Abstract
As the largest organ in human body, skin acts as a physicochemical barrier, offering protection against harmful environmental stressors, such as chemicals, pathogens, temperature and radiation. Nonetheless, skins prominence goes further, with a significant psychosocial role in an increasingly ageing population. Prompted by consumers’ concern regarding skincare, cosmetic industry has been developing new formulas capable of lessening the most visible signs of ageing, including reduction in skin density and elasticity, wrinkling and hyperpigmentation. Allied to skincare is the rising importance set on natural products, sustainably obtained from less environmental impacting methods. Cyanobacteria and microalgae are adding importance in this field, given their ability to biosynthesize secondary metabolites with anti-ageing potential. In this review, we present an overview on the potential of cyanobacteria and microalgae compounds to overcome skin-ageing, essentially by exploring their effects on the metalloproteinases collagenase, elastase, gelatinase and hyaluronidase, and in other enzymes involved in the pigmentation process.
Keywords: Skin ageing, matrix metalloproteinases, collagenase, elastase, hyaluronidase, hyperpigmentation
1. Introduction
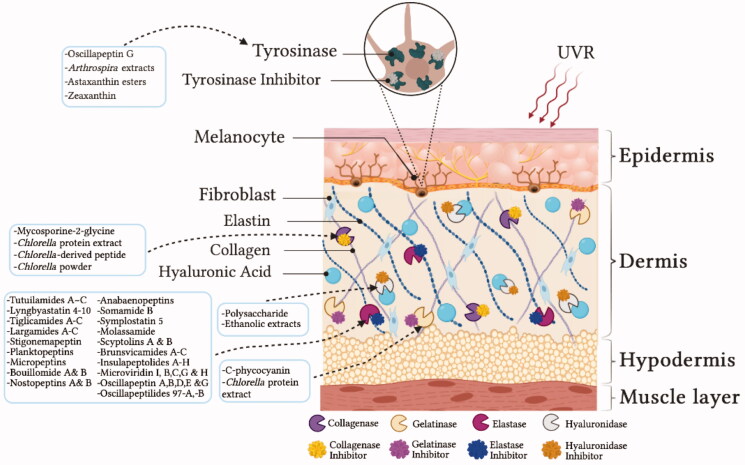
Representing 16% of the total body weight, skin is the largest human organ. Among its several functions, skin works as a physical barrier, offering protection against harmful stressors, such as chemicals, pathogens, cold, heat and ultraviolet radiation (UVR)1. In addition, skin plays a crucial role in the synthesis of vitamin D, essential to the maintenance of calcium homeostasis, as well as in immune, sensorial and body temperature regulation functions2,3. Structurally, skin is composed of three distinct layers: epidermis, dermis and hypodermis4 (Figure 1). The most superficial and exposed layer, epidermis, is a continuous renewing stratified keratinised squamous epithelium, constituted mainly by keratinocytes and melanocytes. Its primary function relies on protection against environmental chemical and biochemical threats, functioning as a physical and adaptive immunologic barrier5. Underlying the epidermis, there is dermis, constituted by connective tissue that includes an extracellular matrix (ECM) and cells like fibroblasts and macrophages. ECM is a three-dimensional network of collagen and elastin fibres surrounded by the ground substances, such as hyaluronic acid (HA), acting together to maintain skin filling, elasticity and flexibility4 (Figure 1). Any imbalance between these main components may result in the loss of skin structure, leading to an unhealthy and aged appearance4. Given its crucial role in personal feature and social welfare, the preservation of all skin layers has become one of the main requirements of modern societies, which has driven the development of new and innovative products by pharmaceutical and cosmetic industries6.
Figure 1.
Schematic representation of skin structure, with emphasis on the main targets of cyanobacteria and microalgae bioactive compounds. UVR: ultraviolet radiation. Created with BioRender.com.
Skin care and beauty products have played important roles in human history7. The oldest records on cosmetics came from Egyptians, who were particularly concerned with physical appearance, namely with the development of facial wrinkles. Due to the dry and hot weather to which population was exposed, the skin care with the use of oils and creams were part of the daily routine8. Over the years, other products like salts, honey and hydroxy and tartaric-acids were also used for skin treatment and cleaning. With origin in the ancient Roman public baths, the term “cosmetic”, meaning to “beautify the body”, came up9. Currently, cosmetic products are defined by the European Commission (EC) regulation No 1223/2009 as “any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair , nails, lips and external genital organs) or with teeth and the mucous membranes of the oral cavity, with a view to clean, perfume, change the appearance, protect, keep in good condition or correct body odours”10. Globally, the cosmetic industry has been one of the least impacted from the oscillation of the financial markets. According to a very recent survey11, it is predicted an economic volume of $805.61 billion by 2023, as a result of an increase in global consumption. Likewise, the rise in average life expectancy has led to a robust demand for anti-ageing products, thus creating room for countless innovations and boosting the industry growth6.
A main society demand concerning skin is in fact the delay of skin-ageing. This slow and complex process is induced by endogenous factors such as genetics, and exogenous factors such as personal habits and environment12. Endogenous ageing is a natural process where skin gradually loses its functional and structural characteristics, as a natural consequence of cellular senescence due to a decrease in cellular metabolism, DNA repair capability, gene mutations, loss of telomeres, chromosomal abnormalities, and hormonal changes13,12. On the other hand, exogenous ageing is caused by chemicals, toxins, pollutants, extreme conditions of cold or heat and radiation14. In both cases, the phenomenon commonly affects the epidermal thickness, structure and pigmentation, as well as the morphology and microstructure of the deeper layers15, resulting in thinning, dryness, flaccidity, enlarged pores, fine lines and wrinkles, dark spots and hyperpigmentation14.
In the last decades, scientific research underwent a significant evolution in the field of anti-ageing products, with focus on natural sources and green processes, free of animal testing and with a green life cycle, including packaging, manufacturing, distribution, post-consumer use, and sourcing16. As a result, a substantial expansion of cosmetic industries emerged and a countless number of new products have been launched to the market6. While plants have been the primary raw material for cosmetics production for centuries, the exhaustion in this over-studied resource led to the use of other organisms such as macroalgae and eukaryotic microalgae, namely from marine origin17. Marine organisms have thus emerged as a prolific source of cosmetic ingredients able to minimise damages that occur during skin ageing, such as the formation and exacerbation of wrinkles4, pigmentation, collagen degradation and loss of elasticity18 and loss of moisture19. Among them, cyanobacteria have gained importance, due to their capacity to produce bioactive secondary metabolites, with unique structures and mechanisms of action. These gram-negative bacteria represent the only group of prokaryotes that can perform oxygenic photosynthesis, similarly to plants, although with a higher photosynthetic rate and biomass production20,21. Their capacity to self-renew, basic nutritional requirements22, minimal cultivation space and low environmental impact17,23, makes them a sustainable choice for skin care products. Their residual biomass can be used as fertiliser or in animal feed, and can generate bio-polyesters, known as “Green Plastics”, thus fitting the concept of circular economy17,24,25. Given this, marine organisms, and particularly, microorganisms, can be seen as a new hope in the search for new and innovative bioactive molecules, able to counteract the reactions leading to skin damage and ageing.
2. Methods
The aim of this review was to compile the available studies on extracts or bioactive compounds produced by cyanobacteria and microalgae to potentially restore the skin ECM and overcome hyperpigmentation. The review was conducted using Scopus, Web of Science, PubMed, ScienceDirect, ResearchGate, and Google Scholar databases. Query terms included "cyanobacteria", "microalgae", "bioactive compounds", "skin-ageing", "metalloproteinases", “collagenase”, “gelatinase”, “elastase”, “hyaluronidase”,“tyrosinase” and "hyperpigmentation". In addition, we have supplemented the search by further exploring references of the articles retrieved from the referred databases.
