Abstract
Recipient diabetes accounts for ~34% of end stage renal disease in patients awaiting renal transplantation and has been linked to poor graft function. We conducted a single center, open label, randomized controlled trial to determine if moderately intense glucose control during allograft reperfusion would reduce the incidence of poor graft function. Adult diabetics undergoing deceased donor renal transplant were randomized to moderately intense glucose control (n=30) or standard control (n=30). The primary outcome was poor graft function (dialysis within seven days of transplant or failure of serum creatinine to fall by 10% for three consecutive days). Recipients with moderately intense glucose control had less poor graft function in the intention to treat (43.3% vs. 73.3%, P =0.02) and per protocol analysis (43.2% vs 81%, P <0.01). Recipients with moderately intense control also had higher GFR at 30 days after transplant in the per-protocol and intention to treat analyses. There were no episodes of severe hypoglycemia in either group and no differences in mortality, seizures, stroke, graft loss or biopsy-proven rejection. Moderately intense glucose control at the time of allograft reperfusion reduces the incidence of poor graft function in diabetic renal transplant recipients and improves glomerular filtration rate at 30 days.
Keywords: Kidney transplantation, poor graft function, delayed graft function, diabetes, hyperglycemia
Introduction
The benefit of tight glucose control in hospitalized patients remains unclear. Initial work supported a mortality benefit for tight glucose control however more recent randomized trials have found that targeting a blood glucose of 80–108 mg/dL increased mortality among ICU patients compared to a more moderate target of 180 mg/dL.1–4 Animal models and clinical studies have consistently demonstrated a reduction in ischemia-reperfusion injury when glucose levels are better controlled. In animal models, both spinal cord injury and the degree of myocardial infarction were reduced when blood glucose was controlled at the time of ischemic injury 5–7. Although these are not models of transplantation, they do support the concept that hyperglycemia increases ischemia-reperfusion injury which is prominent in kidney transplantation. Similarly, we found that rats with hyperglycemia at the time of renal ischemia had higher terminal creatinine levels and histological features of more severe acute tubular necrosis compared to normoglycemic rats8. In human studies, improved glucose control has been linked to improved renal function in multiple patient populations, including patients undergoing cardiopulmonary bypass and those in the surgical ICU 2,9. These studies indicate that hyperglycemia increases ischemia reperfusion injury and that this effect may be limited to a window around the time of injury.
In renal transplantation, poor graft function is defined as the need for dialysis within seven days of transplant or a slow decline in creatinine whereas delayed graft function (DGF) is more specifically defined as the need for dialysis within seven days of transplant10. In the short term, poor initial function commands increased resource utilization, including imaging, dialysis, and prolonged hospitalization. More importantly, DGF is associated with a higher risk of rejection and lower long-term graft survival 11,12. Largely thought to be a result of ischemia reperfusion injury, poor graft function is clearly associated with prolonged cold ischemia time and increasing donor age 13–17. Diabetes and hyperglycemia are linked to poor graft function as well 18,19. In particular, our group found that blood glucose levels measured immediately after transplant are associated with poor graft function, and that blood glucose at the time of reperfusion correlates with markers of renal ischemia20,21. Hyperglycemia itself is quite common after renal transplant as large doses of steroids and the return of renal function can cause an abrupt increase in insulin needs. In fact, glycemic control can be quite bad with blood glucose levels often exceeding 200 mg/dL; making moderate control a significant improvement22. The impact of hyperglycemia and diabetes on graft function is particularly important because diabetes has become the most common cause of end stage renal disease for patients waiting for kidney transplant, accounting for ~ 34% of the wait list 23.
Given the profound hyperglycemia that can occur after transplant and evidence that tight control can be detrimental to patient outcomes, we designed a randomized controlled trial of perioperative glucose control to determine if moderately intense glucose control, with the primary goal of achieving a blood glucose level between 80–160 mg/dL at the time of allograft reperfusion, would reduce the incidence of poor graft function.
MATERIALS AND METHODS
Study Design
We conducted a single-center, open-label randomized controlled trial comparing standard and moderately intense glucose control. The study was approved by the UCSF Committee on Human Research (#11–07978) and registered with clinicaltrials.gov (#NCT01643382). Adult patients with a diagnosis of diabetes mellitus who were admitted for deceased donor renal transplantation were approached (Figure 1). Children and adult candidates enrolled in a concurrent study evaluating the effect of a medication or other intervention on graft function were excluded. All recipients underwent transplant and follow-up at the University of California San Francisco Medical Center.
Figure 1.
