Abstract
Conventional clinical management of complex bone healing scenarios continues to result in 5–10% of fractures forming non-unions. Additionally, the aging population and prevalence of osteoporosis-related fractures necessitate the further exploration of novel ways to augment osteogenesis in this special population. This review focuses on the current clinical modalities available, and the ongoing clinical and pre-clinical research to promote osteogenesis in segmental bone defects, delayed unions, and osteoporosis. In summary, animal models of fracture repair are often small animals as historically significant large animal models, like the dog, continue to gain favor as companion animals. Small rodents have well-documented limitations in comparing to fracture repair in humans, and few similarities exist. Study design, number of studies, and availability of funding continue to limit large animal studies. Osteoinduction with rhBMP-2 results in robust bone formation, although long-term quality is scrutinized due to poor bone mineral quality. PTH 1–34 is the only FDA approved osteo-anabolic treatment to prevent osteoporotic fractures. Limited to 2 years of clinical use, PTH 1–34 has further been plagued by dose-related ambiguities and inconsistent results when applied to pathologic fractures in systematic human clinical studies. There is limited animal data of PTH 1–34 applied locally to bone defects. Gene therapy continues to gain popularity among researchers to augment bone healing. Non-integrating viral vectors and targeted apoptosis of genetically modified therapeutic cells is an ongoing area of research. Finally, progenitor cell therapies and the content variation of patient-side treatments (e.g., PRP and BMAC) are being studied.
Keywords: fracture repair, non-union, gene therapy
Bone has a remarkable capacity for self-renewal and remodeling,1 and has evolved to serve many mechanical, endocrine, and homeostatic functions.2 Although normal bone remodels in response to adverse conditions such as changing biomechanical forces, micro-damage, and fracture, about 5–10% of fractures do not heal conventionally even with clinical interventions resulting in non-union.3 Thus, there is an unmet clinical need for novel approaches to promote rapid repair of complicated long bone fractures and large bone defects. The degree of soft tissue injury and type of fixation utilized, host factors such as age, diabetes, NSAID use, and osteoporosis limit osteogenesis in vivo; often these limiting factors result in clinical sequelae such as increased infection rate, risk of nonunion, and inability to maintain quality of life.4,5
Increasing osteogenesis has been explored through targeted overexpression of growth factor and exogenous hormone delivery—therapeutics mainly aimed at osteoinduction, a substance that results in the commitment of progenitor cells down an osteoblastic lineage. One way osteogenic induction is achieved in vivo is through delivery of growth factors that result in accelerated osteoblast generation from native progenitor cells, and therefore, accelerated bone formation. Bone formation and bone healing can be achieved through various pathways; therefore, a cursory signaling summary of the growth factors to be discussed, BMP-2 and PTH, is provided.
Bone morphogenic proteins, part of the transforming growth factor-β superfamily, induce bone formation through binding complexes of serine threonine kinase receptors to initiate cell signaling.6 The most studied osteogenic BMPs, 2, 4, and 7 bind the same complex of receptors.6 Subsequent SMAD 1/5/8 phosphorylation allows nuclear translocation and binding to specific DNA elements to activate transcription of osteoblast-specific genes.7 Osteogenesis may also occur through activation of TAK-1 and TAB1, which are crucial upstream regulators of MKK its activation of osteogenic gene transcription via p38/MAPK.8 Both canonical (R-smad) and non-canonical (MKK) osteogenic BMP signaling results in the transcription of RunX2, Dlx5, and Osx.9 Bone anabolism via PTH occurs through canonical WNT signaling. WNT-PTH crosstalk results in β-catenin stabilization, nuclear translocation, and subsequent transcription of genes to improve bone formation while decreasing bone resorption. Non-canonical WNT bone anabolism is often achieved with planar cell polarity crosstalk and is implicated in PTH 1–34 response to strain and during skeletal morphogenesis.10 Further discussion of the signaling pathways involved in osteogenesis for bone healing can be reviewed with these references.3,8,11
This review describes approaches used to promote osteogenesis in pathologic and osteoporotic fractures and segmental bone defects using BMP-2 and PTH. Use of appropriate pre-clinical animal models, recombinant protein therapy, gene therapy, and the use of progenitor cells are discussed. Scaffolding materials for bone have recently been comprehensively reviewed and will not be discussed in this manuscript.12,13
ANIMAL MODELS
Research in animal models is a critical component for translation to human clinical trials. No perfect model exists that exactly replicates fracture healing in humans; however, animal models may be utilized to answer specific clinical questions. Tables 1 and 2 provides a descriptive summary of common animal model advantages and disadvantages, and Figure 1 provides pictorial representations of common preclinical models and the method most often utilized to study osteogenesis in segmental bone defects.
Table 1.
Small Animal Model Advantages, Disadvantages, and Translational Relevance
| Animal Model | Advantages | Disadvantages | Cost ($-$$$$) | Most Common Bone Used | Potential Implications of Differences (When Humans are Considered) | References for Further Reading |
|---|---|---|---|---|---|---|
| Mouse | Low cost, genetic engineering of knockout models allows for highly specific pathway and disease study; homogeneity confers statistical power; biomarker availability; genetic engineering of knockout or epitope-tagged strains are helpful in quantifying evidence of changes in promoter specificity, response to transcription activators, and inhibitors, and in heterozygous systems, cis, or trans regulatory elements may also be explored | Lack Haversian systems; skeleton is modeling driven due to permanently open growth plates at the epiphysis of long bones; low on phylogenetic scale | $ | Femur | Persistent epiphysis is a good model for juvenile pathology; light weight, difficult to monitor pain response to implants or fixators | 22,28,29 |
| Rat | Genetically homogenous strains are available, use of xenogenic cells and xenogenic gene sequences in gene therapy studies; homogeneity confers statistical power; biomarker availability. The femur offers a larger proportion of soft tissue coverage than the tibia, and can replicate the soft tissue trauma seen as a risk factor for non-union development in humans | Lack Haversian systems; skeleton is modeling driven due to permanently open growth plates at the epiphysis of long bones; low on phylogenetic scale. Inconsistent use of internal fixation or use of only k-wire/intramedullary pins fails to provide axial or rotational stability | $ | Femur | Persistent epiphysis is a good model for juvenile pathology; light weight; immune response altered when using non-immunocompetent breeds | 21,22,28,29 |
Table 2.
Medium and Large Animal Model Advantages, Disadvantages, and Translational Relevance
| Animal Model | Advantages | Disadvantages | Cost ($-$$$$) | Most Common Bone Used | Potential Implications of Differences (When Humans are Considered) | References for Further Reading |
|---|---|---|---|---|---|---|
| Rabbit | Bone density is similar to humans; bone contains Haversian systems | Size and shape differs greatly from humans; very fast remodeling compared to humans; differences in composition of the microstructure; vascular longitudinal tissue structure. External fixators are used inconsistently, the tibia is more commonly used than the femur, and bones that carry less weight are frequently used in growth factor studies (such as the ulna) | $$ | Femur, Tibia | Faster remodeling may confound the expected speed of healing in the human | 16,20,28,29 |
| Canine | Most similar bone density, extractable protein content (such as IGF-1), and ash weight when compared to humans | Regarded as companion animal; trabecular bone may withstand greater compressive forces than human bone due to the increased plexiform bone structure adjacent to the periosteum | $$$ | Femur | Mixed microstructure, specifically in the vicinity of the periosteum confers greater mechanical strength | 16,21,28,29 |
| Sheep | Most similar body weight when compared to humans; size of bone and weight of animal replicates conditions for human implants and prostheses | High content of plexiform bone conferring greater ability to withstand compression in early life; Haversian remodeling is favored with age; prior to haversian remodeling, sheep bone is comprised mostly of a combination of woven and lamellar bone (primary structure). Ruminant digestive tract affects nutrient cycling and delivery compared to monogastrics; seasonally polyestrous cycle alters bone metabolism | $$$ | Tibia | Mixed microstructure, specifically in the vicinity of the periosteum confers greater mechanical strength; Haversian remodeling increases with age in sheep—average age and sex of sheep must be considered when extrapolating to differing populations of human patients. Human bone structure is mostly secondary osteons formed by the replacement of existing bone. It has been suggested the mechanical differences exist since primary bone structure is formed through cartilage mineralization | 21,22 |
| Horse | Most similar mechanical loading of the musculoskeletal system when compared to humans | Immediately weight bearing; high cost of anesthesia, housing, and routine care. Studies conducted in small cohorts yield lower statistical power, but confer high scientific evidence; seasonally polyestrous cycle alters bone metabolism | $$$$ | Metacarpal IV (MCIV) | Success in the horse likely confers success in the human; if MCIV is used, one must be careful to immediately extrapolate evidence to a persistently weight bearing bone | 30 |
Figure 1.