3. Cyanobacteria and microalgae in skin-ageing
Cyanobacteria and microalgae are prolific sources of natural bioactive compounds with different areas of application22. It is known that cyanobacteria and microalgae synthesise pigments, lipids (polyunsaturated fatty acids - PUFAs, hydrocarbons), proteins, polysaccharides (cellulose, alginates, starch), and other compounds, with proven bioactivities in the pharmaceutical, energy, nutrition and cosmetic fields24,26,27. In relation to energy application, diverse microalgae are being used to produce bioethanol, biogas, and biohydrogen. Due to its high protein and PUFAs content, they can also be used for human and animal nutrition24. In the pharmaceutical field, it is noteworthy the production of grassystatin A–B for lung cancer, kempopeptin A for colon cancer, and dolastatin 15 for breast cancer28. Other studies show that they also have antitumor, anticoagulant, anti-inflammatory, and protease inhibitory activities28. Regarding cosmetics, their bioactive compounds, mostly as extracts, have been reported to be used in shampoos, and body soaps10,19, face lotions, anti-ageing creams, makeup and sun blockers17,19,22. Concerning sunscreens, some of these microorganisms produce UV-absorbing compounds, such as mycosporine-like amino acids (MAAs) and scytonemin, as well as carotenoids, phycobiliproteins and polyphenols, with an important role in preventing oxidative stress through their capacity to scavenge deleterious free radicals29. They also produce exopolysaccharides (EPS), with important moisturising properties17, metalloproteinase inhibitors30, and compounds able to inhibit tyrosinase, and thus avoid skin hyperpigmentation17.
3.1. ECM-target compounds
The dermis is constituted by loose and dense connective tissue in which ECM constitutes the major component. ECM is a gel-like material made of collagen and elastic fibres dispersed in a ground substance made of glycosaminoglycans, proteoglycans, and connective tissue glycoproteins. It is essential to hold cells together, and to provide a pathway for nutrients and oxygen to the epidermis31. Several cell types, such as keratinocytes, fibroblasts, macrophages, endothelial cells, mast cells, eosinophils and neutrophils, are capable of producing specific enzymes responsible for the ECM turnover and, in some situations, leading to the loss of skin structure and appearance of wrinkles4. Recently, there has been more research on metalloproteinases, and in their effect on the dermal matrix structure, as well as in enzymes responsible for skin pigmentation. Both metalloproteinases and skin pigmentation-associated enzymes have become targets for bioactive compounds with anti-ageing potential. Hence, we present below an overview on the potentialities of cyanobacteria and microalgae-derived compounds to overcome skin-ageing, focussing the main enzymes responsible for the maintenance of dermal matrix structure.
3.1.1. Metalloproteinases
Matrix metalloproteinases (MMPs) are a family of extracellular zinc-dependent enzymes, which main function is to remodel and degrade the ECM30. Collagen and elastin are primary proteins of the ECM, responsible for resistance and elasticity of the skin32. Therefore, any alterations in collagen and elastin induced by MMPs, will contribute to the loss of dermal structure, resulting in its damage33. A main skin stress condition is the exposition to UVR, that exacerbates the degradation of the ECM collagen and elastin fibres through the induction of MMPs activity4. Although MMPs are crucial to epidermal differentiation and prevention of wound scars, their up-regulation potentiates the signs of ageing and the development of skin cancer30.
Despite the existence of different subgroups of MMPs, such as collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs (MT-MMPs), among others34, this review will focus on the most relevant regarding skin ageing: collagenase, gelatinase, elastase, and hyaluronidase (Figure 1).
3.1.1.1. Collagenases
There are different subtypes of collagenases, e.g. MMPs-1, -8, -13, and -18, which are proteolytic enzymes responsible for the initiation of collagen fragmentation in human skin, and for the control of collagen turnover35. These enzymes cleave all types of interstitial collagens in the skin (I, II, and III) at a single site. After cleavage, the collagen fragments lose stability at body temperature and their structure is disrupted, contributing to the loss of dermal homeostasis and leading to tissue damage30,36,37. Hence, inhibiting MMPs constitutes a strategy to conserve the dermal matrix structure, avoiding tissues damage and delaying the formation of wrinkles.
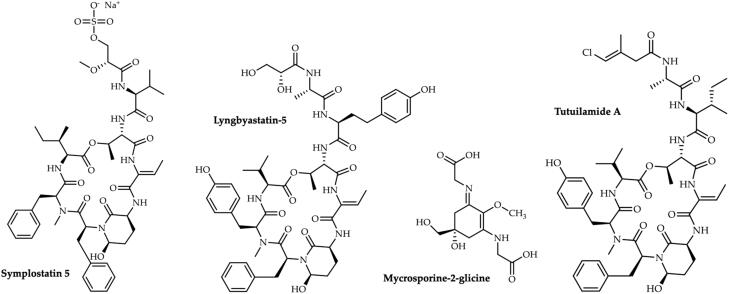
Several recent reports point different compounds isolated from cyanobacteria and microalgae as potent inhibitors of enzymes responsible for the digestion of ECM components, essential to maintain dermal filling, and that are naturally decreased during the ageing process and exposition to deleterious abiotic factors38 (Figure 1, Table 1). An example is the mycosporine-2-glycine (M2G) (Figure 2), isolated from the cyanobacterium Aphanothece halophytica, that presented collagenase inhibitory properties, with a robust IC50 of 0.47 mmol/L, comparable to that of the well-known collagenase inhibitor phenanthroline. It was suggested that the mechanism of enzyme inhibition could be related to the capacity of M2G to chelate calcium ions, and to the efficiency of the compound in inhibiting the formation of glycation-dependent protein-protein cross-linking, a process associated to the development of dull skin and to the decrease in skin elasticity. These results have demonstrated M2G as an alluring candidate for the development of new anti-ageing cosmetics and emphasised its potential in the prevention of skin ageing39.
Table 1.
