period would be stopped or continued.
Among racial and ethnic minorities, after the blanking period rhythm control medications were used in 40.4% of ablation arm participants versus 80.6% of drug arm participants (Table 2). For non-minority participants, the corresponding rates were 46.3% and 89.8%. Racial and ethnic minorities relative to non-minorities in the drug arm were less likely to be treated with dronedarone: 6.5% versus 14.7%; or flecainide: 8.1% versus 23.9%. Conversely, amiodarone was more commonly used in racial and ethnic minority participants in the drug arm: 41.9% racial and ethnic minorities versus 30.2% non-minorities. Overall, sotalol and dofetilide were used in approximately 21.8% and 28.1% of drug-arm participants, respectively, with no notable differences between racial and ethnic minority and non-minority participants (Table 2).
Adverse Events
Non-endpoint adverse events in the ablation and drug treatment groups were infrequent and did not vary significantly between racial and ethnic minorities and non-minorities. The most common adverse events with ablation included hematoma 2.7%, pseudo aneurysm 1.2%, and severe pericardial chest pain 1.9%. The most common treatment-related adverse events in the drug therapy arm included hyper- or hypothyroidism 2.0%, major proarrhythmic event 1.6%, gastrointestinal abnormality excluding moderate/severe diarrhea 1.5%, and allergic reaction 1.2% (Supplemental Table 1).
Cardiac arrest, ventricular fibrillation, or ventricular tachycardia events occurred in 6.3% of racial and ethnic minority patients and 2.3% of non-minorities. None of these events were assessed by site investigators as definitely or probably related to drug therapy.
Outcomes by Treatment Among Racial and Ethnic Minorities
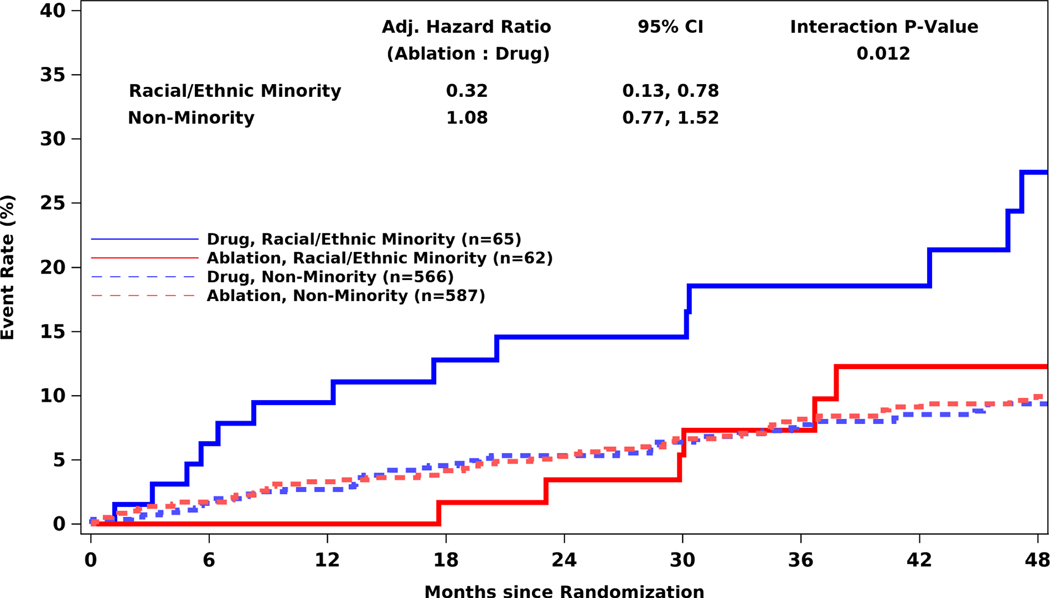
The CABANA primary outcome event (death, disabling stroke, serious bleeding, or cardiac arrest) occurred in 8/62 (12.9%) racial and ethnic minority participants in the catheter ablation arm and in 15/65 (23.1%) in the drug therapy arm (aHR for ablation versus drug therapy 0.32; 95% CI, 0.13 to 0.78) (Table 3, Central Illustration).
Table 3.