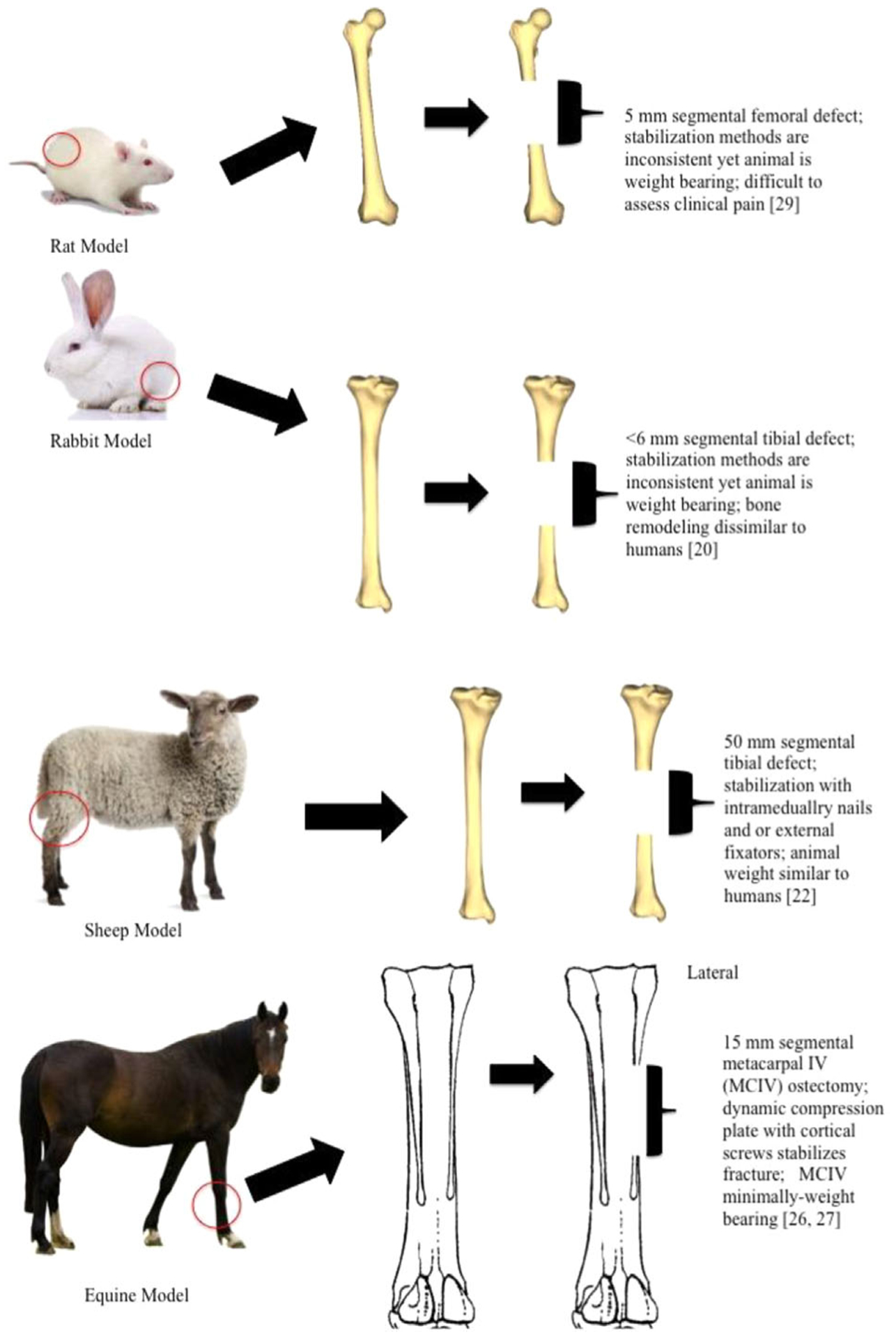
Figure 1 describes common animal models, the corresponding segmental defect size, and the type of fracture stabilization utilized for segmental bone research.
Mouse models in fracture repair are often utilized because the ability to purchase and or design specific genotypes and phenotypes affords researchers the ability to study cells with specific characteristics.14 This is often done through genetically manipulated knock out models and inbred strains.15 Nonetheless, murine bone lacks haversian systems, and it is unknown how this may affect pathophysiologic pathways of bone injury and healing when compared to humans16 (Table 1). Similarly, rat bone is also devoid of haversian systems.16 The rat is a popular model for delayed and non-union fracture repair models, as well as growth factor use in fracture repair. Similar to the mouse, inbred strains of rats may be purchased from commercial vendors, and housing, anesthesia, and pain management are inexpensive compared to large animal models. While it is often assumed inbred rat strains are genetically homogenous, there is some genetic heterogeneity within inbred populations with large variation in the number of single nucleotide repeats (SNPs).17 How SNP variation within genetically inbred populations affects baseline variation is unknown. Other limitations of the rat include size and decreased elasticity compared to human bones.18,19 It is unknown if the lack of haversian systems alters local reaction and nutrient or waste shuttling during pathology (Table 1). The rabbit offers similar advantages as the mouse and rat, such as ease of housing, anesthesia, and pain monitoring20; although they too are dissimilar in size and body weight when compared to humans. Compared with mice and rats, rabbits are a more outbred species. This necessitates larger numbers in a given study to reach statistical power and significance because of individual variation. However, rabbit bone contains haversian systems and more closely replicates large animal models of bone structure. Therefore, success in rabbits may predict success in a larger animal model (Table 2).
When comparing bone composition between human, canine, swine, bovine, ovine, poultry, and rodent bone, canines’ most closely resemble human bone composition when ash weight, extractable proteins, and IGF-1 content are considered.21 Some studies have found that trabecular bone turnover is higher in canines than in humans, and that there is an age-related decrease in the remodeling capabilities.21 At the microstructure level, the secondary osteon structure and presence of plexiform bone adjacent to periosteum, especially during callus formation, allows canine cortical bone to withstand greater compressive forces than human bone.22 Finally, the increased standing of canines as companion animals increases ethical concerns for their continued use in orthopedic research (Table 2).
Sheep are more similar in body weight to humans compared to the models discussed thus far, and the dimensions of their bones allow them to be suitable for surgical implants and biomaterial studies. However, their long bone trabecular density is 1.5–2 times greater than humans, conferring more inherent mechanical strength; and, because they are quadrupeds, weight distribution is dissimilar to humans.22 Despite these limitations, sheep have some advantages. For example, when sheep age, their bone physiology resembles that of humans, with increases in osteoporotic or osteopenic bone loss.22,23 Differences in bone healing as it relates to age in sheep, and stark difference in nutrition status should be taken into careful consideration when researchers are considering the sheep as a large animal model for bone repair (Table 2).
The horse is an FDA recommended model for osteoarthritis and comparative joint research,24 and availability to measure in vivo bone strain as well as the similar haversian remodeling suggest the horse is a good pre-clinical model for fracture repair despite cost-associated drawbacks.25,26 However, horses exhibit rapid periosteal expansion with plexiform bone that is unlike fracture healing in humans27 (Table 2) and the use of minimally weight bearing metacarpal bones should be considered (Figure 1).
RECOMBINANT PROTEINS
Recombinant protein therapy is the use of purified therapeutic protein applied to bone defects to produce union (e.g., rhBMPs) or administered systemically to increase osteo-anabolism (e.g., PTH1–34). Production of recombinant proteins is done through a variety of bacterial (Escherichia coli), eukaryotic (yeast), or mammalian expression systems (Chinese Hamster Ovary cells (CHO), Human Embryonic Kidney cells (HEK), and AD293 cells (a derivative of HEK cells). In 2002 and 2004, respectively, the recombinant proteins to be discussed, PTH (1–34) and rhBMP-2, were approved for use in osteoporosis treatment and open tibial fractures in humans31,32 after extensive preclinical animal studies that demonstrated clinical efficacy (Table 3). The proteins remain of clinical, ethical, and socioeconomic interest.33,34
Table 3.