Bioactive potential of cyanobacteria and microalgae-derived compounds against matrix metalloproteinases.a
| MMP | Compound/extract | Species | Model | Reference |
|---|---|---|---|---|
| Collagenase | Mycosporine-2-glycine |
Aphanothece
halophytica |
Collagenase from Clostridium histolyticum | Tarasuntisuk et al.39 |
| Protein extract | Chlorella minutissima | Human breast cancer cell line MDA-MB231 | Kunte and Desai40 | |
| Chlorella-derived peptide | Chlorella sp. | Human skin fibroblasts 966SK (BCRC 60153) | Chen et al.41 | |
| Chlorella powder | Chlorella pyrenoidosa | Human MMP-1 | Cheng et al.97 | |
| Arthrospira -derived peptide | Arthrospira maxima | Collagenase from Clostridium histolyticum | Montalvo et al.42 | |
| Arthrospira crude protein (SPCP) | Arthrospira platensis | Human dermal fibroblast cell line (CCD-986sk) | Liu et al.98 | |
| Gelatinase | C-phycocyanin extract | Spirulina platensis | HepG2 cell line | Kunte and Desai 44 |
| Protein extract | Chlorella minutissima | HepG2 cell line | Kunte and Desai 40 | |
| Elastase | Tutuilamides A − C |
Schizothrix sp. Coleofasciculus sp. |
PPE | Keller et al.46 |
| Lyngbyastatin 4 Lyngbyastatin 5 Lyngbyastatin 6 Lyngbyastatin 7 Cyclodepsipeptide somamide B Tiglicamides A-C Largamides A-C |
Lyngbya confervoides | PPE | Matthew et al.47 Taori et al.48 Matthew et al.50 Matthew et al.49 |
|
| Lyngbyastatin 8 Lyngbyastatin 9 Lyngbyastatin 10 |
Lyngbya semiplena | PPE | Kwan et al.51 | |
| Bouillomide A Bouillomide B |
Lyngbyabouillonii | PPE | Rubio et al.52 | |
| Symplostatin 5-10 | Symploca sp. | PPE Human neutrophil elastase |
Salvador et al.54 | |
| Stigonemapeptin | Stigonema sp. | PPE | Kang et al.53 | |
| Oscillapeptins A, B, D, E Oscillapeptin G Oscillapeptilides 97-A and -B Microviridin I |
Oscillatoria agardhii | PPE | Itou et al.55 Fujii et al.56 |
|
| Microviridins G and H Nostopeptins A and B |
Nostoc minutum | PPE | Murakami et al.57 Okino et al.58 |
|
| Microviridins B and C Micropeptins HH978, HH960, HH992, and DR1006 |
Microcystis aeruginosa | PPE | Okino et al.59 Lodin-Friedman and Carmeli60 Adiv et al.61 |
|
| Molassamide | Dichothrix utahensis | PPE | Gunasekera et al.62 | |
| Scyptolins A and B | Scytonema hofmanni | PPE | Matern et al.63 | |
| Planktopeptins BL1125, BL843, and BL1061 Anabaenopeptins B and F |
Planktothrix rubescens | HLE/PPE HLE/PPE |
Grach-Pogrebinsky et al.64 Bubik et al.65 |
|
| Brunsvicamides A-C | Tychonema sp. | HLE | Sisay et al.66 | |
| Insulapeptolides A-H | Nostoc insulare | HLE | Mehner et al.67 | |
| Arthrospira crude protein (SPCP) | Arthrospira platensis | Human dermal fibroblast cell line (CCD-986sk) | Liu et al.98 | |
| Hyaluronidase | Polysaccharide | Nostochopsis lobatus MAC0804NAN | Hyaluronidase from bovine testes, type IV-S | Yamaguchi and Koketsu et al.75 |
| Ethanolic extracts |
Spirulina platensis
Chlorella pyrenoidosa Porphyridium purpureum Rhodosorus marinus Dunaliella salina Pleurochrysis carterae |
Hyaluronidase from bovine testes, type IV-S | Fujitani et al.76 | |
| Arthrospira-derived peptide | Arthrospira maxima | Hyaluronidase from bovine testes, type IV-S | Montalvo et al.42 |
aMMP: matrix metalloproteinase; PPE: porcine pancreatic elastase; HLE: human leucocyte elastase.
Figure 2.
Structures of compounds isolated from cyanobacteria, with ability to inhibit matrix metalloproteinases.
A protein extract was able to reduce the expression of MMP-1 at the mRNA and protein levels was obtained from the microalgae Chlorella minutissima40. Also, a Chorella-derived peptide was found to inhibit UVB-induced expression of MMP-1, in UVB irradiated human fibroblasts, by suppressing the expression of the ECM-associated signalling protein CYR61, the transcription factor AP-1, and the production of the chemotactic factor MCP-1. These results are crucial since the up-regulation of CYR61 triggers alterations of type I collagen similarly to those verified in photoaged and chronologically-aged skin, once UV irradiation induces the transcription of AP-1 and MCP-1, which in turn stimulates MMP-1 expression41.
Arthrospira maxima is another example of cyanobacteria able to produce anti-collagenase peptides. The peptide fraction PHS showed anti collagenase activity (92.5%) with an IC50 of 32.5 µg/mL compared with the synthetic inhibitor (57.13%)42. These peptides sequence can resemble the cleavage site in native collagen, and thus prevent the degradation of the ECM. A competition with the enzyme active site was pointed out as the blocking action of the collagenase by these peptides.
3.1.1.2. Gelatinases
Gelatinases (MMPs-2 and −9) degrade basement membrane and denatured structural collagens30,36. These enzymes are essential in digesting collagen fragments after their initial cleavage by collagenases43. Although in lower amount, there are also reports on the potential of cyanobacteria-derived compounds to act upon gelatinases (Figure 1, Table 1). Kunte and Desai44 evaluated the effect of the phycobiliprotein C-phycocyanin containing protein extract (C-PC extract) obtained from the cyanobacteria Spirulina platensis, in the human gelatinases MMP-2 and MMP-9. The authors found that, besides significantly reducing the activity of MMP-2 by 55.13% and of MMP-9 by 57.9%, C-PC extract also reduced the mRNA expression of both gelatinases, in the hepatocellular cancer cell line HepG2. Although the exacts’ mechanism of inhibition remains unknown, these findings can lead to newer insights for S. platensis as a potential source of therapeutically bioactive molecules. A year later, the same authors found another protein extract from Chlorella minutissima that successfully reduced the mRNA expression of human MMP-2 and 9, and also upregulated mRNA expression of the tissue inhibitor of metalloproteinases-3 (TIMP-3)40.
3.1.1.3. Elastase
Elastase (MMPs-12) is a serine protease with unique ability to digest elastin. After collagen, elastin is the most abundant constituent of the connective tissue in the dermis4,37. The degradation of elastin fibres results in the loss of skin elasticity and, consequently, in a sagging and aged appearance. MMP-12 is the most effective MMP against elastin, and it is produced by macrophages and fibroblasts in response to UV radiation45. Several recent reports addressed the ability of natural compounds derived from cyanobacteria and microalgae to overcome elastase overactivity (Figure 1, Table 1). It was recently found that the cyclic depsipeptides tutuilamides A − C, isolated from the cyanobacteria Schizothrix sp. and Coleofasciculus sp., act as potent inhibitors of porcine pancreatic elastase (PPE), through a reversible binding mode similar to those of the natural cyanobacteria compound lyngbyastatin46. Following the National Cancer Institute parameters, we can consider that tutuilamides A − C presented incredible low IC50 values (1.18 nM, 2.05 nM and 4.93 nM), being tutuilamide A (Figure 2) the most effective cyanobacteria-derived compound regarding elastase inhibition. Structural analysis of tutuilamide A complexed with PPE confirmed an additional hydrogen bond between the 4-chloro-3-methylbut-3-enoic acid residue and the backbone amide group of elastase residue R226, that appears to stabilise the ligand and may explain the increased inhibitory potency of the compound. In fact, tutuilamide A showed a higher elastase inhibition potential when compared to other compounds such as lyngbyastatin 7, where this additional interaction does not occur46.
Other compounds such as the cyclic depsipeptides lyngbyastatin-4, -5, -6, and -7,somamide B, tiglicamides A-C, and largamides A-C, produced by Lyngbya spp.,were shown to selectively inhibit PPE in vitro47–49. Lyngbyastatin -5, -6, -7, and somamide B inhibited elastase in a competitive way, following the Michaelis-Menten kinetics. The 2-amino-2-butenoic acid (Abu) moiety of the hexadepsipeptide core appears to be the main contributor to the selectivity for elastase48. The activity of largamides A-C and tiglicamides A-C in elastase inhibition was inferior to lyngbyastatin 4–749,50. Later, three new members of lyngbyastatins, namely lyngbyastatins 8, 9 and 10 isolated from the marine cyanobacteria Lyngbia semiplena, were also found to inhibited PPE, with IC50 values ranging from 120 to 210 nM51. Even though these are high IC50 values, they denote the potentiality of lyngbyastatins for elastase inhibition, and open doors for further studies where chemical modifications may be considered to increase the compounds activity and specificity. Of the lyngbyastatins evaluated so far, lyngbyastatin 5 (Figure 2) and lyngbyastatin 6 were the most effective against elastase, with IC50 values of 3.2 and 3.3 nM, respectively. Within the same genus, Rubio and his team52 isolated two cyclic depsipeptides analogues of dolastatin 13, bouillomides A and B, from the cyanobacteria Lyngbya bouillonii, and found their capacity to selectively inhibit these serine proteases, although with a higher IC50 (1.9 µM). The Abu moiety is also present in these two compounds, which reinforces its role in the selectivity for elastase. Another Abu moiety-containing cyclic depsipeptide, stigonemapeptin, isolated from Stigonema sp., also showed selective elastase inhibitory activity, with an IC50 of 0.26 µM53.