Clinical Endpoint Event Rates Among Racial and Ethnic Minorities by Treatment
| Events, N (%) | Kaplan-Meier 4-Year Event Rate, % | ||||
|---|---|---|---|---|---|
| Ablation (n = 62) | Drug (n = 65) | Ablation (n = 62) | Drug (n = 65) | Adjusted Hazard Ratio* (95% CI) (Ablation : Drug) | |
| Primary Endpoint (all-cause mortality, disabling stroke, serious bleeding, or cardiac arrest) | 8 (12.9) | 15 (23.1) | 12.3 | 27.4 | 0.32 (0.13, 0.78) |
| All-cause mortality | 6 (9.7) | 12 (18.5) | 8.1 | 20.2 | 0.28 (0.10, 0.79) |
| Disabling stroke | 0 (0.0) | 2 (3.1) | |||
| Serious bleeding | 1 (1.6) | 4 (6.2) | |||
| Cardiac arrest | 1 (1.6) | 3 (4.6) | |||
| Secondary Endpoint: Death or cardiovascular hospitalization | 37 (59.7) | 45 (69.2) | 59.4 | 70.7 | 0.73 (0.47, 1.13) |
Hazard ratio and associated CI=confidence interval were calculated using an adjusted Cox model.
Central Illustration: Kaplan-Meier Estimates of the Primary Composite Endpoint Among Racial and Ethnic Minorities and Non-minorities by Randomized Treatment in CABANA.
Kaplan-Meier estimates of the cumulative risk of having a primary endpoint event by intention-to-treat analysis. In the non-minority group, the outcomes do not differ significantly between treatment groups. In the racial and ethnic minority subgroup, patients randomized to ablation have a lower risk of having a primary endpoint event out to 4 years compared to drug therapy alone. CI=confidence interval.
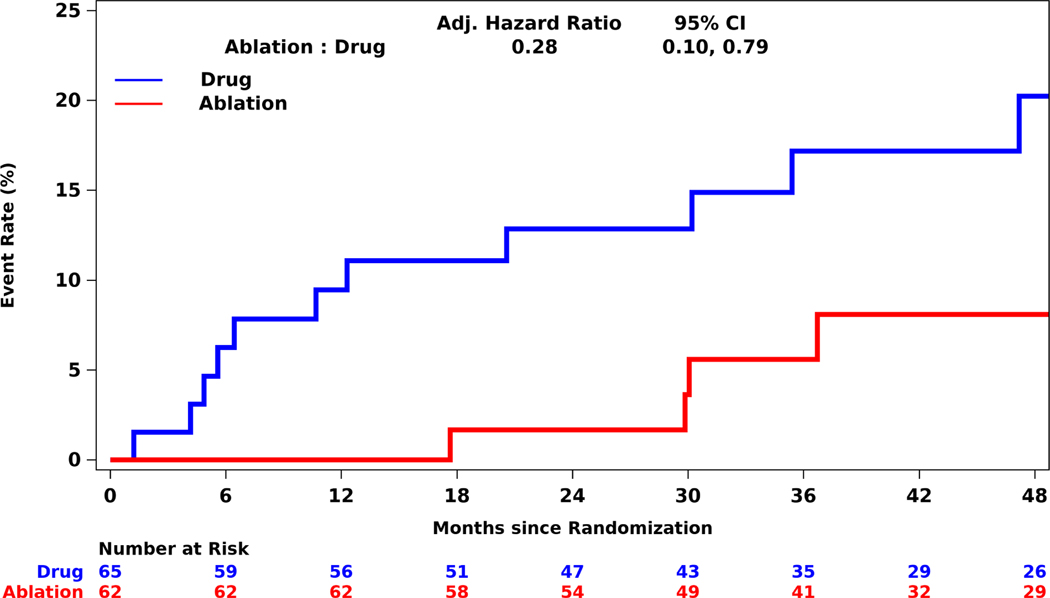
All-cause mortality occurred in 6/62 (9.7%) racial and ethnic minority participants in the ablation arm and 12/65 (18.5%) racial and ethnic minority participants in the drug therapy arm (aHR 0.28; 95% CI, 0.10, 0.79) (Table 3, Figure 2). For the additional components of the combined outcome including disabling stroke, serious bleeding, or cardiac arrest, event rates were low with fewer events among racial and ethnic minority participants treated with ablation compared with drug therapy (Table 3).
Figure 2: Kaplan-Meier Estimates of Total Mortality Among Racial and Ethnic Minorities.
Kaplan-Meier estimates of the cumulative risk of death by intention-to-treat analysis. In the non-minority group, the outcomes do not differ between treatment groups. In the racial and ethnic minority subgroup, patients randomized to ablation had a far lower risk of death out to 4 years compared to drug therapy alone. CI=confidence interval.
Death or cardiovascular hospitalization occurred in 37/62 (59.7%) racial and ethnic minority participants in the catheter ablation arm and 45/65 (69.2%) racial and ethnic minority participants in the drug therapy arm (aHR 0.73; 95% CI, 0.47, 1.13) (Table 3, Supplemental Figure 1). Death from cardiovascular causes occurred in 2/62 (3.2%) for racial and ethnic minority participants in the catheter ablation arm and 7/65 (10.8%) racial and ethnic minority participants in the drug therapy arm (Supplemental Table 2).