Preclinical Studies Utilizing RhBMP-2 or PTH (1–34) Are Grouped According to the Animal Model Used and Described
| Pre-Clinical Animal | Recombinant BMP-2 | Recombinant PTH (1–34) | Selected References for Further Reading: |
|---|---|---|---|
| Mouse | In vitro: Culture with 100 ng/ml rhBMP-2-induced osteocalcin gene and protein expression after 4 days of treatment, and increased intracellular [cAMP] in response to 1–34 PTH (400 ng/ml) after 8 days of treatment.73 In vivo: the addition of 5 or 20 μg rhBMP-2 to porous poly-D,L-lactide-co-glycolide implanted intra-muscularly in immunocompromised nude mice resulted in production of marrow-like tissue, and a greater area of new bone when compared to the carrier matrix alone69 | In vivo: luciferase tagged BMDMSCs were generated into ossicles and implanted in immunocompromised mice. 40 μg/kg/day PTH (1–34) was given SQ for 3 weeks. Three out of four PTH (1–34) treated groups showed significant increases in total bone area within implanted ossicles71 | BMP-2:69,73 PTH (1–34):70–72 |
| Rat | In vitro: RhBMP-2 increased cellular response to PTH (1–34) in an osteoblastic cell line (C20) and in a cell line (C26) capable of undergoing myogenic, adipogenic, and osteogenic differentiation. rhBMP-2 also increased the cellular response to PTH (1–34) in both cell lines, a characteristic of osteoblastic cells.76 In vivo: 5 mm segmental femoral defects showed radiographic, histologic, and mechanical evidence of union through endochondral bone formation in a dose dependent manner with 11 μg rhBMP-2 in guanidine hydrochloride extracted demineralized bone matrix75 | In vivo: in a closed fracture model, 200 μg/kg/day for 20 or 40 days increased ultimate load and external callus volume. A 60 μg/kg/day for 40 days increased ultimate load and external callus volume.61 Treatment with 30 μg/kg/day for 21 days resulted in increased torsional strength, stiffness, bone mineral content and density, and callus volume.74 Doses as low as 10 μg/dg/day for 28 days increased bone mineral content and density, ultimate load to failure of fracture callus32 |
BMP-2:31,75,76,77 PTH (1–34):32,61,74 |
| Rabbit | In vivo: empty defects, autologous bone graft, and rhBMP-2 (0, 17, 35, and 70 μg) loaded onto poly (DL-lactic acid) implant were loaded onto 20 mm radial defects in 96 New Zealand White rabbits. After 8 weeks, autogenous bone graft, 35 and 70 μg rhBMP-2 groups made an equivalent amount of bone and had restored normal architecture81 | In vivo: PTH (1–34) increases cortical bone mass and mechanical strength in female rabbits after 140 days without adversely affecting serum Ca/P ratios. 40 μg/kg/day increased ultimate force, stiffness, and work; while 10 μg/kg/day had a lower elastic modulus not different from the control.78 When treated for only 70 days with 10 μg/kg/day, cortical bone porosity was not increased and cortical bone strength was increased79 | BMP-2:81 PTH (1–34):78,79 |
| Canine | In vivo: bilateral 25 mm radial osteotomies were filled with autologous bone graft or a collagen sponge with 0,150, 600, or 2,400 μgrhBMP-2. Dose-dependent osteoinduction was observed, with higher doses resulting in heterotopic bone formation and cyst-like voids. By 12 and 24 weeks, biomechanical parameters were equal to autologous bone graft. Minimum effective dose of rhBMP-2 in collagen was determined to be between 0 and 150 μg80 | In vivo: 5 μg/kg/day increased titanium alloy implant fixation in the proximal tibia. Increased shear stiffness and energy absorption were observed after 4 weeks of daily SQ PTH (1–34) treatment.83 A combination of the bisphosphonate, zoledronic acid, and PTH (1–84) at 2.3 and 0.1 μg/mL, respectively, increased polar moments of inertia in a canine osteosarcoma cell line, xenografted into athymic rats82 | BMP-2:80 PTH (1–34):82,83 |
| Caprine | In vivo: bilateral closed fractures were created in 16 goats, and 1 cm of periosteum was excised proximal and distal to the fracture. Absorbable collagen sponge with 0.86 mg rhBMP-2 or buffer were applied to the anteriomedial aspect of the fracture, or wrapped circumferentially around it without any stability. Increased callus volume, and moderate increases in strength and stiffness were noted by torsional toughness and higher radiographic scores in the wrapped rhBMP-2 treated tibiae87 | BMP-2:87 PTH (1–34): none authors are aware of |
|
| Equine | In vivo: defects made in the II and IV metatarsals were left empty, filled with autologous bone graft, or calcium phosphate cement/matrix with 2 or 0.5 mg rhBMP-2, respectively. The combination of calcium phosphate and rhBMP-2 had greater maximum torque to failure in torsion scores. Histology suggested increased bone volume and more mature bone in rhBMP-2 treated sites rhBMP-2 dose did not appear to be significant.85 | A1 mg of PTH (1–34) was covalently attached to 1.5 ml volume fibrin hydrogel and implanted into a 5.5 mm subchondral bone cyst that communicated with the joint in the proximal interphalangeal joint. Mild lameness was evident 9 weeks post operatively. Eight months post operatively the cyst was filled with radiopaque material, and the animal ambulated normally84 | BMP-2:85,86
PTH (1–34):84 |
BMP-2
In 1965, Marshall Urist first discovered that proteins within bone could induce osteoid formation when he placed demineralized bone matrix in muscle tissue.35 The proteins that were able to induce osseous metaplasia were given the family name “Bone Morphogenic Proteins.” Since the advent of gene sequencing, BMPs have been further characterized by nucleotide similarity and are thus grouped accordingly (e.g., BMP-2/4 and BMP-5/6/7/8).36 The most potent osteoinductive agent available to clinicians’ today is BMP-2. Table 3 summarizes seminal studies that supported FDA approval of rhBMP-2 for open tibial fractures. RhBMP-2 is most commonly combined with bovine collagen37 and provides an exogenous supraphysiologic dose of osteoinductive growth factor to overcome the challenging clinical environment it is often placed in (e.g., open tibial fractures).
Despite the use of supraphysiologic doses, results are variable.38 Contributing factors include the short half-life, potential for improper folding or post-translational modification that can reduce biologic activity,39 and the presence of natural BMP-2 antagonists, such as Noggin.40 Likewise, there are strong species-associated dose requirements for osteogenesis—the recommended dose to induce osteogenesis in humans (1.5 mg/ml) is at least 3.75 times greater than the required dose in rodents (0.02–0.4 mg/ml).41 Furthermore, rhBMP-2 therapy is complicated as it is often cost-prohibitive, is not covered by insurance,42 is associated with a high degree of inflammation and ectopic bone formation,43 and wide-spread off-label use is documented and often results in unwanted side effects.44,45
RhBMP-2 use often results in bony union, but continuous corticies and non-remodeled trabeculae are often thin46; as the ultimate goal of fracture healing is to have mechanically functional bone, rhBMP-2 generated bone quality is in question. Osteolysis and subsistence are reported in spinal fusion47–49—although, spinal use of rhBMP-2 is beyond the scope of this review, it is prudent to note similar findings found in long-bone fracture repair—principally cystic bone formation. There are accounts of greater trabecular bone spacing50 and evidence of BMP-2 signaling induced osteoclastogenesis and inflammatory cytokine expression induction51–53—traits not overcome, and potentially worsened, by supraphysiologic rhBMP-2 doses. There are reports of BMP-2 induced adipogenesis54 and clinical accounts of adipose tissue scattered throughout BMP-2 regenerated bone.55 Although the concentration of BMP-2 required to overcome its native antagonists is unknown, the current research trends to low-dose BMP-2 with moderate success.56,57 Furthermore, phase II and III clinical trials were completed with objectives to decrease the dose of rhBMP-2 from 1.5 to 1.0 mg/ml in patients undergoing internal fixation surgeries to repair closed diaphyseal tibial fractures. To its favor, retrospective analyses of on-label spinal fusion and open tibial fracture surgeries show a decreased rate of secondary interventions when rhBMP-2 is used instead of autograft alone. Additionally, operation time and hospital stay were reduced with rhBMP-2 use.38,58
There is no current consensus on rhBMP-2 treatment, utilization, or effectiveness among clinicians or researchers. Further study is needed to elucidate if combination therapy may allow a lower dose of rhBMP-2, if rhBMP-2 could be more effective if administered via another mechanism (e.g., gene therapy), and if bone quality and subsistence limit the long-term effectiveness of treatment.