Salvador and co-workers54 demonstrated that symplostatin 5–10 (Abu containing cyclic depsipeptides) (Figure 2), isolated from the cyanobacteria Symploca sp., potently inhibited the proteolytic activity of elastase (IC50 of 37 to 89 nM), which was comparable to the activity of the related compounds lyngbyastatin 4 and 7. It was also shown that compounds containing N-Me-Tyr (symplostatin 8–10) were slightly more potent than their N-Me-Phe (symplostatin 5–7) congeners in inhibiting PPE elastase and human neutrophil elastase. These compounds, with high specificity for elastase, attenuated the effects of elastase in receptor activation, exhibited a superior activity than the clinically approved elastase inhibitor sivelestat, in short-term assays, and also demonstrated superior sustained activity in longer term assays54.
The cyclic depsipeptides oscillapeptins A, B, D, and E, isolated from Oscillatoria agardhii, inhibited elastase with IC50 values of 0.3, 0.05, 30, and 3.0 µg/mL, respectively. The structure/activity analysis of these compounds suggested that the presence of an amino acid residue between Thr and the 3-amino-6- hydroxy-2-piperidone (Ahp) unit is essential in the selectivity55. Tricyclic peptide microviridin I showed inhibitory activity on elastase with an IC50 of 0.34 µg/mL. The cyclic depsipetides containing the Ahp moiety such as oscillapeptin G and oscillapeptilides 97-A, -B, were also recognised as elastase inhibitors (IC50 =0.73, 0.42 and 1.12 µg/mL)56. Other microviridin-type peptides (G and H) and nostopeptins (A and B), produced by Nostoc minutum, have also demonstrated ability to prevent elastin degradation through elastase inhibition57,58. The cyanobacteria Microcystis aeruginosa was also shown to produce microviridins, namely microviridins B and C, which inhibited elastas with IC50 = 0.044 and 0.084 µg/mL, and micropeptins HH978, HH960, HH992, and DR1006, withIC50 = 17.6, 55.5, 16.9 and 13.0 µM, respectively. Microviridins B and C had similar IC50 against elastase as G and H, and this observation can be explained, at least in part, by the molecular structure: it was reported that the amino acid sequence of X-Thr-Y affects elastase inhibitory activity, and both microviridins B, C, G, and H presented a hydrophilic amino acid residue in the place of X, and a Leu in the place of Y59–61.
A new peptide, molassamide, from Dichothrix utahensis, was found to have serine protease inhibitory activity against elastase with IC50 = 0.032 µM, and a similar selectivity profile as those previously described for lyngbyastatin 4–7, maybe due to their structural similarity62. Two other cyclic depsipeptides with activity against elastase were isolated from Scytonema hofmanni, and designated scyptolin A and B63. A correlation between the molecular structure and the bioactivity can also be predicted, once two distinguish features were observed: the fifth position replaced by Leu, as previously reported in microviridins, and a 3-chloronated N-methyl-Tyr residue in eighth position. As in the previous studies, PPE was used as model, and scyptolin A and B were reported to block elastase activity at low concentrations. It has been shown that scyptolins bind directly into the active centre of the target peptidase, in a substrate-like manner, however, the molecular basis of this selectivity is still unclear63.
Planktothrix rubescens is another cyanobacteria that produces elastase inhibitors (planktopeptin BL1125, BL843, and BL106) with IC50 values of 96 nM, 1.7 µM, and 40 nM, respectively. After the examination of the molecular structure of these compounds, it was possible to predict a structure-activity relationship, being revealed that the flexible side chain of the molecules showed marginal selectivity for elastase. BL1125 is a liner competitive tight-binding inhibitor of human leukocyte (HLE) (Ki =2.9 nM) and pancreatic (Ki =7.2 nM) elastase, and is effective in inhibiting the cleavage, not only of the synthetic substrate, but also of elastin of natural provenance. HLE has become more relevant due to its involvement in several pathological processes so, finding inhibitors for this enzyme has a strategic therapeutic interest64. Years later, Bubik and co-workers65, discovered the peptides anabaenopeptins B and F from the same cyanobacteria strain, with also the ability to inhibit HLE and PPE, although in a lesser extent than PPBL1125. The inhibition profiles of HLE showed competitive inhibition, with the Ki values between 0.1–1 µM. Regarding PPE, the profiles revealed a sigmoid shape, which describes the binding of two inhibitor molecules to the enzyme. The first inhibitor molecule had a Ki ranging from 1–2 µM and, the second, presented Ki values approximately 50-fold higher65.
Inhibition of HLE was also achieved with brunsvicamides A-C, produce by Tychonema sp. These compounds were highly selective for HLE with Ki values of 1.1, 0.70, and 1.6 µM, respectively, calculated assuming competitive inhibition. It was also reported that brunsvicamides may act as alternate HLE substrates with a strongly decelerated diacylation66. Another HLE inhibition was found with the Nostoc insulare cyanopeptolins, insulapeptolides A-H. Insulapeptolides A-D had IC50 values between 85 (Ki value of 36 nM) and 140 nM, hence being highly potent inhibitors, whereas insulapeptolides E-H were less active, with IC50 values varying between 1.6 and 3.5 µM. Therefore, it can be concluded that these compounds occupy the substrate-binding site of HLE, suggesting that the insulapeptolides act as competitive inhibitors by gorming non-covalent enzyme-inhibitor complexes with HLE67 (Table 1).
The cyclic depsipeptides isolated from cyanobacteria have revealed a huge potential to avoid and slow down elastin degradation through both direct elastase inhibition and interference at the level of enzyme expression. In some situations, the high specificity for the enzyme put these compounds at the forefront for the development of effective and innovative anti-ageing formulations, with potential to maintain and improve dermal filling, and delay the establishment of wrinkles. Of the molecules presented before, tutuilamides and lyngbyastatins seem worthy of further studies by the pharmaceutical and cosmetic industries, in view of their proved potency against this enzyme.
3.1.1.4. Hyaluronidases
Excessive superficial water lost by evaporation greatly contributes to skin ageing. Evaporated water is replaced with water from the innermost epidermal layers and dermis, leading to cell shrinkage, and in a worst scenario to cell death68,69. The relationship between skin hydration and occurrence of wrinkles, demonstrated that skin hydration significantly reduce the depth of wrinkle furrows70.
An adequate skin moisture is, in part, achieved through the preservation of hyaluronic acid (HA), due its unique capacity of retaining water. Hyaluronidases (HASEs) are enzymes that break-down polymers by cleaving high molecular weight HA into smaller fragments4. HA is the key molecule involved in skin moisture71. Its function is, among others, to bind water and to lubricate movable parts of the body72. As already stated, HA is degraded into fragments of varying sizes by HASEs73. HA is found in young skin at the periphery of collagen and elastin fibres. Aged skin, which is less plump than youthful skin, is characterised by decreased levels of HA. The decrease in HA levels may be involved in the changes noted in aged skin, including wrinkling, altered elasticity, and reduced turgidity74. Beating the enzymes responsible for HA degradation seems then an effective strategy to delay skin-ageing and improve the appearance of the skin at all ages.