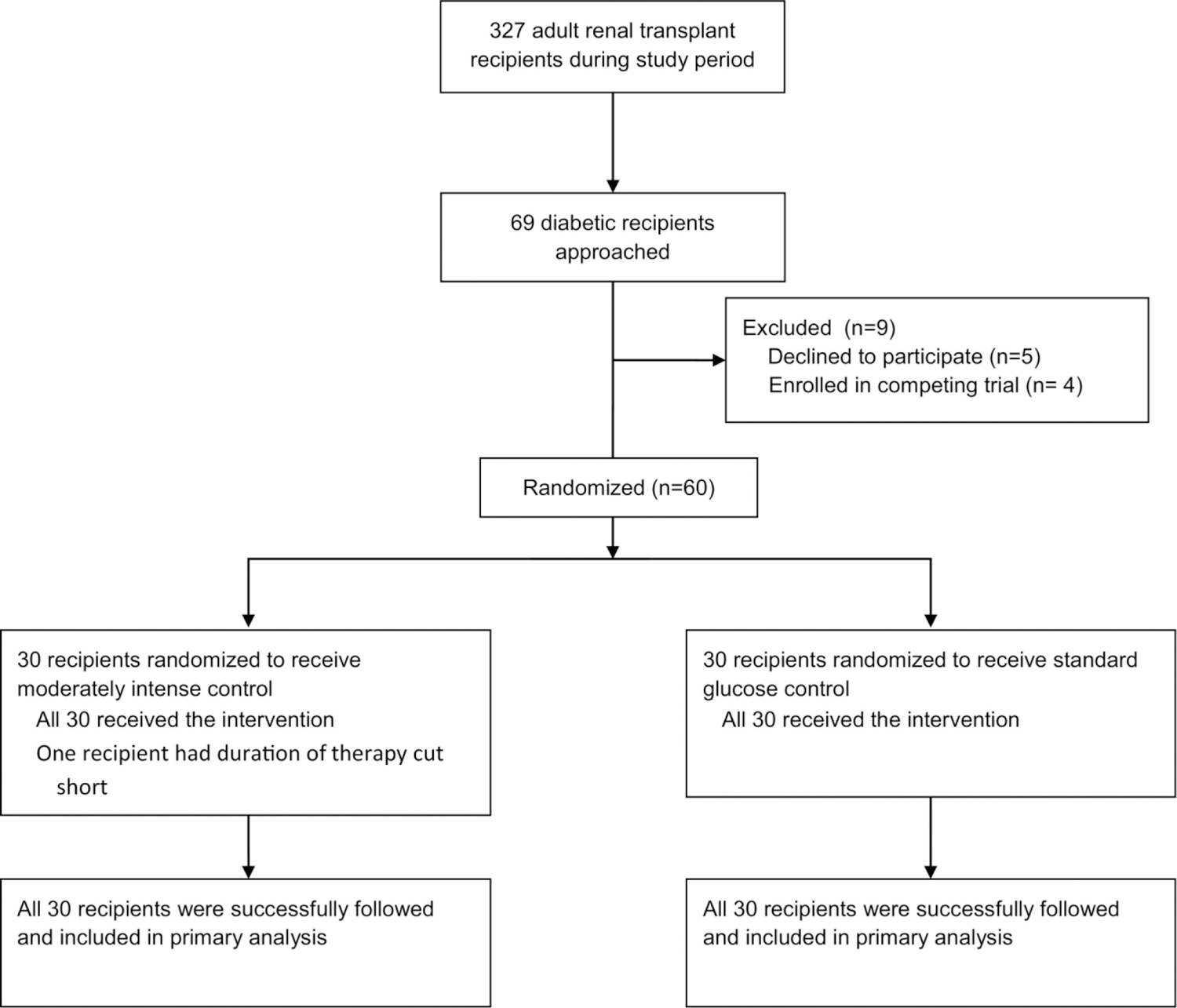
Flow Diagram for Study Enrollment and Outcomes
Participant flow of perioperative glucose control trial.
Randomization
We used a block randomization strategy (block size of four) to ensure equal distribution of patients between study arms. The randomization was generated per computer algorithm and the randomization scheme was hidden in an electronic file until each patient was enrolled.
Interventions
We sought to implement a trial that was based on current practices at our institution and used existing protocols that did not require expertise outside of the usual course of care. Before the trial began we observed that standard pre-operative management at our institution consisted of ordering an insulin sliding scale or no intervention unless blood glucose exceeded 200 mg/dL. Intra-operative management varied widely, from insulin infusions to bolus insulin therapy, to no monitoring or therapy at all. Post-operative management routinely consisted of a simple scale for the first day after transplant and long acting insulin started on the night of post-operative day one as needed. With these practices in mind, we devised our study using existing insulin treatment algorithms that were already in use at our center.
Once patients were admitted to the hospital for transplant, they were approached to participate in the study and enrolled by a member of the research team. Final randomization was performed once consent was obtained and the plan to move forward with transplant was confirmed. Recipients were then randomized to either standard glucose control or moderately intense glucose control. All blood glucose measurements were taken at the point of care using Accuchek Hospital Meters (Roche Diagnostics, Indianapolis, Indiana).
Preoperatively, recipients enrolled in the control arm were placed on a standard insulin sliding scale when serum blood glucose exceeded 200 mg/dL. During the operation, anesthesiologists were advised to check the blood glucose at the time of reperfusion and treat at their discretion via intravenous insulin if the glucose was above 200 mg/dL. In practice, no insulin was administered. Post-operatively, all recipients were placed on a standard sliding scale for 24 hours. After 24 hours, the primary transplant team was given complete control of glucose management and allowed to start insulin as they deemed appropriate.
Pre-operatively, recipients in the moderately intense glucose control group were started on an insulin infusion if their blood glucose was greater than 120 mg/dL. To avoid prolonged treatment, the infusion was started no earlier than four hours before the anticipated start of the transplant. Intra-operatively, the anesthesia team was advised to check the blood glucose every 30–45 min with the goal of keeping it between 80–160 mg/dL. The decision to target a blood glucose of less than 160 mg/dL was based on our previous work that indicated a threshold effect above that level; resulting in greater rates of DGF and markers of ischemic injury20,21. The use of bolus therapy or insulin infusion was left to the discretion of the anesthesia team. Post-operatively, all recipients were placed on an insulin infusion (existing protocol already approved by the medical center for use on standard medical-surgical wards)) that targeted blood glucose levels of 100–180 mg/dL. This infusion was continued for 24 hours post-operatively. After 24 hours of the infusion, the primary transplant team was given complete control over glucose management.