PTH 1–34
The production of recombinant PTH 1–34, teriparatide, the amino terminal of the full-length PTH peptide (84 amino acids), is utilized to encourage osteo-anabolism when administered systemically and intermittently for the treatment of osteoporosis.
Off-label, PTH (1–34) has been studied in clinical trails to assess fracture repair59; however, therapy is limited by dosing ambiguities and mixed results. In one human clinical trial treating distal radius fractures, a higher dose (40 μg) of PTH 1–34 was no better than the vehicle control, while the lower dose (20 μg) shortened time to cortical continuity.59 In patients with pelvic fractures, a dose of 100 μg of PTH (1–84) and concurrent vitamin D and calcium supplementation accelerated fracture healing and functional outcome.60 In rat tibial fractures, lower doses (60 μg) of PTH 1–34 produced less external callus volume and ultimate load when compared to higher doses (200 μg).61 These ambiguities and mixed results have led to the termination of several of the clinical trials in long bone fracture repair; although, PTH (1–34) may still be clinically indicated in other clinical scenarios. For instance, PTH (1–34) increases bone formation around implants, helping them assimilate into grafts62–65 and increases flexural thickness and overall cortical thickness66 predominantly through the proliferation of bone lining cells67 and inhibition of osteoblast apoptosis.68 It may also regulate bone formation around bone implants through strain specific osteoblastic induction.10
An important limitation of PTH (1–34) is that it that treatment is approved for only 24 months of use due to an increased incidence of osteosarcoma and a dose-related increase in osteoblastoma and osteoma in female and male Fischer rats (https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021318s015lbl.pdf). While this limitation may not be relevant in long bone fracture since patients should be well-within healthy bone remodeling, patients at risk of osteoporotic fractures may require long-term therapy.
GENE THERAPY
The transformation of cells, as observed by differing phenotypes, dates back to at least 1928, and was first observed and characterized in bacteria by Frederick Griffith.88 Today, the term gene therapy encompasses a variety of techniques that utilize viruses, plasmids, and gene activated matrices to deliver therapeutic cDNA into host cells. This review will focus on gene therapy utilizing viruses only. Targets of gene therapy may be somatic or germ cells, and the distinction is important as the FDA currently allows gene therapy on somatic cells only. In challenging bone-healing environments it would be beneficial to deliver therapeutic osteo-anabolic genes over several weeks as opposed to only a few days.
The purpose of viral gene therapy is to either (i) replace defective native gene sequence, or to (ii) provide an extra gene copy and drive over expression. Although different vectors and their associated therapies are designed with specific therapeutic targets in mind, transduction should result in transgene expression at therapeutic quantities89 and be highly specific to the cellular target.90 Viral gene therapy utilizes the efficiency of viruses to gain entrance into cells and to have quick production of protein from the genetically modified cell. When compared to transfection by plasmid or another cDNA containing element, the efficiency of viral vectors to transduce target cells is superior91; further, vectors with different serotypes have been shown to selectively transduce a number of cell types, including mesenchymal stem cells, chondrocytes, and synoviocytes.92 Gene therapy to induce bone formation has been delivered in vivo directly to a defect as a suspension,93,94 in vivo lyophilized to an allograft implant scaffold,95 or through an ex vivo approach, where cell-type transduction is controlled in vitro, and then applied to a defect some time later in a dual surgery process (traditional),89,96 or tissue may be selectively isolated, transduced, and re-implanted in a single surgery (expedited).91,97,98 Figure 2 shows how a traditional, allogeneic ex vivo approach might be utilized to study fracture repair in situ.
Figure 2.
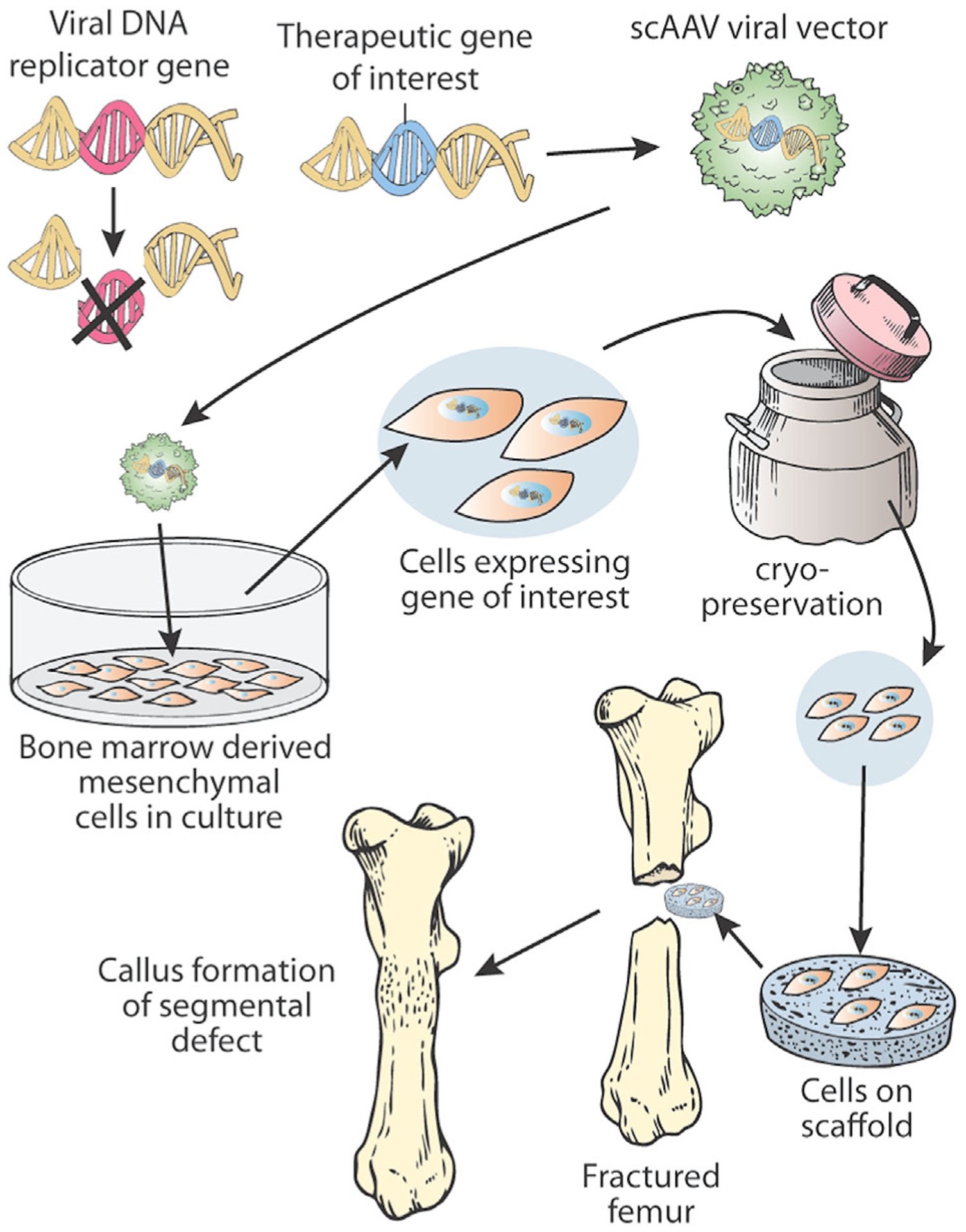
Figure 2 describes how an ex vivo technique may be utilized with cryopreservation to provide a gene therapy approach to segmental bone defects in fracture repair.