Regarding HASE inhibitory activity, Yamaguchi and Koketsu75 found that the cyanobacteria Nostochopsis lobatus MAC0804NAN produce a large amount of a polysaccharide with a high inhibitory effect (IC50= 7.18 µg/mL) on HASE, being about 14.5 times stronger than the natural inhibitor disodium cromoglycate. Being an edible species, upholds its use for cosmetic purposes, as well as its acceptance by consumers, since it already has a known safety profile. Besides pure compounds, it was also noticed that extracts, namely ethanol-insoluble fractions, could inhibit the activity of HASE, as shown by Fujitani et al. in a study conducted with seven different genera of microalgae76 (Table 1). The IC50 of Spirulina platensis, Porphyridium purpureum, Rhodosorus marinus, Chlorella pyrenoidosa, Dunaliella salina, and Pleurochrysis carterae was 0.15, 0.18, 0.26, 0.94, 0.15 and 0.41 mg/mL, respectively, with S. platensis and D. salina presenting similar values to those of the natural HASE inhibitor. It was reported that the ethanol-insoluble fraction included macromolecules such as polysaccharides, which may be involved in hyaluronidase inhibition.
The use of effective extracts as active ingredients for cosmetics production can constitute an asset face to isolated compounds, due to the higher extraction yield and lower processing costs.
3.2. Hyperpigmentation
Skin-whitening, as well as an aesthetically pleasing and uniform skin pigmentation, has been a primary focus of many cosmetic industries. Skin often gets irregularly darkened because of the UV radiation, ageing, and pregnancy. Although hyperpigmentation is not harmful in any way, sometimes it may cause serious problems, such as melanoma. As a result, several treatment modalities are being investigated for their efficacy to treat skin pigmentation disorders10,77.
Melanogenesis occurs in melanocytes, located at the base of epidermis, in a process involving several chemical and enzymatic reactions to produce melanin, a major component of skin colour78. Hyperpigmentation can occur through an increase in the number of melanocytes, or through the overactivity of melanogenic enzymes - Tyrosinase79,80 (Figure 1). The accumulation of abnormal amount of melanin is mainly caused by UVR exposure, which increases reactive oxygen species (ROS) production78. ROS are produced in the epidermis of the skin and stimulate melanocytes to convert tyrosine into melanin by oxidation, through the action of tyrosinase10. Tyrosinase is a crucial enzyme that catalyses melanin synthesis in melanocytes. Therefore, skin pigmentation can be prevented by tyrosinase inhibitors10,81. Some of the well-known tyrosinase inhibitors are hydroquinone (HQ), kojic acid, and arbutin. Although being effective as depigmenting agents, these compounds are not devoid of harmful effects. It has already been demonstrated that HQ has mutagenic effects and cytotoxicity against mammalian V79 cells82, causes DNA damage83 and has some evidence of carcinogenic activity84. Regarding kojic acid, skin irritation and allergic dermatitis were developed after using skincare products containing it85. In relation to arbutin, the application of higher concentrations caused skin irritation and hyperpigmentation86. Additionally, they have high toxicity, low stability, poor skin penetration, and insufficient activity87. Face to the exposed, it is extremely important to find alternatives to overcome hyperpigmentation, or to find new tyrosinase inhibitors with effectivity and less harmful side effects. The research on tyrosinase inhibition by cyanobacteria and microalgae-derived compounds has been very limited to date, and the majority of the available studies explore mushroom tyrosinase as enzymatic model, making it difficult to translate the results to human environment. However, some promising compounds and bioactive extracts from cyanobacteria and microalgae have emerged in the last years (Table 2).
Table 2.
Bioactive potential of cyanobacteria and microalgae-derived extracts and isolated compounds in skin-whitening.a
| Compound/extract | Species | Model | Reference |
|---|---|---|---|
| Ethanol and water extracts | Arthrospira platensis | Mushroom tyrosinase | Sahin87 |
| Oscillapeptin G | Oscillatoria agardhii | Mushroom tyrosinase | Sano and Kaya88 |
| C-phycocyanin Vitamins C and E |
Spirulina sp. | B16F10 murine melanoma cells - |
Wu et al.89 Babadzhanov et al.95 |
| PHORMISKIN Bioprotech G® | Phormidium persicinum | Humans | Phormiskin bioprotech g91 |
| Mono and Diesters of Astaxanthin | Haematococcus pluvialis | Mushroom tyrosinase | Rao et al.92 |
| Zeaxanthin in submicronsized precipitates | Nannochloropsis oculata | Mushroom tyrosinase | Shen et al.93 |
| Purified peptide | Pavlova lutheri | Hydroxyl radical scavenging activity ABTS B16F10 murine melanoma cells |
Oh et al.90 |
| Vitamins C and E | Chlorella vulgaris | Muscle and hepatopancreas of the M. rosenbergii | Radhakrishnan et al.96 |
aABTS: 2,2’-azino bis (3-ethylbenzothiazoline-6-sulfonic acid) as substrate in a colorimetry assay.
Examples of the potential of cyanobacteria in hyperpigmentation include crude extracts of Arthrospira platensis. Sahin87, found that ethanol and water extracts of A. platensis presented IC50 values in a comparable scale to those of kojic acid. It was found that some phenolic compounds produced by this species, e.g. caffeic and ferulic acids, and present in the extracts, are considered to be the most effective inhibitors of the enzyme tyrosinase, with IC50 values significantly lower than those of the drugs kojic acid and arbutin. Although the authors have undertaken the study in a non-human enzyme model, the comparison of their results with those of the human tyrosinase inhibitors, points A. platensis extracts as alternative precursors in obtaining both effective and safer inhibitors for tyrosinase activity87.
In 1996, a study performed with Oscillatoria agardhii demonstrated that oscillapeptin G exhibited tyrosinase inhibition in 55% face to the untreated control, but no mechanistic or reference drugs were explored88. A more complex research in this thematic has been undertaken by Wu and co-workers, who explored the anti-melanogenic effect of C-PC from Spirulina sp., using B16F10 murine melanoma cells. The authors found that C-PC inhibits melanin biosynthesis by a dual mechanism, one promoting the degradation of MITF protein, the transcription factor of tyrosinase, through the up-regulation of MAPK/ERK signalling pathway, and the other by suppressing the activation of CREB, the transcription factor of MITF, via the down-regulation of p38 MAPK pathway89. Oh and co-workers90 explored a novel peptide isolated from Pavlova lutheri in ROS generation and expression of melanogenic specific proteins. The authors found that the peptide demonstrated inhibitory properties against α-Melanocyte Stimulating Hormone-induced melanogenesis via melanin content, tyrosinase inhibition in B16F10 melanoma cells, and also decreased melanogenesis-related proteins. Therefore, this protein has potential whitening effects and prominent protective effects on oxidative stress-induced cell damage, which can be used as an effective natural source in cosmeceutical and pharmaceutical products.
Despite the scarce in vitro trials in the thematic of hyperpigmentation using cyanobacteria and microalgae-derived compounds, the company CODIF Research & Nature took a step forward with a trial involving human volunteers. This biotechnological company, that explores sea resources for cosmetics production, developed a biotechnological extract, PHORMISKIN Bioprotech G®, from the cyanobacteria Phormidium persicinum, able to reduce melanin synthesis. In a study, 15 volunteers aged between 25 and 46 years old applied the extract, in a concentration of 2%, during 28 consecutive days. After the experimental period, the skin tone became more uniform and the skin brighter. The extract also stimulated the synthesis of the protein thioredoxin, which is known for its antioxidant and detoxifying properties91.