AF Recurrence and Burden
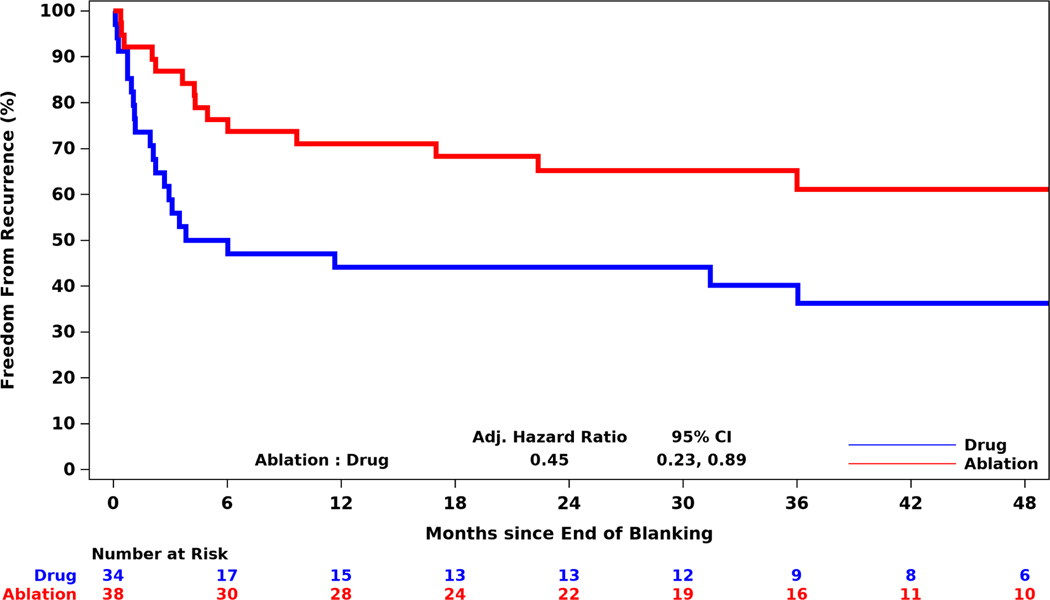
Among racial and ethnic minorities, by 12 months after the blanking period the cumulative incidence of AF recurrence was 28.9% in the ablation arm versus 55.9% in the drug therapy arm (Figure 3). At 48 months, the corresponding incidences were 38.9% (ablation arm) and 63.7% (drug arm). Overall, in racial and ethnic minority participants, the ablation arm had a 55% relative reduction in first AF recurrence when compared with the drug arm (aHR 0.45; 95% CI, 0.23, 0.89).
Figure 3: First Recurrence of Atrial Fibrillation Post Blanking Period Among Racial and Ethnic Minorities.
Cumulative incidence estimates using death as a competing risk were derived using Fine-Gray competing risks methodology. Cumulative freedom from recurrence of atrial fibrillation following a 90-day post-treatment blanking period is shown by randomized treatment group for the 72 racial or ethnic minority patients enrolled in North America who used the CABANA Box electrocardiogram event recorders. Patients randomized to ablation were at lower risk of recurrence of atrial fibrillation through 48 months of follow-up.