Transplantation
All recipients received a single deceased donor renal allograft. All allografts were transported via cold storage without use of a perfusion pump. Induction immunosuppression was dictated by the operating surgeon and consisted of 500 mg of Solumedrol and either Thymoglobulin (total dose 6 mg/kg) or Simulect (two doses of 20mg) administered at the time of the transplant. All allografts were placed in the extra-peritoneal location with ureteroneocystostomy for the ureteral anastomosis. Post-operative immunosuppression consisted of a steroid taper (Solumedrol 250mg POD 1, Solumedrol 125mg POD 2, then Prednisone 60mg POD 3 followed by Prednisone 30mg for one week, 20mg for one week, 10mg for one week and then 5mg), mycophenolate mofetil (one gram twice daily), and tacrolimus (goal serum trough of 8–10 µg/L). The need for dialysis was determined by the nephrology team without input from the surgeon or members of the research team and was based on recipient hyperkalemia, volume overload, and uremia.
Outcome Measurement
The primary outcome for the trial was poor graft function defined by the need for dialysis within seven days of transplant or a failure of the serum creatinine to drop by more than 10% for three consecutive days24. The primary safety measure was the number of severe hypoglycemic events (blood glucose <40 mg/dL).
Secondary outcomes were DGF (need for dialysis within seven days of transplant), peri-operative death, stroke, and seizure, as well as serum creatinine and estimated glomerular filtration rate (GFR), using the Modification of Diet in Renal Disease (MDRD) study calculation, at 30 days, six months and one year. Graft-specific outcomes were biopsy-proven rejection and graft loss.
Statistical Analysis
Both an intention to treat and a per-protocol analysis based on achieving a blood glucose level less than 160mg/dL were planned. Continuous variables were compared using Student’s t test and Welch’s t test for variables with unequal variance and sample sizes. Dichotomous variables were compared with the chi squared test. Kaplan Meier analysis and the log rank test were used to analyze death, graft loss and rejection events. All calculations were performed with the Stata 11 statistical software package (StataCorp, College Station, TX). Two-sided P values of ≤ 0.05 were considered statistically significant.
Based on our retrospective study of blood glucose and its effect on poor initial graft function, we estimated an absolute difference of 33% between recipients with glucose levels less than 160 mg/dL (26% poor function) and those with levels greater than 160 mg/dL (59% poor function) at the time of reperfusion. With a power of 80% and P value of 0.05, we estimated a needed sample size of 40 recipients per study group.
Given the potential risks of hypoglycemia, we planned for three interim analyses; only starting to evaluate outcomes after the second analysis or 50% recruitment. Stopping the trial would be based on the data monitoring and safety board’s (DMSB) review of the number of adverse events in either group or a statistically significant difference in our primary outcome based on the O’Brien-Fleming approach to multiple testing in clinical trials using an α of 0.05 and four total analyses.
RESULTS
Patients
A total of 60 participants were recruited between August 15, 2012 and March 20, 2014, all of whom underwent transplant and were successfully followed at our center through June 18, 2014 (Figure 1). There were no statistically significant differences in baseline characteristics between treatment groups (Table 1). All study participants remained on their assigned treatment protocol except for one in the moderately intense treatment group whose insulin infusion was stopped at six hours due to a misinterpretation of orders by nursing staff.
Table 1.
Baseline Characteristics of Randomized Patients
| Standard | Moderately Intense | P-value | |
|---|---|---|---|
| Recipient Characteristics | |||
| Recipient Age (years: mean ± SD) | 60.7 ±11 | 60.9 (9.1) | 0.95 |
| Recipient Male Gender: n (%) | 20(67%) | 20(67%) | 1.0 |
| Recipient BMI (kg/m2: mean ± SD) | 29.23 ± 6.24 | 27.33 ± 4.19 | 0.18 |