There are also some studies with bioactive extracts and compounds isolated from microalgae. One of them uses astaxanthin from Haematococcus pluvialis, and shows the multitarget action of this xanthophyll, namely in the inhibition of ROS accumulation and down-regulation of tyrosinase. In this study, astaxanthin diesters had the highest tyrosinase inhibitory activity than monoesters, presenting IC50 values of 2.12 and 3.5 µg/mL, respectively. Hence the mentioned properties may prevent the uncontrolled proliferation and accumulation of melanocytes, and consequently of melanin92. Also, other survey with zeaxanthin from Nannochloropsis oculata reported tyrosinase inhibitory activity in a dose-dependent manner93, assuming the potential of xanthophylls as brightening agents.
Another approach to prevent melanosome formation in the skin is by using vitamins C and E94. In this regard, it can be assumed that Spirulina sp.89,95 and Chlorella vulgaris96 constitute great candidates for cosmetic purposes, due to their significant content in these vitamins.
In an attempt to point a possible structure-activity relationship, and taking into account the IC50 values found for the different cyanobacteria and microalgae-derived compounds, it seems that phenolic acids like caffeic and ferulic acid, present in bioactive extracts, and with a molecular structure more similar to kojic acid, are more effective in inhibiting tyrosinase than peptides.
4. Future perspectives
The concern in delaying the effects of ageing has been the fuel for the investment in the search for new, innovative, effective and eco-friendly compounds, aiming the discovery of the perfect anti-ageing formulation. Allied to this, the growing research in natural sources, more specifically from marine origin, has provided a countless number of new molecules with promising bioactivities worth of further exploitation in the field of skin ageing. Besides the inherent advantages of using cyanobacteria and microalgae as metabolic producers, it has been demonstrated herein their huge potential to target specific enzymes involved in the ageing process, most of the times with an activity significantly higher than that of the reference drugs currently in use. In this regard, it seems unquestionable that further toxicological and biotechnological studies will be the next steps to evaluate the safety and effectiveness of these molecules in anti-ageing formulations, and that they will much probably revolutionise the cosmetic market.
Acknowledgements
This work was supported by ALGAVALOR-MicroALGAs: integrated production and valorisation of biomass and its various applications-SI I&DT n.o 352234-supported by the PORTUGAL 2020 through the European Regional Development Fund, and by BLUEHUMAN–BLUE biotechnology as a road for innovation on HUMAN’s health aiming smart growth in Atlantic Area–EAPA_151/2016 of the Interreg Atlantic Area Programme funded by the European Regional Development Fund. CIIMAR acknowledges the strategic funding UIDB/04423/2020 and UIDP/04423/2020.
Funding Statement
This work was supported by ALGAVALOR-MicroALGAs: integrated production and valorisation of biomass and its various applications-SI I&DT n.o 352234-supported by the PORTUGAL 2020 through the European Regional Development Fund. CIIMAR acknowledges the strategic funding UIDB/04423/2020 and UIDP/04423/2020.
Disclosure statement
The authors report no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Berthon JY, Nachat-Kappes R, Bey M, et al. . Marine algae as attractive source to skin care. Free Radic Res 2017;51:555–67. [DOI] [PubMed] [Google Scholar]
- 2.Wickett RR, Visscher MO.. Structure and function of the epidermal barrier. Am J Infect Control 2006;34:S98–S110. [Google Scholar]
- 3.Holick MF.Vitamin D: a millenium perspective. J Cell Biochem 2003;88:296–307. [DOI] [PubMed] [Google Scholar]
- 4.Lourith N, Kanlayavattanakul M.. Biopolymeric agents for skin wrinkle treatment. J Cosmet Laser Ther 2016;18:301–10. [DOI] [PubMed] [Google Scholar]
- 5.Baroni A, Buommino E, De Gregorio V, et al. . Structure and function of the epidermis related to barrier properties. Clin Dermatol 2012;30:257–62. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S.Exploratory analysis of global cosmetic industry: major players, technology and market trends. Technovation 2005;25:1263–72. [Google Scholar]
- 7.Draelos ZD.Cosmetics and skin care products. A historical perspective. Dermatologic Clinics 2000;18:557–9. [DOI] [PubMed] [Google Scholar]
- 8.Jain N, Chaudhri S.. History of cosmetics. Asian J Pharmac 2009;3:164. [Google Scholar]
- 9.Blanco-Davila F.Beauty and the body: the origins of cosmetics. Plastic Reconstruc Surg 2000;105:1196–204. [DOI] [PubMed] [Google Scholar]
- 10.Yarkent C, Gurlek C, Oncel SS.. Potential of microalgal compounds in trending natural cosmetics: a review. Sustain Chem Pharm 2020;17:100304. [Google Scholar]
- 11.Haddara M, Hsieh J, Fagerstrøm A, et al. . Exploring customer online reviews for new product development: the case of identifying reinforcers in the cosmetic industry. Manag Decision Econ 2020;41:250–73. [Google Scholar]
- 12.Mehta RC, Fitzpatrick RE.. Endogenous growth factors as cosmeceuticals. Dermatol Ther 2007;20:350–9. [DOI] [PubMed] [Google Scholar]
- 13.Makrantonaki E, Zouboulis CC.. William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007;214:352–60. [DOI] [PubMed] [Google Scholar]
- 14.Makrantonaki E, Zouboulis CC.. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci 2007;1119:40–50. [DOI] [PubMed] [Google Scholar]
- 15.Pensalfini M, Rotach M, Hopf R, et al. . How cosmetic tightening products modulate the biomechanics and morphology of human skin. Acta Biomaterialia 2020;115:299–316. [DOI] [PubMed] [Google Scholar]
- 16.Bom S, Jorge J, Ribeiro HM, Marto J.. A step forward on sustainability in the cosmetics industry: a review. J Cleaner Produc 2019;225:270–90. [Google Scholar]
- 17.Morone J, Alfeus A, Vasconcelos V, Martins R.. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals - a new bioactive approach. Algal Res Biomass Biofuels Bioproducts 2019;41:101541. [Google Scholar]
- 18.Souza C, Campos P.. Development and photoprotective effect of a sunscreen containing the antioxidants spirulina and dimethylmethoxy chromanol on sun-induced skin damage. Eur J Pharm Sci 2017;104:52–64. [DOI] [PubMed] [Google Scholar]
- 19.Mourelle ML, Gómez CP, Legido JL.. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017;4:46. [Google Scholar]
- 20.Chlipala GE, Mo S, Orjala J.. Chemodiversity in freshwater and terrestrial cyanobacteria - a source for drug discovery. Curr Drug Targets 2011;12:1654–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schopf JW, The ecology of cyanobacteria. Netherlands: Springer. [Google Scholar]
- 22.Nowruzi B, Sarvari G, Blanco S.. The cosmetic application of cyanobacterial secondary metabolites. Algal Res Biomass Biofuels Bioproducts 2020;49:101959. [Google Scholar]
- 23.Zhang LF, Selao TT, Nixon PJ, Norling B.. Photosynthetic conversion of co2 to hyaluronic acid by engineered strains of the cyanobacterium synechococcus sp. Pcc 7002. Algal Res Biomass Biofuels Bioproducts 2019;44:101702. [Google Scholar]
- 24.Koller M, Muhr A, Braunegg G.. Microalgae as versatile cellular factories for valued products. Algal Res 2014;6:52–63. [Google Scholar]