Therapeutic genes used in gene therapy were initially successful in their use as recombinant proteins, and were then explored with gene therapy because it was hypothesized they may better effect clinical success with an alternate delivery.99 At the site of large bone defects, recombinant proteins require specific temporal and spatial delivery mechanisms to decrease diffusion of the protein from the site of interest. While genetically modified cells will produce protein that also diffuses, the continuous and persistent production that is achieved for the lifetime of the genetically modified cell eliminates the need to deliver one-time supraphysiologic doses as occurs with recombinant protein therapy. Further, transduction and gene production by host cell machinery is more likely to undergo genuine post-translational modification, and may have greater biological activity compared to their recombinant counterparts.39,100
Despite removal of virulence factors from therapeutic vectors, there is concern viruses may revert to pathogenicity if they transduce a cell that has previously been, or becomes co-infected with another virus and that allows pathogenic replication within the patient. Some vectors, especially commonly used adenoviruses are pro-inflammatory even after removal of virulence factors, a trait attributed to the production of non-therapeutic, non-pathogenic viral genes.101,102
The following viruses have been the most commonly used in segmental bone defects as delivery vectors. Table 4 provides seminal gene therapy references utilizing BMP-2 and PTH (1–34) as therapeutics for segmental bone defects.
Table 4.
Pre-Clinical Animal Studies Are Described and Grouped According to Method of Transgene Administration
| Gene Therapy Modality | Pre-Clinical Studies | Therapeutic Gene | References for Further Reading |
|---|---|---|---|
| In vivo: suspension | In rats, femoral defects were treated with Ad-BMP-2 at the time of defect creation or 24 h after. Mean bending and mean stiffness were increased, and bone mineral content was similar to contralateral control femora. In rabbit ulnae, segmental defects were treated 7 days after defect creation with Ad-BMP-6. At 6 and 8 weeks, treated ulnae had increased bone area, mineral content, and were stronger in torsional stiffness than control ulnae. In the equine, Ad-BMP-2/7 treated metacarpal IV did not heal better than untreated controls | BMP-2, BMP-2/7, BMP-6 | 93,94,119,120 |
| In vivo: GAM | In mice, scAAV-BMP-2.5 lyophilized to allograft healed segmental femoral defects, cortical shells formed were equivalent to unfractured femurs and had equivalent torsional rigidity. PTH (1–34) on a collagen sponge increased periosteal bone in equine cortical defects after 13 weeks, but did not heal subchondral defects exposed to the articular surface | BMP-2, PTH (1–34) | 95,118 |
| Ex vivo: traditional | Utilizing the traditional ex vivo technique, rat femoral defects treated with LV-BMP-2 had significantly higher peak torque and torque to failure biomechanics than femora treated with BMDMSCs alone. Further, a variety of cell types including muscle and human adipose can be transduced and utilized in mice and rats to produce bony union after 8 weeks | BMP-2, BMP-4 | 89,96,116,122,123 |
| Ex vivo: expedited | Muscle, fat, and buffy coat hematologic components were transduced the same day as defect creation and implanted into critical sized bone defects. Radiographic union was displayed as early as 10 days and was earlier when compared to ex vivo traditional methods. Within 8 weeks, bone volume and torsion testing suggest mechanical stability comparable to control femora | BMP-2, LMP-1 | 97,98,121 |
Adeno Vectors
The non-enveloped adenovirus is a double stranded DNA (dsDNA) virus. Several of the early transcript genes of adenovirus are required for adeno-associated vectors to replicate, although the two are unrelated. Adenoviruses are relatively ubiquitous and do not cause any known disease in humans, making them incredibly useful during the early experiments of gene therapy. However, adenoviruses do elicit a large immune response, leading to immune destruction of transduced target cells.103,104 Newer vector constructs are often utilized.
Adeno-Associated Vectors
The adeno-associated virus (AAV) is a small, single-stranded DNA parvovirus that elicits minimal immunogenic reaction. The many serotypes available add to its allure as a therapeutic vector since targeted tissue tropism is conferred.90 Serotype 2 is the most commonly utilized serotype in musculoskeletal tissues and is used with serum free media for maximal transduction. Several genes including BMP-2,105 BMP-4,106 and BMP-7107 are used in AAV vectors to induce osteogenesis, although BMP-2 is by far the most widely used due to its ability to induce de novo osteogenesis in vitro108 and in vivo.109 However, it has been observed that AAV vectors might not produce enough protein to heal large segmental defects. As previously mentioned, AAV vectors utilize genes from Adenovirus (termed “helper genes”) to ensure viral replication; although, high titers of recombinant adeno-associated viral vectors have been produced in the absence of adenovirus helper genes.110
Self-Complementary Adeno-Associated Vectors
Self-complementary adeno-associated virus (scAAV) is an AAV vector that has been engineered to contain coding and non-coding strands of DNA. Therefore, scAAV does not require DNA polymerase to produce a complementary DNA strand before mRNA is produced. This ultimately results in more efficient protein expression.111 scAAV vectors have been used in vivo to produce the interleukin-1 receptor antagonist (IL-1ra) protein transgene in normal joints112; and it has been shown that repeat dosing can be achieved without immunogenic reaction when the serotype of the repeat dose is modified.112 In bone healing, scAAV vectors have been used to deliver various DNA molecules that showed improved bone integration histologically. Yazici et al.95 used scAAV2.5-BMP-2 coated allografts to increase incorporation when compared to autograft. Further, Yazici et al.95 showed that scAAV-BMP-2 treated femurs had increased torsional rigidity when compared to the control femur in post mortem analysis. In contrast to integrated vectors, scAAV vectors remain episomal within the nucleus and the transgene is not replicated with subsequent cell divisions. Although this may seem like a disadvantage, scAAV transduced cells increased IL-1ra transgene expression in the equine model 183 days after in vivo injection.112 Therefore, long-term expression results from scAAV gene therapy even though it does not integrate into the cells genome. Figure 2 shows how scAAV may be used clinically to augment bone healing.
Lentiviral Vectors
Lentiviral vectors are RNA viruses that transduce dividing and non-dividing cells, and each virion inserts two transgene copies into host chromosomes. Host chromosome integration leads to prolonged transgene expression, however insertion is currently not controlled and may lead to insertional mutagenesis. Insertional mutagenesis occurs when the viral transgene integrates near potential proto-oncogenes, altering the nuclear regulation of transcripts, and ultimately resulting in unwanted neoplasia. This random insertion into the genome affects the safety profile of this vector91 and could limit its efficacy; however non-integrating lentiviral vectors have been produced113,114 and studies are being performed using antiviral pro-drugs that are metabolized to toxic compounds within transduced cells only.115 Such advancements in vector technology show an effort to provide a clinical approach to eliminating therapeutic cells after the transgene is no longer needed.
Retroviral Vectors
Retroviral vectors are RNA viruses that preferentially transduce and integrate into the genome of actively dividing cells. While bone is in a constant state of turnover, it is not dividing at a rate that allows it to be a suitable target of retroviral vectors delivered in vivo. However, similar to lentiviral vectors, transgene expression may also be mutagenic.116,117
PROGENITOR/STEM CELLS
The term “stem cell” exists in scientific literature dating back to 1868. Stem cells, or perhaps more correctly, tissue progenitor cells, utilized in modern research are derived from a variety of tissues (e.g., are digested away from lipo-aspirate, muscle, blood vessels, and other organs124,125) and are selected for by adherence to culture plates. To properly denote plastic adherent tissue-based cells isolated from any source as true mesenchymal stem cells, characterization would include expression of CD105, CD73, and CD90.126 Additionally, cells would lack expression of the following markers: CD45, CD34, CD14/11b, CD79-alpha/CD19, and Human Leukocyte Antigen-DR,126 must self-renew, re-populate, and undergo tri-lineage differentiation in vitro.127 Ease of isolation, replication potential (telomere length, pluripotent markers), planned therapeutic use, and other clinical indications often dictate the site of progenitor cell harvest, though there is evidence to suggest differences in cell differentiation between progenitor cells from different sources.128–130 While each source can undergo tri-lineage differentiation into adipocytes, chondroblasts, and osteoblasts, there is evidence of lineage biases of progenitor cells isolated from bone marrow when osteoinduction is the goal131 and it is likely epigenetic gene regulation confers some sort of tissue-source memory, as some groups have successfully changed the osteogenic differentiation capacity of adipose derived progenitor cells to rival bone marrow derived progenitors through use of histone deacetylase inhibitors.132
Patients presenting with several risk factors for non-union formation or those presenting for a second surgery to repair a failed fracture consolidation are considered to have clinical indications for progenitor cell therapies133; however, patients cannot receive culture-expanded BMDMSCs unless they are part of a clinical trial. In lieu—clinicians are utilizing patient-side progenitor cell therapies, such as bone marrow aspirate concentrate and stromal vascular fraction—increased concentrations of cytokines and a small population of stem cells characterize both treatments.134 While these patient-side treatments are minimally manipulated and autogenic, they have the potential to be heavily influenced by patient co-morbidities.