| Recipient Race | 0.17 | ||
| Caucasian: n (%) | 5(16.7%) | 2(6.9%) | |
| Hispanic: n (%) | 13(43.3%) | 11(37.9%) | |
| Black: n (%) | 6(20%) | 3(10.3%) | |
| Asian/Islander: (%) | 6(20%) | 13(44.8%) | |
| Duration Dialysis (years: mean ± SD) | 5.7 ± 2.7 | 5.9 (2.6) | 0.73 |
| Duration Diabetes (years: mean ± SD) | 21.1 ± 9.9 | 18.6 ± 11 | 0.36 |
| Hemoglobin A1C: mean ± SD | 6.7 ± 1 | 6.5 ± 1.2 | 0.36 |
| Preoperative Diabetic Regimen | 0.92 | ||
| Insulin: n (%) | 17(56.7%) | 17(56.7%) | |
| Oral: n (%) | 7(23.3%) | 6(20%) | |
| None: n (%) | 6(20%) | 7(23.3%) | |
|
| |||
| Transplant Factors | |||
| PRA %: mean ± SD | 14.9 ± 28.1 | 18.6 ± 24.2 | 0.59 |
| HLA mismatch: mean ± SD | 3.4 ± 2.2 | 4.4 ± 1.8 | 0.07 |
| Thymoglobulin induction: n (%) | 14(46.7%) | 11(36.7%) | 0.43 |
| Tacrolimus Start Day (mean ± SD) | 1.72 ± 0.81 | 1.67 ± 0.76 | 0.79 |
| Tacrolimus level (ng/ : mean ± SD) | |||
| Day 3 | 4.67 ± 3.45 | 6.18 ± 3.87 | 0.12 |
| Day 7 | 9.16 ± 4.28 | 9.41 ± 4.71 | 0.83 |
| Day 14 | 10.43 ± 4.64 | 10.77 ± 4.81 | 0.78 |
| Day 30 | 8.12 ± 2.78 | 9.28 ± 2.31 | 0.08 |
| Cold Ischemia time (hours: mean ± SD) | 10.8 (4.6) | 10.5 (4.2) | 0.77 |
| Warm ischemia time (min: mean ± SD) | 31.2 (8.9) | 28.8 (8.7) | 0.28 |
|
| |||
| Donor Factors | |||
| Donor Age (years: mean ± SD) | 40.6 ± 15.2 | 44.2 ± 13.1 | 0.33 |
| Donor Male Gender: n (%) | 22(73%) | 18(60%) | 0.28 |
| Donor Race | |||
| Caucasian: n (%) | 7(23.3%) | 14(46.7%) | 0.30 |
| Hispanic: n (%) | 14(46.7%) | 10(33.3%) | |
| Black: n (%) | 5(16.7%) | 3(10%) | |
| Asian/Islander: n (%) | 4(13.3%) | 3(10%) | |
| Donor Hypertension: n (%) | 5(16.7%) | 11(36.7%) | 0.08 |
| Donor Diabetes Mellitus: n (%) | 1(3.3%) | 4(13.3%) | 0.16 |
| Donor BMI (kg/m2: mean ± SD) | 26.84 ± 7.77 | 27.42 ± 9.07 | 0.79 |
| Extended Criteria Donor: n (%) | 3(10%) | 4(13.3%) | 0.69 |
| Donation after Cardiac Death: n (%) | 3(10%) | 5(16.7%) | 0.45 |
| KDPI, mean (SD) | 47.6 (26.1) | 51.1 (21.6) | 0.56 |
| Terminal Creatinine (mg/dL: mean ± SD) | 1.2 (0.47) | 1.1 (0.88) | 0.53 |
Abbreviations: SD, standard deviation; BMI, body mass index; PRA, Panel Reactive Antibody: KDPI, Kidney Donor Profile Index
Interim analysis at 25% and 50% recruitment showed no difference in adverse events. At 50% recruitment, there was an absolute difference in poor graft function of 20% between treatment groups but the test statistic failed to exceed the pre-set cutoff so the DMSB recommended continuation of the trial. The trial was then stopped after 60 patients or 75% of the planned total recruitment as the criterion for stopping the trial based on the primary outcome was met. Specifically, the chi-square test for our primary outcome multiplied by 0.75 (the percentage of patients recruited) exceeded the pre-determined value calculated by the O’Brien-Fleming multiple testing procedure using the pre-determined plan of four total analyses and an α of 0.05.
Glucose Results
Recipient blood glucose (BG) values are displayed in Figure 2. In the intention to treat analysis, pre-operative BG levels did not differ significantly between treatment groups, but by the time of allograft reperfusion, levels were significantly lower in the moderately intense glucose control arm (Table 2). A larger percentage of recipients in the moderately intense arm also achieved a BG of <160 mg/dL at the time of reperfusion (80% v 46.4%, p <0.01). Similarly, recipients in standard treatment group were more likely to have a BG of greater than 200 mg/dL (3.3% v 28.6%, p 0.01). However, many recipients were normoglycemic or even hypoglycemic pre-operatively without intervention. As previously mentioned ~46% of recipients in the standard treatment group had a BG < 160 mg/dL at reperfusion. Furthermore, only 53% of patients in the moderately intense arm had glucose levels high enough to start insulin infusion pre-operatively. After transplant, average glucose levels were significantly lower during each time period for the moderately intense group, but the impact of the insulin infusion was most prominent at 12–24h hours – a difference of 82 mg/dL between moderately intense and standard treatment groups (Table 2).
Figure 2.