- 25.Lau NS, Matsui M, Abdullah AAA.. Cyanobacteria: photoautotrophic microbial factories for the sustainable synthesis of industrial products. Biom Res Inter 2015;2015:754934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morone J, Lopes G, Preto M, et al. . Exploitation of filamentous and picoplanktonic cyanobacteria for cosmetic applications: potential to improve skin structure and preserve dermal matrix components. Marine Drugs 2020;18:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes G, Clarinha D, Vasconcelos V.. Carotenoids from cyanobacteria: a biotechnological approach for the topical treatment of psoriasis. Microorganisms 2020;8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijayakumar S, Menakha M.. Pharmaceutical applications of cyanobacteria—a review. J Acute Med 2015;5:15–23. [Google Scholar]
- 29.Gao X, Jing X, Liu XF, Lindblad P.. Biotechnological production of the sunscreen pigment scytonemin in cyanobacteria: progress and strategy. Marine Drugs 2021;19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philips N, Auler S, Hugo R, Gonzalez S.. Beneficial regulation of matrix metalloproteinases for skin health. Enzyme Res 2011;2011:427285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maquart FX, Monboisse JC.. Extracellular matrix and wound healing. Pathol Biol 2014;62:91–5. [DOI] [PubMed] [Google Scholar]
- 32.Deniz FSS, Salmas RE, Emerce E, et al. . Evaluation of collagenase, elastase and tyrosinase inhibitory activities of cotinus coggygria scop. Through in vitro and in silico approaches. South African J Bot 2020;132:277–88. [Google Scholar]
- 33.Sin BY, Kim HP.. Inhibition of collagenase by naturally-occurring flavonoids. Arch Pharm Res 2005;28:1152–5. [DOI] [PubMed] [Google Scholar]
- 34.Herouy Y.Matrix metalloproteinases in skin pathology (review). Inter J Mol Med 2001;7:3–15. [PubMed] [Google Scholar]
- 35.Fisher GJ, Quan T, Purohit T, et al. . Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009;174:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philips N, Conte J, Chen YJ, et al. . Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-beta by polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch Dermat Res 2009;301:487–95. [DOI] [PubMed] [Google Scholar]
- 37.Shingleton WD, Hodges DJ, Brick P, Cawston TE.. Collagenase: a key enzyme in collagen turnover. Biochem Cell Biol 1996;74:759–75. [DOI] [PubMed] [Google Scholar]
- 38.Hetta M.Hyaluronidase inhibitors as skin rejuvenating agents from natural source. Inter J Phytocosmetics Natural Ingred 2020;7(1):4. [Google Scholar]
- 39.Tarasuntisuk S, Patipong T, Hibino T, et al. . Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett Appl Microbiol 2018;67:314–320. [DOI] [PubMed] [Google Scholar]
- 40.Kunte M, Desai K.. The protein extract of chlorella minutissima inhibits the expression of mmp-1, mmp-2 and mmp-9 in cancer cells through upregulation of timp-3 and down regulation of c-jun. Cell Journal 2018;20:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CL, Liou SF, Chen SJ, Shih MF.. Protective effects of chlorella-derived peptide on uvb-induced production of mmp-1 and degradation of procollagen genes in human skin fibroblasts. Regul Toxicol Pharmacol 2011;60:112–119. [DOI] [PubMed] [Google Scholar]
- 42.Montalvo GEB, Thomaz-Soccol V, Vandenberghe LPS, et al. . Arthrospira maxima of15 biomass cultivation at laboratory and pilot scale from sugarcane vinasse for potential biological new peptides production. Bioresource Technology 2019;273:103–113. [DOI] [PubMed] [Google Scholar]
- 43.Pittayapruek P, Meephansan J, Prapapan O, et al. . Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Inter J Mol Sci 2016;17:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunte M, Desai K.. The inhibitory effect of c-phycocyanin containing protein extract (c-pc extract) on human matrix metalloproteinases (mmp-2 and mmp-9) in hepatocellular cancer cell line (hepg2). Prot J 2017;36:186–195. [DOI] [PubMed] [Google Scholar]
- 45.Taddese S, Weiss AS, Jahreis G, et al. . In vitro degradation of human tropoelastin by mmp-12 and the generation of matrikines from domain 24. Matrix Biol 2009;28:84–91. [DOI] [PubMed] [Google Scholar]
- 46.Keller L, Canuto KM, Liu CX, et al. . Tutuilamides a-c: vinyl-chloride-containing cyclodepsipeptides from marine cyanobacteria with potent elastase inhibitory properties. ACS Chem Biol 2020;15:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthew S, Ross C, Rocca JR, et al. . Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium lyngbya confervoides. J Nat Products 2007;70:124–127. [DOI] [PubMed] [Google Scholar]
- 48.Taori K, Matthew S, Rocca JR, et al. . Lyngbyastatins 5-7, potent elastase inhibitors from floridian marine cyanobacteria, lyngbya spp. J Nat Prod 2007;70:1593–1600. [DOI] [PubMed] [Google Scholar]
- 49.Matthew S, Pau VJ, Luesch H.. Largamides a-c, tiglic acid-containing cyclodepsipeptides with elastase-inhibitory activity from the marine cyanobacterium lyngbya confervoides. Planta Medica 2009;75:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthew S, Paul VJ, Luesch H.. Tiglicamides a-c, cyclodepsipeptides from the marine cyanobacterium lyngbya confervoides. Phytochemistry 2009;70:2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwan JC, Taori K, Paul VJ, Luesch H.. Lyngbyastatins 8-10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium lyngbya semiplena. Marine Drugs 2009;7:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubio BK, Parrish SM, Yoshida W, et al. . Depsipeptides from a guamanian marine cyanobacterium, lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron Lett 2010;51:6718–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang HS, Krunic A, Orjala J.. Stigonemapeptin, an ahp-containing depsipeptide with elastase inhibitory activity from the bloom-forming freshwater cyanobacterium stigonema sp. J Nat Prod 2012;75:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salvador LA, Taori K, Biggs JS, et al. . Potent elastase inhibitors from cyanobacteria: structural basis and mechanisms mediating cytoprotective and anti-inflammatory effects in bronchial epithelial cells. J Med Chem 2013;56:1276–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itou Y, Ishida K, Shin SJ, Murakami M.. Oscillapeptins a to f, serine protease inhibitors from the three strains of oscillatoria agardhii. Tetrahedron 1999;55:6871–6882. [Google Scholar]
- 56.Fujii K, Sivonen K, Naganawa E, Harada K.. Non-toxic peptides from toxic cyanobacteria, oscillatoria agardhii. Tetrahedron 2000;56:725–733. [Google Scholar]
- 57.Murakami M, Sun Q, Ishida K, et al. . Microviridins, elastase inhibitors from the cyanobacterium nostoc minutum (nies-26). Phytochemistry 1997;45:1197–1202. [Google Scholar]
- 58.Okino T, Qi S, Matsuda H, et al. . Nostopeptins a and b, elastase inhibitors from the cyanobacterium nostoc minutum. J Nat Prod 1997;60:158–161. [Google Scholar]
- 59.Okino T, Matsuda H, Murakami M, Yamaguchi K.. New microviridins, elastase inhibitors from the blue-green alga microcystis aeruginosa. Tetrahedron 1995;51:10679–10686. [Google Scholar]
- 60.Lodin-Friedman A, Carmeli S.. Metabolites from microcystis aeruginosa bloom material collected at a water reservoir near kibbutz hafetz haim, israel. J Nat Prod 2013;76:1196–1200. [DOI] [PubMed] [Google Scholar]