It is currently unknown how many progenitor cells are needed to affect segmental bone healing, from what source progenitor cells should come from, at what concentration, and in what vehicle should cells be delivered in. It is known those progenitors cells alone and without a carrier are not sufficient to alter segmental defects, and alone are not a suitable intervention. Studies have found correlations between number of osteoprogenitor cells, concentration of fibroblast colony forming units, and final fracture consolidation135–137 although there remains no consensus on how many cells are needed to fill a defect. In general, it is considered that mineralized callus formation is correlated to the number of progenitor cells within the bone marrow aspirate, especially when utilized in the absence of concentration.138 Therefore, it is reasonable to postulate that culture-expanded cells may be especially helpful in instances of healing segmental bone defects when higher concentrations of cellular therapies have affected clinical success.139,140
Bone marrow aspirate concentrate (BMAC) is one minimally manipulated therapy, and considered an alternative to autologous bone graft (ABG). ABG has many well-documented co-morbidities and an upper limit of available graft material.141 BMAC contains several cell type precursors, including platelet alpha-granules that contain numerous growth factors, and a small population of mesenchymal stem cells.142 Recently, one group found that nucleated cell counts of BMAC samples were not predictive of colony forming units, suggesting the healing property of BMAC is correlated to growth factors contained within platelet granules such VEGF and IL-1ra.134 In an equine model it was demonstrated that the addition of culture-expanded BMDMSCs and autologous PRP resulted in bone formation in chondral defects.143 The role of nucleated cells, expanded BMDMSCs, and platelets (and platelet-based patient-side therapies) remains to be elucidated but may have the potential to encourage bone healing. It is judicious to note that none of these therapies have been tested in a challenging bone healing environment.
CONCLUSION
Promotion of osteogenesis to heal segmental bone defects and osteoporosis in humans and animals is a complex issue. While many studies have been performed, there are a myriad of unanswered questions. Successful clinical therapies may predominantly move towards cell-based growth factor delivery systems that address the need for sustained osteoinduction, especially in large defects to augment risk factors for non-unions. The barrier to this may be regulations that are associated with FDA rules governing autologous and allogeneic cell implantation in addition to genetically modified cells. The frequencies of nonunions (5–10% of fractures annually) remain an unmet challenge with serious socioeconomic impact, and the aging population necessitates that a more critical emphasis must be put on osteoporotic fractures and disease progression. Cellular-based therapeutic approaches require further intensive investigation, as there is no clear solution. Future testing in preclinical models and additional clinical trials of bone repair will elucidate safety and efficacy of alterations in dosing and route of administration of the recombinant proteins BMP-2 and PTH 1–34, gene therapy, and progenitor cell therapies.
ACKNOWLEDGMENT
Author AB supported by NIH T32: 5 T32 OD 12201-3, the Colorado State University College Research Council, and generous donations by the Bender Family Fund.
REFERENCES
- 1.Hadjidakis DJ. Androulakis Ioannis I II. 2006. Bone remodeling. Ann N Y Acad Sci 1092:385–396. [DOI] [PubMed] [Google Scholar]
- 2.Doherty AH, Ghalambor CK, Donahue SW. 2015. Evolutionary physiology of bone: bone metabolism in changing environments. Physiology (Bethesda) 30:17–29. [DOI] [PubMed] [Google Scholar]
- 3.Bayer EA, Gottardi R, Fedorchak MV, et al. 2015. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Control Release 219:129–140. [DOI] [PubMed] [Google Scholar]
- 4.Copuroglu C, Calori GM, Giannoudis PV. 2013. Fracture non-union: who is at risk? Injury 44:1379–1382. [DOI] [PubMed] [Google Scholar]
- 5.Sirkin M, Sanders R, DiPasquale T, et al. 1999. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma 13:78–84. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt JM, Hwang K, Winn SR, et al. 1999. Bone morphogenetic proteins: an update on basic biology and clinical relevance. J Orthop Res 17:269–278. [DOI] [PubMed] [Google Scholar]
- 7.Carreira AC, Alves GG, Zambuzzi WF, et al. 2014. Bone morphogenetic proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys 561:64–73. [DOI] [PubMed] [Google Scholar]
- 8.Hayrapetyan A, Jansen JA, van den Beucken JJ. 2015. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev 21:75–87. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Chen G, Li YP. 2016. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galea GL, Meakin LB, Savery D, et al. 2015. Planar cell polarity aligns osteoblast division in response to substrate strain. J Bone Miner Res 30:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsell R, Einhorn TA. 2011. The biology of fracture healing. Injury 42:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Censi R, Di Martino P, Vermonden T, et al. 2012. Hydrogels for protein delivery in tissue engineering. J Control Release 161:680–692. [DOI] [PubMed] [Google Scholar]
- 13.Oryan A, Alidadi S, Moshiri A, et al. 2014. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes C, Kobayashi T, Kronenberg HM. 2007. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci 1116:149–164. [DOI] [PubMed] [Google Scholar]
- 15.Wang RN, Green J, Wang Z, et al. 2014. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis 1:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce AI, Richards RG, Milz S, et al. 2007. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater 13:1–10. [DOI] [PubMed] [Google Scholar]
- 17.Smits BM, van Zutphen BF, Plasterk RH, et al. 2004. Genetic variation in coding regions between and within commonly used inbred rat strains. Genome Res 14:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller TS, Mao Z, Spengler DM. 1990. Young’s modulus, bending strength, and tissue physical properties of human compact bone. J Orthop Res 8:592–603. [DOI] [PubMed] [Google Scholar]
- 19.Rohl L, Larsen E, Linde F, et al. 1991. Tensile and compressive properties of cancellous bone. J Biomech 24:1143–1149. [DOI] [PubMed] [Google Scholar]
- 20.Mapara M, Thomas BS, Bhat KM. 2012. Rabbit as an animal model for experimental research. Dent Res J (Isfahan) 9:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aerssens J, Boonen S, Lowet G, et al. 1998. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139:663–670. [DOI] [PubMed] [Google Scholar]
- 22.Liebschner MA. 2004. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 25:1697–1714. [DOI] [PubMed] [Google Scholar]
- 23.Clarke B 2008. Normal bone anatomy and physiology. Clin J Am Soc Nephrol, 3:S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIlwraith CW, Frisbie DD, Kawcak CE. 2012. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res 1:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Thomas CD, Clement JG, et al. 2016. A mechanostatistical approach to cortical bone remodelling: an equine model. Biomech Model Mechanobiol 15:29–42. [DOI] [PubMed] [Google Scholar]
- 26.Harrison SM, Whitton RC, Kawcak CE, et al. 2014. Evaluation of a subject-specific finite-element model of the equine metacarpophalangeal joint under physiological load. J Biomech 47:65–73. [DOI] [PubMed] [Google Scholar]
- 27.Hinchcliff KW, Kaneps AJ, Geor RJ, et al. 2014. Equine sports medicine and surgery basic and clinical sciences of the equine athlete. Philadelphia, PA: Saunders. pp. 145–165. [Google Scholar]
- 28.Bagi CM, Berryman E, Moalli MR. 2011. Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med 61:76–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Peric M, Dumic-Cule I, Grcevic D, et al. 2015. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 70:73–86. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara A, Zekas LJ, Litsky AS, et al. 2010. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res 28:403–411. [DOI] [PubMed] [Google Scholar]
- 31.Yasko AW, Lane JM, Fellinger EJ, et al. 1992. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. Bone Joint Surg Am 74:659–670. [PubMed] [Google Scholar]
- 32.Nakajima A, Shimoji N, Shiomi K, et al. 2002. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J Bone Miner Res 17:2038–2047. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman JR, Daluiski A, Einhorn TA. 2002. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am 84-A:1032–1044. [DOI] [PubMed] [Google Scholar]
- 34.Turner CH. 2002. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int 13:97–104. [DOI] [PubMed] [Google Scholar]