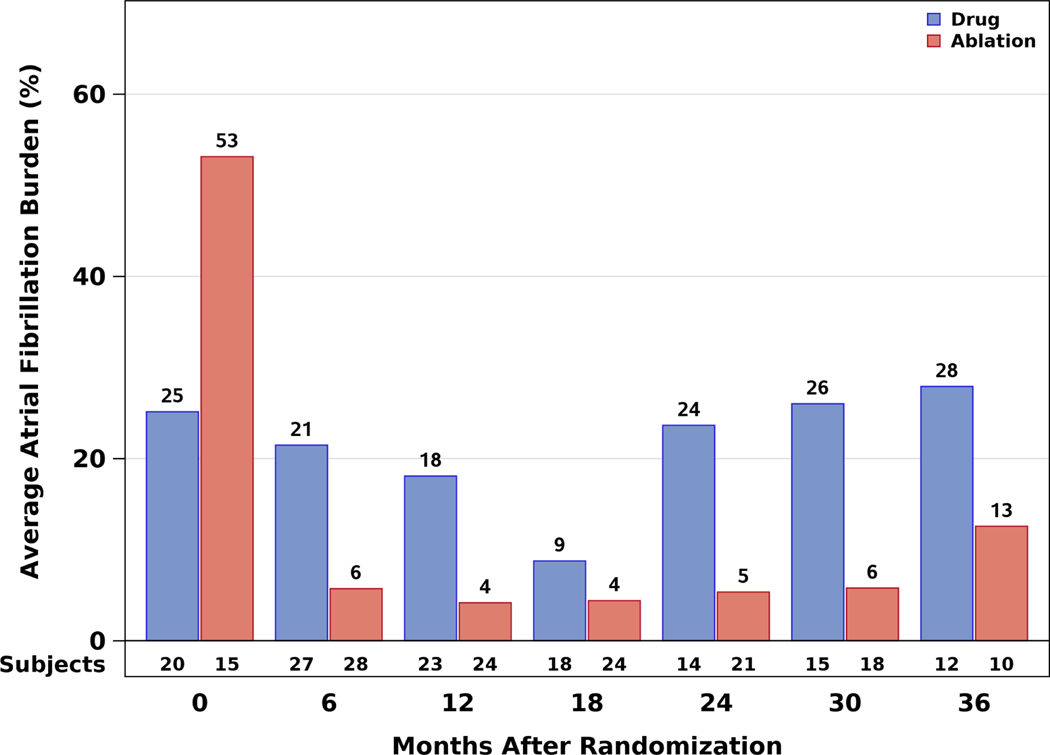
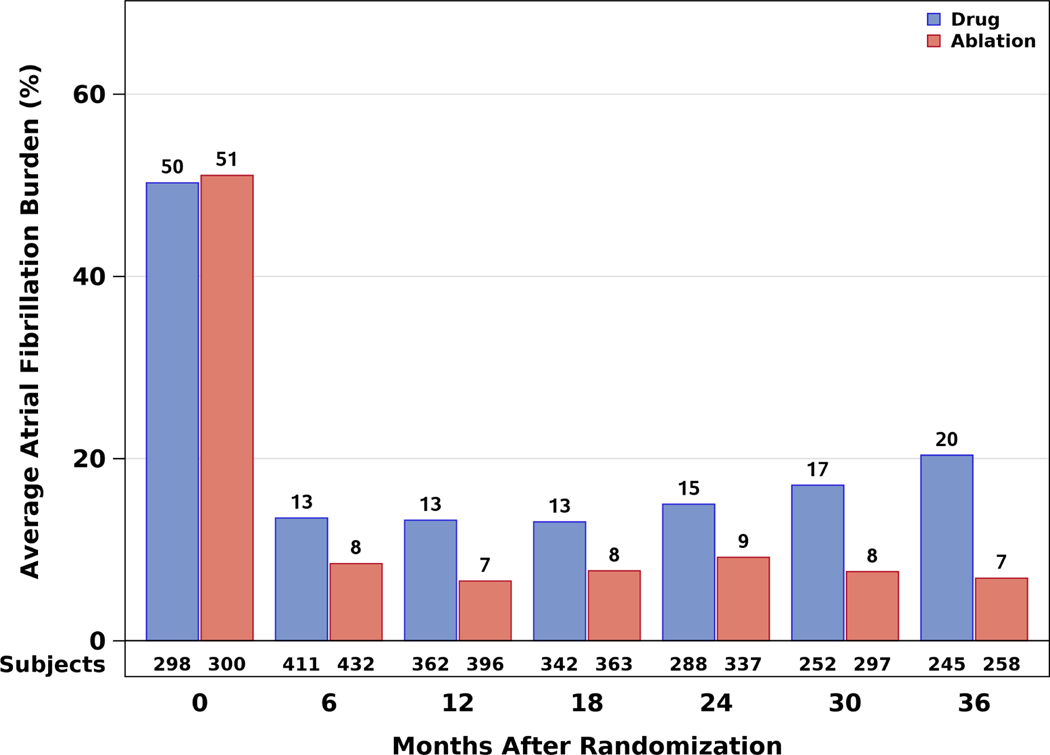
At baseline, for racial and ethnic minorities, an average of 37.1% of the CABANA Holter recording time was spent in AF (25.1% ablation arm, 53.1% drug arm) (Figure 4A). At baseline for non-minorities, an average of 50.5% of recording time was spent in AF (51.1% ablation, 50.3% drug arm) (Figure 4B). At 12 months, AF burden averaged 4.2% in the ablation arm and 18.1% in the drug therapy arm. At 3 years, the corresponding percentages were 12.6% and 27.9%, respectively. At all follow-up time points over the first 3 years, the AF burden was lower in the ablation arm than in the drug therapy arm. After 3 years, sample sizes were insufficient to generate reliable estimates.
Figure 4: Atrial Fibrillation Burden by Time and Randomization Assignment Who Used the CABANA-Box.