- 61.Adiv S, Aharonv-Nadborny R, Carmeli S.. Micropeptins from microcystis aeruginosa collected in dalton reservoir, israel. Tetrahedron 2010;66:7429–7436. [Google Scholar]
- 62.Gunasekera SP, Miller MW, Kwan JC, et al. . Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium dichothrix utahensis. J Nat Prod 2010;73:459–462. [DOI] [PubMed] [Google Scholar]
- 63.Matern U, Oberer L, Falchetto RA, et al. . Scyptolin a and b, cyclic depsipeptides from axenic cultures of scytonema hofmanni pcc 7110. Phytochemistry 2001;58:1087–1095. [DOI] [PubMed] [Google Scholar]
- 64.Grach-Pogrebinsky O, Sedmak B, Carmeli S.. Protease inhibitors from a slovenian lake bled toxic waterbloom of the cyanobacterium planktothrix rubescens. Tetrahedron 2003;59:8329–8336. [Google Scholar]
- 65.Bubik A, Sedmak B, Novinec M, et al. . Cytotoxic and peptidase inhibitory activities of selected non-hepatotoxic cyclic peptides from cyanobacteria. Biol Chem 2008;389:1339–1346. [DOI] [PubMed] [Google Scholar]
- 66.Sisay MT, Hautmann S, Mehner C, et al. . Inhibition of human leukocyte elastase by brunsvicamides a-c: cyanobacterial cyclic peptides. Chem Med Chem 2009;4:1425–1429. [DOI] [PubMed] [Google Scholar]
- 67.Mehner C, Muller D, Kehraus S, et al. . New peptolides from the cyanobacterium nostoc insulare as selective and potent inhibitors of human leukocyte elastase. Chem Bio Chem 2008;9:2692–2703. [DOI] [PubMed] [Google Scholar]
- 68.Draelos ZD.The science behind skin care: moisturizers. J Cosm Dermatol 2018;17:138–144. [DOI] [PubMed] [Google Scholar]
- 69.Foster AR, El Chami C, O'Neill CA, Watson REB.. Osmolyte transporter expression is reduced in photoaged human skin: implications for skin hydration in aging. Aging Cell 2020;19:e13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JW, Kwon SH, Huh CH, et al. . The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Tech 2013;19:E349–E355. [DOI] [PubMed] [Google Scholar]
- 71.Papakonstantinou E, Roth M, Karakiulakis G.. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol 2012;4:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fayad S, Morin P, Nehme R.. Use of chromatographic and electrophoretic tools for assaying elastase, collagenase, hyaluronidase, and tyrosinase activity. J Chromatogr A 2017;1529:1–28. [DOI] [PubMed] [Google Scholar]
- 73.Stern R, Jedrzejas MJ.. Hyaluronidases: their genomics, structures, and mechanisms of action. Chemical Reviews 2006;106:818–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baumann L.Skin ageing and its treatment. J Pathol 2007;211:241–251. [DOI] [PubMed] [Google Scholar]
- 75.Yamaguchi Y, Koketsu M.. Isolation and analysis of polysaccharide showing high hyaluronidase inhibitory activity in nostochopsis lobatus mac0804nan. J Biosci Bioeng 2016;121:345–348. [DOI] [PubMed] [Google Scholar]
- 76.Fujitani N, Sakaki S, Yamaguchi Y, Takenaka H.. Inhibitory effects of microalgae on the activation of hyaluronidase. J Appl Phycol 2001;13:489–492. [Google Scholar]
- 77.Ebanks JP, Wickett RR, Boissy RE.. Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Inter J Mol Sci 2009;10:4066–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa JAV, Barbieri Moro GM, de Moraes Vaz Batista Filgueira D, et al. . The potential of spirulina and its bioactive metabolites as ingested agents for skin care. Industr Biotechnol 2017;13:244–252. [Google Scholar]
- 79.Briganti S, Camera E, Picardo M.. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res 2003;16:101–110. [DOI] [PubMed] [Google Scholar]
- 80.Wang HMD, Chen CC, Huynh P, Chang JS.. Exploring the potential of using algae in cosmetics. Bioresource Tech 2015;184:355–362. [DOI] [PubMed] [Google Scholar]
- 81.Choe SY, Hong JH, Gu YR, et al. . Hot water extract of glehnia littoralis leaf showed skin-whitening and anti-wrinkle properties. South African J Bot 2019;127:104–109. [Google Scholar]
- 82.Curto EV, Kwong C, Hermersdorfer H, et al. . Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem Pharmacol 1999;57:663–672. [DOI] [PubMed] [Google Scholar]
- 83.Akhtar MN, Sakeh NM, Zareen S, et al. . Design and synthesis of chalcone derivatives as potent tyrosinase inhibitors and their structural activity relationship. J Mol Struc 2015;1085:97–103. [Google Scholar]
- 84.Program NT.Ntp toxicology and carcinogenesis studies of hydroquinone (cas no. 123-31-9) in f344/n rats and b6c3f1 mice (gavage studies). Natl Toxicol Prog Techni Report Series 1989;366:1–248. [PubMed] [Google Scholar]
- 85.Nakagawa M, Kawai K, Kawai K.. Contact allergy to kojic acid in skin care products. Contact Dermatitis 1995;32:9–13. [DOI] [PubMed] [Google Scholar]
- 86.Migas P, Krauze-Baranowska M.. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett 2015;13:35–40. [Google Scholar]
- 87.Sahin SC.The potential of arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. South African J Bot 2018;119:236–243. [Google Scholar]
- 88.Sano T, Kaya K.. Oscillapeptin g, a tyrosinase inhibitor from toxic oscillatoria agardhii. J Nat Prod 1996;59:90–92. [DOI] [PubMed] [Google Scholar]
- 89.Wu LC, Lin YY, Yang SY, Weng YT, et al. . Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of erk and downregulation of p38 mapk signaling pathways. J Biomed Sci 2011;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh GW, Ko SC, Heo SY, Nguyen VT, Kim G, et al. . A novel peptide purified from the fermented microalga pavlova lutheri attenuates oxidative stress and melanogenesis in b16f10 melanoma cells. Proc Biochem 2015;50:1318–1326. [Google Scholar]
- 91.Phormiskin bioprotech g. http://www.codif-tn.com/en/principesactifs/phormiskin-bioprotech-g/
- 92.Rao AR, Sindhuja HN, Dharmesh SM, et al. . Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga haematococcus pluvialis. J Agric Food Chem 2013;61:3842–3851. [DOI] [PubMed] [Google Scholar]
- 93.Shen CT, Chen PY, Wu JJ, et al. . Purification of algal anti-tyrosinase zeaxanthin from nannochloropsis oculata using supercritical anti-solvent precipitation. J Supercritical Fluids 2011;55:955–962. [Google Scholar]
- 94.Quevedo WC, Holstein TJ, Dyckman J, et al. . Inhibition of uvr-induced tanning and immunosuppression by topical applications of vitamins c and e to the skin of hairless (hr/hr) mice. Pigment Cell Res 2000;13:89–98. [DOI] [PubMed] [Google Scholar]
- 95.Babadzhanov AS, Abdusamatova N, Yusupova FM, et al. . Chemical composition of spirulina platensis cultivated in uzbekistan. Chem Nat Compounds 2004;40:276–279. [Google Scholar]
- 96.Radhakrishnan S, Saravana Bhavan P, Seenivasan C, et al. . replacement of fishmeal with spirulina platensis, chlorella vulgaris and azolla pinnata on non-enzymatic and enzymatic antioxidant activities of macrobrachium rosenbergii. J Basic Appl Zool 2014;67:25–33. [Google Scholar]
- 97.Cheng FC, Feng JJ, Mizoguchi T, et al. . Effects of chlorella on activities of protein tyrosine phosphatases, matrix metalloproteinases, caspases, cytokine release, b and t cell proliferations, and phorbol ester receptor binding. J Med Food 2004;7:146–152. [DOI] [PubMed] [Google Scholar]
- 98.Liu P, Lee MK, Choi JW, et al. . Crude protein from spirulina increases the viability of ccd‑986sk cells via the egfr/mapk signaling pathway. Int J Mol Med 2019;43:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]