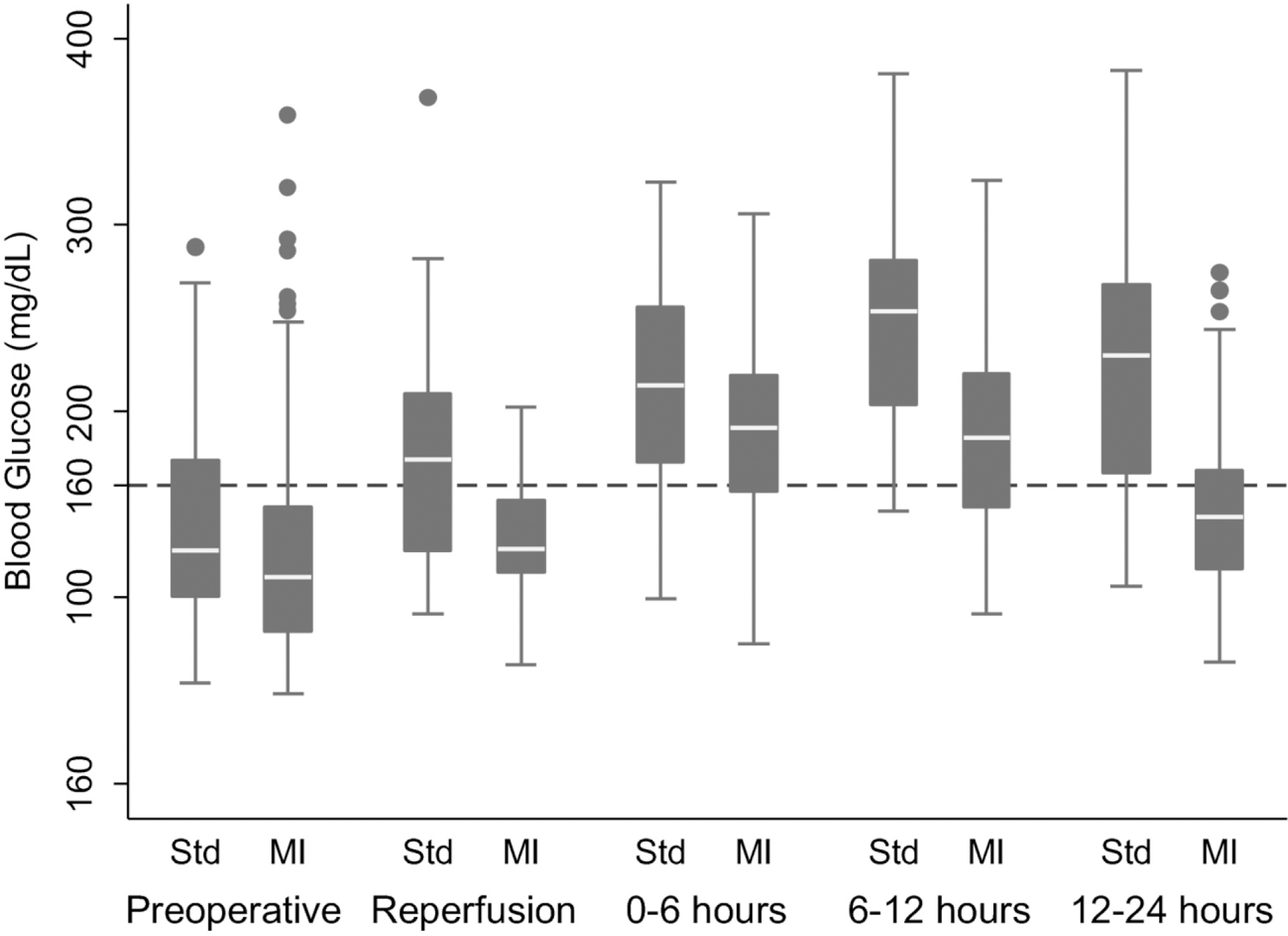
Peri-operative Blood Glucose Levels in Kidney Transplant Recipients
Abbreviations: MI, moderately intense
Box-plot of recipient peri-operative blood glucose levels at multiple time points and divided by treatment group. Each box represents values between the 25th and 75th percentile. Whiskers span the 5th and 95th percentiles of all data and outliers are represented by dots. Abbreviations: Std, standard glucose control; MI, moderately intense glucose control.
Table 2.
Peri-operative Blood Glucose Measurements in Kidney Transplant Recipients
| Intention to Treat | Per Protocol | |||||
|---|---|---|---|---|---|---|
| Moderately Intense | Standard | P value | <160 mg/dL | >160 mg/dL | P value | |
|
| ||||||
| Pre-operative: (mg/dL: mean ± SD) | 125.3 ± 59.1 | 138.6 ± 52.3 | 0.12 | 122.9 ± 47.5 | 146.4 ± 71 | 0.01 |
|
| ||||||
| Reperfusion: (mg/dL: mean ± SD) | 137.4 ± 33.8 | 182.2 ± 67.7 | <0.01 | 125.1 ± 23.3 | 212 ± 45.2 | <0.01 |
|
| ||||||
| 0–6 hours: (mg/dL: mean ± SD) | 182.3 ± 49.6 | 205.7 ± 60.5 | <0.01 | 175.1 ± 51.7 | 219.3 ± 46.9 | <0.01 |
|
| ||||||
| 6–12 hours: (mg/dL: mean ± SD) | 183.6 ± 45.6 | 247.5 ± 51.1 | <0.01 | 193.3 ± 51.3 | 218.9 ± 59.7 | <0.01 |
|
| ||||||
| 12–24 hours: (mg/dL: mean ± SD) | 143.2 ± 40.1 | 225.5± 65 | <0.01 | 154 ± 51 | 183.7 ± 58 | <0.01 |
In the per protocol analysis, BG at reperfusion differed significantly between recipients who achieved the primary goal of BG less than 160 mg/dL and those who did not. This difference was more pronounced than that seen in the intention to treat analysis (average difference of 87 mg/dL vs. 44.8 mg/dL). Recipients who achieved a glucose level less than 160 mg/dL at reperfusion also had lower average BG levels at each time point compared to those recipients whose levels were above 160 mg/dL (Table 2).
In total, we accumulated 1964 blood glucose measurements, none of which were less than 40 mg/dL in either group. There were no reported symptoms of hypoglycemia in any study participant.
Recipient and Graft Outcomes
In the intention to treat analysis, there was a 30% absolute difference between the moderately intense and standard glucose control groups for poor graft function (43.3% vs. 73.3%, P = 0.02). We also looked at the indication for dialysis and found no statistically significant difference between treatment groups. Hyperkalemia was the most common indication in both the moderately intense and standard glucose control groups (50% vs 63%) followed by volume overload (33% vs. 21%) and uremia (8% vs. 16%) (p-value 0.47). On average, length of hospitalization and duration of dialysis were one day shorter in the moderately intense group, but this difference did not reach statistical significance. There were no strokes or seizures in either group.