The percentage of atrial fibrillation burden is shown according to randomized treatment groups as assessed at each of the 6-month Holter recording timepoints for (A) Racial and ethnic minority participants in North America and (B) Non-minority participants in North America.
Comparison of Racial and Ethnic Minority and Non-minority Outcomes Within Treatment Groups
Examining event rates for racial and ethnic minority and non-minority participants suggested that ablation outcomes were comparable in both subgroups (Central Illustration, Supplemental Figure 2). In the ablation arm, the racial and ethnic minority and non-minority primary endpoint 4-year Kaplan-Meier rates were 12.3% versus 9.9%, and the all-cause mortality 4-year Kaplan-Meier rates were 8.1% versus 6.7%. However, in the drug arm, the racial and ethnic minority and non-minority primary endpoint 4-year Kaplan-Meier rates were 27.4% versus 9.4%, and the corresponding all-cause mortality 4-year Kaplan-Meier rates were 20.2% versus 4.5%.
DISCUSSION
This paper presents the first randomized multicenter assessment of the efficacy and safety of catheter ablation for the treatment of AF in North American racial and ethnic minorities. Among racial and ethnic minority participants with atrial fibrillation, catheter ablation relative to drug therapy produced significant reductions in the combined primary endpoint of death, disabling stroke, serious bleeding, or cardiac arrest (68% relative risk reduction), all-cause mortality (72% relative risk reduction), and time to first recurrence of AF (55% relative risk reduction). Treatment-related adverse events in both arms were low and did not differ between racial and ethnic minorities and non-minorities.
One of the principal objectives of the CABANA trial was to test whether effectively treating the AF state with catheter ablation could reduce the excess mortality risk associated with AF (14). While the effect of ablation on all-cause mortality compared with drug therapy was small and statistically inconclusive in the overall 2204 patient CABANA cohort (HR 0.85, 95% CI 0.60, 1.21), the mortality reduction with catheter ablation among NA racial and ethnic minorities was large (HR 0.28, 95% CI 0.10, 0.79). Conversely, the effects of catheter ablation on mortality for NA non-minorities was indeterminate, consistent with the overall trial results (HR 1.25, 95% CI 0.81–1.92) (Supplemental Figure 2). Post hoc analyses performed to explore this apparent clinical advantage of racial and ethnic minority participants randomized to ablation revealed that the difference relative to non-minority participants was primarily created by substantially worse outcomes in the drug therapy arm for racial and ethnic minorities (Central Illustration, Supplemental Figure 2). Among racial and ethnic minorities randomized to ablation, the primary endpoint 4-year Kaplan Meier rate was 12.3% and the non-minority rate was 9.9%. The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%. However, in racial and ethnic minorities randomized to drug therapy compared with non-minorities, a large discrepancy in outcomes was found: primary endpoint 4-year Kaplan-Meier rate of 27.4% versus 9.4% respectively and corresponding 4-year mortality rates of 20.2% and 4.5%. Racial and ethnic minorities in the drug arm had an early acceleration of mortality during the first 12 months following randomization (Figure 2), which accounted for most of the excess events producing the discrepancy with non-minority drug arm participants.
Explaining the Results
Given the provocative nature of our findings that suggest an unanticipated heterogeneity of treatment effect by racial and ethnic minority status in CABANA, we consider several potential explanations.
Associated Heart Disease and Importance of Heart Failure
While randomization resulted in relatively well-balanced baseline characteristics between racial and ethnic minorities and non-minorities, they differed in a few notable variables. Racial and ethnic minorities were younger, had more hypertension, and more HF with NYHA class ≥II symptoms. Among those who had EF assessments racial and ethnic minorities were more likely to have an EF ≤40% than non-minorities. HF with and without left ventricular dysfunction in racial and ethnic minorities, particularly Blacks, is typically due to hypertensive heart disease contrasted with non-Hispanic Whites in which HF is overwhelmingly associated with coronary artery disease (19–21). Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AF might account for differences in the benefits observed with ablation therapy. A growing body of both clinical trial and observational data has suggested that AF in the setting of heart failure, both with reduced and preserved EF, has substantially better clinical outcomes with ablation relative to drug therapy (22–31). Unfortunately, most of these reports either did not report racial and ethnic demographics or enrolled exceedingly low numbers of racial and ethnic minorities. Thus, possibility that the large relative advantage of ablation and the worse outcomes with drug therapy seen in NA racial and ethnic minority patients in CABANA are related to their increased prevalence of and/or type of heart failure cannot at present be corroborated using published trial data.