- 35.Urist MR. 1965. Bone: formation by autoinduction. Science 150:893–899. [DOI] [PubMed] [Google Scholar]
- 36.Mueller TD, Nickel J. 2012. Promiscuity and specificity in BMP receptor activation. FEBS Lett 586:1846–1859. [DOI] [PubMed] [Google Scholar]
- 37.Geiger M, Li RH, Friess W. 2003. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev 55:1613–1629. [DOI] [PubMed] [Google Scholar]
- 38.Garrison KR, Donell S, Ryder J, et al. 2007. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess 11:1–150. iii–iv. [DOI] [PubMed] [Google Scholar]
- 39.Evans C 2014. Using genes to facilitate the endogenous repair and regeneration of orthopaedic tissues. Int Orthop 38:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Kim J, Cheng C, et al. 2006. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 39:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKay W 2004. National research council. In: Scarborough BA, editor. Proceedings from the workshop on science-based assessment: accelerating product development of combination medical devices. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 42.Chen FM, Zhang M, Wu ZF. 2010. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 31:6279–6308. [DOI] [PubMed] [Google Scholar]
- 43.Garrison KR, Shemilt I, Donell S, et al. 2010. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev 2010:CD006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boraiah S, Paul O, Hawkes D, et al. 2009. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res 467:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brannan PS, Gaston RG, Loeffler BJ, et al. 2016. Complications with the use of BMP-2 in scaphoid nonunion surgery. J Hand Surg Am 41:602–608. [DOI] [PubMed] [Google Scholar]
- 46.Shen J, James AW, Zhang X, et al. 2016. Novel wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol 186:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burkus JK, Sandhu HS, Gornet MF. 2006. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine (Phila Pa 1976) 31:775–781. [DOI] [PubMed] [Google Scholar]
- 48.McClellan JW, Mulconrey DS, Forbes RJ, et al. 2006. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech 19:483–486. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Guo J, Zhou Y, et al. 2014. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev 20:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James AW, Zara JN, Zhang X, et al. 2012. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med 1:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itoh K, Udagawa N, Katagiri T, et al. 2001. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 142:3656–3662. [DOI] [PubMed] [Google Scholar]
- 52.Hori M, Sawai H, Tsuji Y, et al. 2006. Bone morphogenetic protein-2 counterregulates interleukin-18 mRNA and protein in MC3T3-E1 mouse osteoblastic cells. Connect Tissue Res 47:124–132. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto M, Murai J, Yoshikawa H, et al. 2006. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res 21:1022–1033. [DOI] [PubMed] [Google Scholar]
- 54.Jeon MJ, Kim JA, Kwon SH, et al. 2003. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem 278:23270–23277. [DOI] [PubMed] [Google Scholar]
- 55.Zara JN, Siu RK, Zhang X, et al. 2011. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SS, Huang BJ, Kaltz SR, et al. 2013. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 34: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decambron A, Fournet A, Bensidhoum M, et al. 2017. Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J Orthop Res 35:2637–2645. [DOI] [PubMed] [Google Scholar]
- 58.Wei S, Cai X, Huang J, et al. 2012. Recombinant human BMP-2 for the treatment of open tibial fractures. Orthopedics 35:e847–e854. [DOI] [PubMed] [Google Scholar]
- 59.Aspenberg P, Johansson T. 2010. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 81:234–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peichl P, Holzer LA, Maier R, et al. 2011. Parathyroid hormone 1–84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am 93: 1583–1587. [DOI] [PubMed] [Google Scholar]
- 61.Andreassen TT, Ejersted C, Oxlund H. 1999. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 14:960–968. [DOI] [PubMed] [Google Scholar]
- 62.Fahlgren A, Yang X, Ciani C, et al. 2013. The effects of PTH, loading and surgical insult on cancellous bone at the bone-implant interface in the rabbit. Bone 52:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dayer R, Brennan TC, Rizzoli R, et al. 2010. PTH improves titanium implant fixation more than pamidronate or renutrition in osteopenic rats chronically fed a low protein diet. Osteoporos Int 21:957–967. [DOI] [PubMed] [Google Scholar]
- 64.Dayer R, Badoud I, Rizzoli R, et al. 2007. Defective implant osseointegration under protein undernutrition: prevention by PTH or pamidronate. J Bone Miner Res 22:1526–1533. [DOI] [PubMed] [Google Scholar]
- 65.Zeng X, He H, Zhang L, et al. 2011. A potential therapeutic approach to overload-induced bone loss around implant: parathyroid hormone (PTH). Med Hypotheses 77:701–704. [DOI] [PubMed] [Google Scholar]
- 66.Burr DB, Hirano T, Turner CH, et al. 2001. Intermittently administered human parathyroid hormone(1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 16:157–165. [DOI] [PubMed] [Google Scholar]
- 67.Dobnig H, Turner RT. 1995. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136:3632–3638. [DOI] [PubMed] [Google Scholar]
- 68.Manolagas SC. 2000. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137. [DOI] [PubMed] [Google Scholar]
- 69.Boyan BD, Lohmann CH, Somers A, et al. 1999. Potential of porous poly-D,L-lactide-co-glycolide particles as a carrier for recombinant human bone morphogenetic protein-2 during osteoinduction in vivo. J Biomed Mater Res 46: 51–59. [DOI] [PubMed] [Google Scholar]
- 70.Kaback LA, Soung do Y, Naik A, et al. 2008. Teriparatide (1–34 human PTH) regulation of osterix during fracture repair. J Cell Biochem 105:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettway GJ, Meganck JA, Koh AJ, et al. 2008. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone 42: 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugiyama T, Saxon LK, Zaman G, et al. 2008. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone 43:238–248. [DOI] [PubMed] [Google Scholar]
- 73.Thies RS, Bauduy M, Ashton BA, et al. 1992. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20–17 stromal cells. Endocrinology 130:1318–1324. [DOI] [PubMed] [Google Scholar]
- 74.Alkhiary YM, Gerstenfeld LC, Krall E, et al. 2005. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am 87:731–741. [DOI] [PubMed] [Google Scholar]
- 75.Uludag H, D’Augusta D, Palmer R, et al. 1999. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res 46:193–202. [DOI] [PubMed] [Google Scholar]
- 76.Yamaguchi A, Katagiri T, Ikeda T, et al. 1991. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol 113:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Einhorn TA, Majeska RJ, Mohaideen A, et al. 2003. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am 85-A:1425–1435. [DOI] [PubMed] [Google Scholar]
- 78.Hirano T, Burr DB, Turner CH, et al. 1999. Anabolic effects of human biosynthetic parathyroid hormone fragment (1–34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res 14:536–545. [DOI] [PubMed] [Google Scholar]
- 79.Mashiba T, Burr DB, Turner CH, et al. 2001. Effects of human parathyroid hormone (1–34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone 28: 538–547. [DOI] [PubMed] [Google Scholar]
- 80.Sciadini MF, Johnson KD. 2000. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res 18:289–302. [DOI] [PubMed] [Google Scholar]
- 81.Zegzula HD, Buck DC, Brekke J, et al. 1997. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2). J Bone Joint Surg Am 79: 1778–1790. [DOI] [PubMed] [Google Scholar]
- 82.Curtis RC, Custis JT, Ehrhart NP, et al. 2016. Combination therapy with zoledronic acid and parathyroid hormone improves bone architecture and strength following a clinically-relevant dose of stereotactic radiation therapy for the local treatment of canine osteosarcoma in athymic rats. PLoS ONE 11:e0158005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daugaard H, Elmengaard B, Andreassen T, et al. 2011. Parathyroid hormone treatment increases fixation of orthopedic implants with gap healing: a biomechanical and histomorphometric canine study of porous coated titanium alloy implants in cancellous bone. Calcif Tissue Int 88:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuerst A, Derungs S, von Rechenberg B, et al. 2007. Use of a parathyroid hormone peptide (PTH(1–34))-enriched fibrin hydrogel for the treatment of a subchondral cystic lesion in the proximal interphalangeal joint of a warmblood filly. J Vet Med A Physiol Pathol Clin Med 54:107–112. [DOI] [PubMed] [Google Scholar]