Recipients were followed for an average of 1.16 years; 51(83.3%) patients were followed for at least 6 months and 42 (70%) were followed for at least one year. Renal function at 30 days was better in the moderately intense glucose control group as measured both by serum creatinine and GFR). However, at six months and one year, serum creatinine and GFR did not differ significantly between groups (Table 3).
Table 3.
Recipient and Graft Outcomes
| Intention to Treat Analysisa | |||
|---|---|---|---|
|
| |||
| Standard | Moderately Intense | P value | |
|
| |||
| Delayed Graft Function: n (%) | 22(73.3%) | 13(43.3%) | 0.02 |
| Dialysis within 7 Days: n (%) | 19(63.3%) | 13(43.3%) | 0.12 |
|
| |||
| Dialysis within 7 days (minimum 2 sessions): n(%) | 13(43.3%) | 10(30%) | 0.28 |
|
| |||
| Dialysis Duration: (days: mean ± SD) | 9.8 ± 8.8 | 8.8 ± 8.8 | 0.75 |
|
| |||
| Length of Stay: (days: mean ± SD) | 5 ± 2.4 | 4.1 ± 1.9 | 0.13 |
|
| |||
| Renal Function – 30 days | n=29 | n=29 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.78 ± 0.7 | 1.4 ± 0.6 | 0.04 |
| GFR: (mL/min/1.73 m2: mean ± SD) | 46.9 ± 19.3 | 57.6 ± 20.6 | 0.05 |
|
| |||
| Renal Function – 6 months | n=24 | n=23 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.13 |
| GFR: (mL/min/1.73 m2: mean ± SD) | 60.1 ± 22 | 65.1 ± 18.4 | 0.4 |
|
| |||
| Renal Function – 1 year | n=18 | n=18 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.32 ± 0.5 | 1.2 ± 0.3 | 0.43 |
| GFR: (mL/min/1.73 m2: mean ± SD) | 60.5 ± 21.9 | 65.1 ± 20.5 | 0.53 |
|
| |||
| Per Protocol Analysisa | |||
|
| |||
| >160 mg/dL (n=21) |
< 160 mg/dL (n=37) |
P value | |
|
| |||
| Delayed Graft Function: n (%) | 17(81%) | 16(43.2%) | <0.01 |
|
| |||
| Dialysis within 7 Days: n (%) | 15(71.4%) | 15(41%) | 0.02 |
|
| |||
| Dialysis within 7 days (minimum 2 sessions): n (%) | 11(52.4%) | 9(24.3%) | 0.03 |
|
| |||
| Dialysis Duration: (days: mean ± SD) | 7.6 ± 8.9 | 3.1 ± 6.4 | 0.03 |
|
| |||
| Length of Stay: (days: mean ± SD) | 5.2 ± 2.8 | 4.1 ± 1.8 | 0.09 |
|
| |||
| Renal Function – 30 days | n=20 | n=36 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.9 ± 0.9 | 1.4 ± 0.5 | <0.01 |
| GFR: (mL/min/1.73 m2: mean ± SD) | 42.5 ± 19.5 | 57.9 ± 19.5 | 0.006 |
|
| |||
| Renal Function – 6 months | n=19 | n=27 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.15 |
| GFR: (mL/min/1.73 m2: mean ± SD) | 57.9 ± 17.5 | 66.5 ± 21.7 | 0.16 |
|
| |||
| Renal Function – 1 year | n=16 | n=20 | |
|
| |||
| Creatinine: (mg/dL: mean ± SD) | 1.3 ± 0.5 | 1.2 ± 0.3 | 0.52 |
|
| |||
| GFR: (mL/min/1.73 m2: mean ± SD) | 59.6 ± 20 | 65.3 ± 22 | 0.42 |
Abbreviations: DGF, delayed graft function; GFR, glomerular infiltration rate.
n=30 unless otherwise noted in the table
There were no deaths or graft loss in the moderately intense glucose control group, but there were two deaths with associated graft loss in the standard group (P = 0.91). Biopsy-proven rejection did not differ between the moderately intense (3 episodes) and standard (4 episodes) groups (P = 0.68). There was no difference in surgical complications. There were no urine leaks or strictures in either group and there was one wound infection in both the standard and moderately intense treatment group (P = 0.99).
Since many recipients in the control arm maintained a blood glucose level below 160 mg/dL and only 47% of recipients in the moderately intense arm were eligible for a pre-operative insulin infusion according to the treatment algorithm, we performed a per-protocol analysis based on recipients who achieved the primary treatment goal of a blood glucose less than 160 mg/dL at reperfusion. Consistent with the intention to treat analysis, recipients with blood glucose less than 160 mg/dL were far less likely to suffer poor graft function. Furthermore, these recipients had a shorter duration of of dialysis and a lower serum creatinine and higher GFR at 30 days. Again, recipients with better blood glucose control at the time of reperfusion were discharged home a day earlier on average but this difference failed to reach statistical significance. There was no statistically significant difference in renal function between groups at six months or one year (Table 3).
Given this high incidence of poor graft function in the study, we performed one post-hoc analysis using a more stringent definition of DGF (the need for dialysis within 7 days of transplant with at least two sessions of dialysis needed) to minimize any potential bias 25. In the intention to treat analysis, recipients of moderately intense glucose control had a lower but not statistically different incidence of this more rigorously defined DGF (30% vs. 43.3%, p-value 0.28). In the per protocol analysis, recipients of moderately intense glucose control did have a statistically significant decrease in this more rigorously defined DGF (24.3% vs 52.4%, p-value 0.03) (Table 3). We also evaluated the indication for dialysis in each group.