Racial and ethnic minorities also had a somewhat higher rate of cardiac arrest/ventricular tachycardia or ventricular fibrillation events (6.3% vs 2.3% for non-minorities), which could also be indicative of differences in underlying heart disease. Cardiac arrest endpoint events in racial and ethnic minorities were lower in the ablation arm relative to the drug arm, but the small numbers involved precludes any possibility of demonstrating causal relationship.
Differences in Medical Therapy Arm
Following the blanking period, racial and ethnic minorities relative to non-minorities were more likely to be treated with amiodarone. The mode of death analysis of The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study showed a significant increase in fatal non-cardiovascular events in the rhythm-control arm in which amiodarone was the most common drug used (32). The survival curves between the rhythm and rate control arms diverged at 1 year, and at the end of 5 years of follow-up there was a 50% increase in non-cardiovascular deaths in the rhythm-control participants. Amiodarone has previously been associated with an increased noncardiac mortality rate. For example, in the post–myocardial infarction EMIAT trial (European Myocardial Infarct Amiodarone Trial), noncardiac mortality was 37% higher, although statistically insignificant, in the participants treated with amiodarone (33). In contrast, in the present analysis, non-cardiovascular mortality rates among racial and ethnic minorities were equivalent in the two treatment arms and the mortality differences were almost exclusively confined to cardiovascular deaths (Supplemental Table 2).
AF Recurrence
In the subgroup of participants using the CABANA Box monitor post blanking period, catheter ablation relative to drug therapy reduced the hazard of first recurrence of AF among racial and ethnic minorities (aHR 0.45, 95% CI 0.23, 0.89) and non-minorities (aHR 0.54, 95% CI 0.46, 0.64). Bukari et al. conducted a single center retrospective study to assess the impact of race/ethnicity on recurrence of AF after a single AF ablation procedure. They found no significant differences in rates of early or late recurrences between Whites and Blacks or within racial groups (34).
Quality of Treatment
Inter- and intra-institutional differences in the quality of management of AF between racial and ethnic minority participants may have impacted outcomes. For example, if some centers were more proficient in optimizing control of co-morbidities or AF risk factors such as hypertension, sleep apnea, weight loss, or diabetes, this could produce differential outcomes in racial and ethnic subgroups that could be masked in the context of randomization.
The Systematic Review and Meta-analysis of Ablation Strategy Heterogeneity in AF (SMASH-AF) study was conducted to describe geographic and racial representation and single-procedure ablation success rates by country. The authors searched PubMed, Scopus, and Cochrane databases from 1/1/1990 to 8/1/2016 for trials and observational studies reporting AF ablation outcomes. The analysis cohort included 306 studies (49,227 participants) from 28 countries. Reporting of race or ethnicity demographics and outcomes was rare (1 study, 0.3%) and reporting of outcomes was nonexistent (3). Given the paucity of data on outcomes for racial and ethnic minorities undergoing catheter ablation, the CABANA data fills a critical gap in the AF literature.
Limitations
Several important caveats should be considered in the interpretation of our study. First, due to the small number of individuals in each racial and ethnic minority group represented in the NA CABANA population, we combined racial and ethnic minority groups for the analysis. However, there is no medical/physiological basis for this grouping. Further, cultural differences that could impact social determinants of health as well as approaches to care delivery may vary importantly between the racial and ethnic minority groups represented. Such differences may have been obscured by pooling underrepresented racial and ethnic groups into one broad category. This shortcoming notwithstanding, this is the largest cohort racial and ethnic participants in a randomized trial examining catheter ablation as a management strategy for AF, and the findings should be interpreted in this context. Second, due to the small sample size, chance may have produced spurious results for the subgroup analyses and interaction testing. Third, as with many randomized clinical trials, participants who were enrolled in CABANA include a selected group of individuals who meet specific inclusion and exclusion criteria. The findings may not be directly generalizable to non-trial participants, particularly those undergoing catheter ablation (35). This may be especially relevant to racial and ethnic minority participants who historically have been systematically and pervasively underrepresented in clinical trials (36). Fourth, the possibility exists that the unexpected higher mortality rate in the racial and ethnic minority drug arm patients may represent, in part at least, an adverse interaction between one or more rhythm control drugs, hypertensive myocardial disease, and symptomatic heart failure. Our sample size is too small to allow the interaction of these factors to be evaluated. Rhythm control drug adherence is a potential confounder that was not measured in CABANA. This factor could also be different between racial and ethnic minorities and non-minorities and may have impacted the study results. However, to our knowledge there are no published data that suggest that racial and ethnic minorities in clinical trials have different adherence/compliance to medical therapies than non-minorities. Fifth, the unexpectedly low AF burden at baseline among minority patients in the drug therapy arm who used the CABANA Box system (Figure 4A) is likely an artifact of the small numbers of subjects represented and higher rates of missing baseline data. This pattern was not seen in the larger non-minority group (Figure 4B), which supports the conclusion that the imbalance is an artifact. If those baseline data for the racial and ethnic minority subgroup are disregarded, the AF burden pattern in follow-up is consistent with the experience in CABANA overall and shows that racial and ethnic minorities can expect a substantially greater AF burden reduction with ablation over drug therapy.
Conclusions
In a racially and ethnically diverse population of participants with AF, catheter ablation compared with drug therapy in racial and ethnic minorities resulted in clinically meaningful reductions in the primary endpoint of death, disabling stroke, severe bleeding and cardiac arrest, and in all-cause mortality. These clinical benefits were not seen in non-minority participants and appear due primarily to worse mortality outcomes in racial and ethnic minority participants randomized to the drug therapy arm.
Supplementary Material
Clinical Perspectives.
Competency in Patient Care and Procedural Skills:
Catheter ablation is safe and possibly more effective than anti-arrhythmic drug therapy alone for patients from racial and ethnic minority groups with atrial fibrillation (AF).
Translational Outlook:
Further studies are needed to confirm these findings, which derive from a subgroup analysis of a randomized trial, and to identify the reasons members of certain racial and ethnic minority groups experience poor outcomes during antiarrhythmic drug therapy for AF.
Acknowledgements
The authors are indebted to the investigators at the CABANA sites and to the people who participated and made this study possible.
Sources of Funding
NIH: (U01HL89709, U01HL089786, U01HL089907, and U01HL089645)
St Jude Medical Foundation and Corporation
Biosense Webster Inc.
Medtronic Inc.
Boston Scientific Corporation
The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or the Department of Health and Human Services.
Abbreviations:
- aHR
adjusted hazard ratio
- AF
atrial fibrillation
- AI/AN
American Indian/ Alaskan Native
- CABANA
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation
- HF
heart failure
- NIH
National Institutes of Health
- NA
North America
- PI
Pacific Islander
Footnotes
Financial Disclosures
Kevin L. Thomas, MD: reports grants from the NIH/NHLBI, PCORI, American Heart Association, Paid consulting from Sanofi Aventis, Boehringer Ingleheim, Janssen, Bristol Myers Squibb, Medtronic during the conduct of the study.
Hussein R. Al-Khalidi, PhD: reports grants from the NIH/NHLBI and Mayo Clinic, during the conduct of the study.
Adam P. Silverstein, MS: reports none.
Kristi H. Monahan, RN: reports grants from NIH/NHLBI, St. Jude Foundation and Corporation, Biosense Webster, Inc., Medtronic, Inc., and Boston Scientific Corp., during the conduct of the study; consulting without compensation from Biosense Webster, Inc.; personal fees from Thermedical outside the submitted work.
Tristram D. Bahnson, MD: grants from NIH/NHLBI and Mayo clinic for conduct of the study. Grants from Boston Scientific Corp., St. Jude Medical Corporation, Biosense Webster, Inc., and Medtronic Inc; compensated consulting from Cardiofocus, Inc and Ventrix; during conduct of the study but outside of the submitted work.
Jeanne E. Poole, MD: reports grants from ATriCure, outside the submitted work.
Daniel B. Mark, MD, MPH: Dr. Mark reports grants from NIH/NHLBI and Mayo Clinic for conduct of the study; grants from Merck and HeartFlow, outside the submitted work.
Douglas L. Packer, MD: reports grants from NIH/NHLBI, St. Jude Medical Corporation and Foundation, Biosense Webster, Inc., Medtronic Corp., and Boston Scientific Corp., during the conduct of the study; grants from Abbott, Biosense Webster, Inc., Boston Scientific Corp., CardioFocus, Medtronic, Inc., St. Jude Medical, CardioInsight, NIH, Siemens, Thermedical, Endosense, Robertson Foundation, and Hansen Medical; advisory board without compensation from Abbott, Biosense Webster, Inc., Boston Scientific Corp., CardioFocus, Medtronic, Inc., St. Jude Medical, Spectrum Dynamics, Siemens, Thermedical, Johnson & Johnson, and SigNum Preemptive Healthcare, Inc.; speaking with honorarium from Biotronik and MediaSphere Medical, LLC; royalties from Wiley & Sons, Oxford, and St. Jude Medical; Dr Packer and Mayo Clinic jointly have equity in a privately held company, External Beam Ablation Medical Devices, outside the submitted work; in addition, Dr Packer has mapping technologies with royalties paid.