Discussion
This study is the first randomized trial to compare moderately intense glucose control with standard control at the time of renal transplantation. The results demonstrate improved short term graft function in recipients who had moderately well controlled glucose at the time of renal transplant compared to recipients with less tight control. Furthermore, recipients in the moderately intense group had a lower creatinine and higher GFR at 30 days after transplant. Recipients in the moderately intense glucose control group were also discharged home one day earlier on average, though the difference did not reach statistical significance. These findings are supported by both the intention to treat and per-protocol analysis.
Particularly important is that short term graft function improved with only moderate control, using existing hospital treatment algorithms carried out by the primary team and without needing to consult diabetes experts. Compared to intense glucose control studies, which typically targets a blood glucose of 80–120 mg/dL, this moderate level of control resulted in significant benefit without increased risk of severe hypoglycemia or adverse events such as seizure, stroke or death. Furthermore, we saw no difference in the rate of biopsy-proven rejection between treatment groups. This contrasts with findings from the transplant group at the Medical University of South Carolina (MUSC), who reported an increased rate of rejection in recipients getting intense glucose control when they compared intense glucose control (70–110 mg/dL) to moderate control (70–180 mg/dL) over three days after transplant 26. We believe this discrepancy is due to the inclusion of living donor transplants which result in a lower incidence of DGF and that the difference in blood glucose control between treatment groups was not enough to have a significant impact on graft function. As discussed in the methods section, our prior work leads us to believe there is a threshold effect around a blood glucose of 160 mg/dL so the moderate control (70–180 mg/dL) in the MUSC study was likely adequate to minimize injury.
Overall, glucose control in this population was fairly easy. Most patients in either arm had normal blood glucose levels pre-operatively, likely because they were called in to the hospital on short notice and told to take nothing by mouth, preventing them from properly adjusting their usual diabetic medications. Furthermore, only 53% of patients in the moderately intense control group required an insulin infusion pre-operatively. However, the insulin infusion was critical to achieving the target glucose in recipients who were admitted with hyperglycemia. Once reasonable glucose levels were obtained, it was fairly easy to maintain them based on the preferences of the anesthesia team intra-operatively, and then with insulin infusion post-operatively.
Several limitations could affect how the results of our study are interpreted. Although randomized, the treatment was not blinded to recipients or the treating team. The use of insulin infusion on standard medical-surgical wards is well established at our center as a standard protocol but may not be readily available at other centers without expert consultation or the need to keep patients in a higher level of care. This study is also limited by the population treated at our center, which was largely Hispanic (40%).
The incidence of poor graft function in this study is also high; raising the question of selection and/or institutional bias. Some institutional practices may contribute to this finding. In particular, no allografts underwent cold perfusion pumping prior to transplant whereas many centers commonly use perfusion pumping for extended criteria donors and in situations where extended preservations times are anticipated. Furthermore, we are an aggressive center that routinely uses non-standard donors. In fact, 25% of the allografts came from either extended criteria (7 donors) or donation after cardiac death (8 donors), which are well known to have a much higher rate of DGF than standard donors. Our study also did not include living donors as recipients of living donor kidneys have a very low rate of poor graft function. We doubt hyperglycemia alone is enough of an insult to overcome the relatively high quality kidneys and short ischemic times that come with living donation.
In order to minimize the potential impact of selection and/or institutional bias, we performed an additional analysis using DGF defined as the need for at least two dialysis sessions. Even with this more rigorous definition, recipients achieving a blood glucose less than 160 mg/dL at reperfusion had a lower rate of DGF in the per protocol analysis. While selection and/or institutional bias may have had an impact on our primary outcome, the benefit of glucose control was also seen in the duration of dialysis and GFR at 30 days – both of which are markers of short term renal function that are based on outpatient measures. Specifically, the duration of dialysis was longer in the recipients receiving standard insulin therapy and the need for continued dialysis was determined in the outpatient setting without knowledge of participation in this trial. Furthermore, the GFR at 30 days was significantly lower in both the per-protocol and intention to treat analysis. This finding is consistent with greater short term injury in recipients who had higher blood glucose levels at reperfusion and is an objective measure that is free from potential bias.
The duration of moderately intense glucose control after transplant required to improve short term function also remains in question. In both the per-protocol and intention to treat analysis, the blood glucose at time of reperfusion had a clear effect on short term graft function, but the per-protocol analysis did not include the secondary treatment goal of maintaining moderate glucose control for 24 hours after transplantation. However, when the recipients whose blood glucose was less than 160mg/dL at the time of reperfusion were examined at later time points, their average blood glucose remained well controlled. We chose 24 hours to maximize potential benefit, but given the importance of blood glucose at reperfusion in both analyses, the period during which relative moderate control should be maintained remains a question.