Twitter account: @DanMarkMD
Tweet: North American racial/ethnic minorities in CABANA experienced significantly improved major clinical outcomes with catheter ablation treatment compared to drug therapy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm 2017;14:e445–e494. [DOI] [PubMed] [Google Scholar]
- 2.Sarraju A, Maron DJ, Rodriguez F. Under-Reporting and Under-Representation of Racial/Ethnic Minorities in Major Atrial Fibrillation Clinical Trials. JACC Clin Electrophysiol 2020;6:739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leef GC, Perino AC, Cluckey A et al. Geographic and racial representation and reported success rates of studies of catheter ablation for atrial fibrillation: Findings from the SMASH-AF meta-analysis study cohort. J Cardiovasc Electrophysiol 2018;29:747–755. [DOI] [PubMed] [Google Scholar]
- 4.Hoyt H, Nazarian S, Alhumaid F et al. Demographic profile of patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:994–8. [DOI] [PubMed] [Google Scholar]
- 5.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm 2015;12:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Qazi M, Erande A et al. Racial Differences in the Prevalence and Outcomes of Atrial Fibrillation in Patients Hospitalized With Heart Failure. Am J Cardiol 2016;117:1468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrani A, Soma S, Althouse AD, Leef G, Qin D, Saba S. Impact of Race on Outcome of Patients Undergoing Rhythm Control of Atrial Fibrillation. Journal of Immigrant and Minority Health 2018:14–19. [DOI] [PubMed] [Google Scholar]
- 8.Golwala H, Jackson LR 2nd, Simon DN et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am Heart J 2016;174:29–36. [DOI] [PubMed] [Google Scholar]
- 9.Naderi S, Rodriguez F, Wang Y, Foody JM. Racial disparities in hospitalizations, procedural treatments and mortality of patients hospitalized with atrial fibrillation. Ethn Dis 2014;24:144–9. [PubMed] [Google Scholar]
- 10.Tamariz L, Rodriguez A, Palacio A, Li H, Myerburg R. Racial disparities in the use of catheter ablation for atrial fibrillation and flutter. Clin Cardiol 2014;37:733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer DL, Mark DB, Robb RA et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark DB, Anstrom KJ, Sheng S et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole JE, Bahnson TD, Monahan KH et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol 2020;75:3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer DL, Mark DB, Robb RA et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial: Study rationale and design. Am Heart J 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Assoc 1958:457–481. [Google Scholar]
- 16.Cox D. Regression models and life-tables (with discussion). J Royal Statist Soc B 1972;34:187–220. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc 1999;94:496–509. [Google Scholar]
- 18.Mark DB, Lee KL, Harrell FE Jr. Understanding the role of P values and hypothesis tests in clinical research. JAMA Cardiol 2016;1:1048–1054. [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW. Heart failure in African Americans: pathophysiology and treatment. J Card Fail 2003;9:S210–5. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KL, East MA, Velazquez EJ et al. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol 2005;96:956–63. [DOI] [PubMed] [Google Scholar]
- 21.Bibbins-Domingo K, Pletcher MJ, Lin F et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black-Maier E, Ren X, Steinberg BA et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018;15:651–657. [DOI] [PubMed] [Google Scholar]
- 24.Di Biase L, Mohanty P, Mohanty S et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 25.Hunter RJ, Berriman TJ, Diab I et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 26.Jones DG, Haldar SK, Hussain W et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 27.Marrouche NF, Brachmann J, Andresen D et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 28.Packer DL, Piccini JP, Monahan KH et al. Ablation versus drug therapy for atrial fibrillation in heart failure: Results from the CABANA trial. Circulation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhu S, Taylor AJ, Costello BT et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg BA, Li Z, O’Brien E et al. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turagam MK, Garg J, Sartori S, Dukkipati SR, Reddy VY. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure. Ann Intern Med 2019;171:76–77. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg JS, Sadaniantz A, Kron J et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1973–80. [DOI] [PubMed] [Google Scholar]
- 33.Julian DG, Camm AJ, Frangin G et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet 1997;349:667–74. [DOI] [PubMed] [Google Scholar]
- 34.Bukari A, Nayak H, Aziz Z, Deshmukh A, Tung R, Ozcan C. Impact of race and gender on clinical outcomes of catheter ablation in patients with atrial fibrillation. Pacing Clin Electrophysiol 2017;40:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noseworthy PA, Gersh BJ, Kent DM et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J 2019;40:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chastain DB, Osae SP, Henao-Martinez AF, Franco-Paredes C, Chastain JS, Young HN. Racial Disproportionality in Covid Clinical Trials. N Engl J Med 2020;383:e59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.