Overall, this study is consistent with our prior work which demonstrates greater ischemic injury in the setting of hyperglycemia 18,20,21. The underlying mechanism is likely multivariable as multiple animal studies have shown that hyperglycemia is associated with greater oxidative stress, increased apoptosis and variable expression of cytokines which are known to regulate ischemia reperfusion injury. Despite this negative impact, moderately intense glucose control close to the time of transplant reduces injury and leads to improved short term graft function with no apparent increase in adverse events. Therefore, we recommend efforts to achieve a glucose level of 80–160 mg/dL at the time of reperfusion. Given the lack of adverse events, we also believe it is clinically reasonable to maintain moderate control after transplant via either infusion or subcutaneous insulin, although the needed duration remains unclear. Another question has to do with the impact of hyperglycemia on non-diabetic patients. We have previously documented that more than one-third of non-diabetic patients have blood glucose levels above 160 mg/dL in the immediate post-transplant setting while more than 90% develop blood glucose exceeding 200 mg/dL in the extended post-operative setting 21,22,27. Ultimately, a larger study is warranted to establish if these results can be replicated and if all transplant recipients may benefit from more rigorous peri-operative glucose control
Footnotes
Clinical Trial Registration - clinicaltrials.gov #NCT01643382
References
- 1.Van den Berghe G, Schetz M, Vlasselaers D, et al. Clinical review: Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? The Journal of clinical endocrinology and metabolism. 2009;94(9):3163–3170. [DOI] [PubMed] [Google Scholar]
- 2.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. The New England journal of medicine. 2001;345(19):1359–1367. [DOI] [PubMed] [Google Scholar]
- 3.Investigators N-SS, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. The New England journal of medicine. 2009;360(13):1283–1297. [DOI] [PubMed] [Google Scholar]
- 4.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2004;10Suppl 2:21–33. [DOI] [PubMed] [Google Scholar]
- 5.Nagamizo D, Tsuruta S, Matsumoto M, Matayoshi H, Yamashita A, Sakabe T. Tight glycemic control by insulin, started in the preischemic, but not postischemic, period, protects against ischemic spinal cord injury in rabbits. Anesthesia and analgesia. 2007;105(5):1397–1403, table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Marfella R, D’Amico M, Di Filippo C, et al. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45(8):1172–1181. [DOI] [PubMed] [Google Scholar]
- 7.Di Filippo C, Marfella R, Cuzzocrea S, et al. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54(3):803–810. [DOI] [PubMed] [Google Scholar]
- 8.Hirose R, Xu F, Dang K, et al. Transient hyperglycemia affects the extent of ischemia-reperfusion-induced renal injury in rats. Anesthesiology. 2008;108(3):402–414. [DOI] [PubMed] [Google Scholar]
- 9.Lecomte P, Van Vlem B, Coddens J, et al. Tight perioperative glucose control is associated with a reduction in renal impairment and renal failure in non-diabetic cardiac surgical patients. Critical care. 2008;12(6):R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. 2013;96(10):885–889. [DOI] [PubMed] [Google Scholar]
- 11.Scientific Registry of Transplant Recipients. 2010Annual Data Report.
- 12.Yarlagadda SG, Coca SG, Formica RN Jr., Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(3):1039–1047. [DOI] [PubMed] [Google Scholar]
- 13.Sung RS, Guidinger MK, Leichtman AB, et al. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys. Transplantation. 2007;84(9):1138–1144. [DOI] [PubMed] [Google Scholar]
- 14.Ghods AJ, Savaj S, Abbasi M, Heidari H, Rokhsatyazdi H. The incidence and risk factors of delayed graft function in 689 consecutive living unrelated donor renal transplantation. Transplantation proceedings. 2007;39(4):846–847. [DOI] [PubMed] [Google Scholar]
- 15.Koning OH, Ploeg RJ, van Bockel JH, et al. Risk factors for delayed graft function in cadaveric kidney transplantation: a prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. European Multicenter Study Group. Transplantation. 1997;63(11):1620–1628. [DOI] [PubMed] [Google Scholar]
- 16.Kyllonen LE, Salmela KT, Eklund BH, et al. Long-term results of 1047 cadaveric kidney transplantations with special emphasis on initial graft function and rejection. Transplant international : official journal of the European Society for Organ Transplantation. 2000;13(2):122–128. [DOI] [PubMed] [Google Scholar]
- 17.Roels L, Waer M, Coosemans W, Christiaens MR, Vanrenterghem Y. The influence of donor age on initial and long-term renal allograft outcome. Leuven Collaborative Group for Transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 1994;7Suppl 1:S303–305. [DOI] [PubMed] [Google Scholar]
- 18.Parekh J, Bostrom A, Feng S. Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(2):298–303. [DOI] [PubMed] [Google Scholar]
- 19.Brennan TV, Freise CE, Fuller TF, Bostrom A, Tomlanovich SJ, Feng S. Early graft function after living donor kidney transplantation predicts rejection but not outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(6):971–979. [DOI] [PubMed] [Google Scholar]
- 20.Parekh J, Niemann CU, Dang K, Hirose R. Intraoperative hyperglycemia augments ischemia reperfusion injury in renal transplantation: a prospective study. Journal of transplantation. 2011;2011:652458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh J, Roll GR, Feng S, Niemann CU, Hirose R. Peri-operative hyperglycemia is associated with delayed graft function in deceased donor renal transplantation. Clinical transplantation. 2013;27(4):E424–430. [DOI] [PubMed] [Google Scholar]
- 22.Chakkera HA, Weil EJ, Castro J, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009;4(4):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scientific Registry of Transplant Recipients. 2012Annual Data Report
- 24.Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnuelle P, Gottmann U, Hoeger S, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. Jama. 2009;302(10):1067–1075. [DOI] [PubMed] [Google Scholar]
- 26.Hermayer KL, Egidi MF, Finch NJ, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. The Journal of clinical endocrinology and metabolism. 2012;97(12):4399–4406. [DOI] [PubMed] [Google Scholar]
- 27.Hecking M, Haidinger M, Doller D, et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. Journal of the American Society of Nephrology : JASN. 2012;23(4):739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]