- 85.Perrier M, Lu Y, Nemke B, et al. 2008. Acceleration of second and fourth metatarsal fracture healing with recombinant human bone morphogenetic protein-2/calcium phosphate cement in horses. Vet Surg 37:648–655. [DOI] [PubMed] [Google Scholar]
- 86.Seo JP, Tsuzuki N, Haneda S, et al. 2014. Osteoinductivity of gelatin/beta-tricalcium phosphate sponges loaded with different concentrations of mesenchymal stem cells and bone morphogenetic protein-2 in an equine bone defect model. Vet Res Commun 38:73–80. [DOI] [PubMed] [Google Scholar]
- 87.Welch RD, Jones AL, Bucholz RW, et al. 1998. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res 13:1483–1490. [DOI] [PubMed] [Google Scholar]
- 88.Griffith F 1928. The significance of pneumococcal types. J Hyg (Lond) 27:113–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieberman JR, Daluiski A, Stevenson S, et al. 1999. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81:905–917. [DOI] [PubMed] [Google Scholar]
- 90.Hemphill DD, McIlwraith CW, Samulski RJ, et al. 2014. Adeno-associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured mono-layers. Sci Rep 4:5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans CH. 2012. Gene delivery to bone. Adv Drug Deliv Rev 64:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nixon AJ, Goodrich LR, Scimeca MS, et al. 2007. Gene therapy in musculoskeletal repair. Ann N Y Acad Sci 1117:310–327. [DOI] [PubMed] [Google Scholar]
- 93.Bertone AL, Pittman DD, Bouxsein ML, et al. 2004. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. J Orthop Res 22:1261–1270. [DOI] [PubMed] [Google Scholar]
- 94.Baltzer AW, Lattermann C, Whalen JD, et al. 2000. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther 7:734–739. [DOI] [PubMed] [Google Scholar]
- 95.Yazici C, Takahata M, Reynolds DG, et al. 2011. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther 19:1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen HC, Peng H, Usas A, et al. 2004. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone 34:982–992. [DOI] [PubMed] [Google Scholar]
- 97.Virk MS, Sugiyama O, Park SH, et al. 2011. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther 19:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Viggeswarapu M, Boden SD, Liu Y, et al. 2001. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am 83-A:364–376. [DOI] [PubMed] [Google Scholar]
- 99.Carofino BC, Lieberman JR. 2008. Gene therapy applications for fracture-healing. J Bone Joint Surg Am 90: 99–110. [DOI] [PubMed] [Google Scholar]
- 100.Walsh G, Jefferis R. 2006. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 24:1241–1252. [DOI] [PubMed] [Google Scholar]
- 101.Mitani K, Graham FL, Caskey CT. 1994. Transduction of human bone marrow by adenoviral vector. Hum Gene Ther 5:941–948. [DOI] [PubMed] [Google Scholar]
- 102.Wilson JM. 1996. Adenoviruses as gene-delivery vehicles. N Engl J Med 334:1185–1187. [DOI] [PubMed] [Google Scholar]
- 103.Kay MA, Holterman AX, Meuse L, et al. 1995. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet 11:191–197. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y, Wilson JM. 1995. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol 155:2564–2570. [PubMed] [Google Scholar]
- 105.Chen Y, Luk KD, Cheung KM, et al. 2003. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther 10: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 106.Tian K, Qi M, Wang L, et al. 2015. Two-stage therapeutic utility of ectopically formed bone tissue in skeletal muscle induced by adeno-associated virus containing bone morphogenetic protein-4 gene. J Orthop Surg Res 10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang M, Ma QJ, Dang GT, et al. 2005. Adeno-associated virus-mediated bone morphogenetic protein-7 gene transfer induces C2C12 cell differentiation into osteoblast lineage cells. Acta Pharmacol Sin 26:963–968. [DOI] [PubMed] [Google Scholar]
- 108.Dragoo JL, Choi JY, Lieberman JR, et al. 2003. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res 21:622–629. [DOI] [PubMed] [Google Scholar]
- 109.Muller CW, Hildebrandt K, Gerich T, et al. 2015. BMP-2-transduced human bone marrow stem cells enhance neo-bone formation in a rat critical-sized femur defect. J Tissue Eng Regen Med 11:1122–1131. [DOI] [PubMed] [Google Scholar]
- 110.Xiao X, Li J, Samulski RJ. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 72:2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kay JD, Gouze E, Oligino TJ, et al. 2009. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med 11:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goodrich LR, Grieger JC, Phillips JN, et al. 2015. ScAA-VIL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther 22:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sarkis C, Philippe S, Mallet J, et al. 2008. Non-integrating lentiviral vectors. Curr Gene Ther 8:430–437. [DOI] [PubMed] [Google Scholar]
- 114.Matrai J, Cantore A, Bartholomae CC, et al. 2011. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53:1696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alaee F, Sugiyama O, Virk MS, et al. 2014. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther 21:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howe SJ, Mansour MR, Schwarzwaelder K, et al. 2008. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest 118:3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montini E, Cesana D, Schmidt M, et al. 2009. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 119:964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Backstrom KC, Bertone AL, Wisner ER, et al. 2004. Response of induced bone defects in horses to collagen matrix containing the human parathyroid hormone gene. Am J Vet Res 65:1223–1232. [DOI] [PubMed] [Google Scholar]
- 119.Betz OB, Betz VM, Nazarian A, et al. 2006. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am 88:355–365. [DOI] [PubMed] [Google Scholar]
- 120.Southwood LL, Kawcak CE, Hidaka C, et al. 2012. Evaluation of direct in vivo gene transfer in an equine metacarpal IV ostectomy model using an adenoviral vector encoding the bone morphogenetic protein-2 and protein-7 gene. Vet Surg 41:345–354. [DOI] [PubMed] [Google Scholar]
- 121.Evans CH, Liu FJ, Glatt V, et al. 2009. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater 18:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsu WK, Sugiyama O, Park SH, et al. 2007. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone 40:931–938. [DOI] [PubMed] [Google Scholar]
- 123.Peterson B, Zhang J, Iglesias R, et al. 2005. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 11:120–129. [DOI] [PubMed] [Google Scholar]
- 124.Crisan M, Yap S, Casteilla L, et al. 2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313. [DOI] [PubMed] [Google Scholar]
- 125.Kern S, Eichler H, Stoeve J, et al. 2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 126.Dominici M, Le Blanc K, Mueller I, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. [DOI] [PubMed] [Google Scholar]
- 127.Barry FP, Murphy JM. 2004. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584. [DOI] [PubMed] [Google Scholar]
- 128.Trivanovic D, Jaukovic A, Popovic B, et al. 2015. Mesenchymal stem cells of different origin: comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci 141:61–73. [DOI] [PubMed] [Google Scholar]
- 129.Billing AM, Ben Hamidane H, Dib SS, et al. 2016. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep 6:21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao HT, Chen CT. 2014. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 6:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elahi KC, Klein G, Avci-Adali M, et al. 2016. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int 2016:5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu X, Zhang X, Dai L, et al. 2013. Histone deacetylase inhibitor trichostatin A promotes the osteogenic differentiation of rat adipose-derived stem cells by altering the epigenetic modifications on Runx2 promoter in a BMP signaling-dependent manner. Stem Cells Dev 22:248–255. [DOI] [PubMed] [Google Scholar]
- 133.Gazdag AR, Lane JM, Glaser D, et al. 1995. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg 3:1–8. [DOI] [PubMed] [Google Scholar]
- 134.Cassano JM, Kennedy JG, Ross KA, et al. 2016. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc 26:333–342. [DOI] [PubMed] [Google Scholar]
- 135.Hernigou P, Poignard A, Beaujean F, et al. 2005. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–1437. [DOI] [PubMed] [Google Scholar]
- 136.Guimaraes JA, Duarte ME, Fernandes MB, et al. 2014. The effect of autologous concentrated bone-marrow grafting on the healing of femoral shaft non-unions after locked intramedullary nailing. Injury 45:S7–S13. [DOI] [PubMed] [Google Scholar]
- 137.Saxer F, Scherberich A, Todorov A, et al. 2016. Implantation of stromal vascular fraction progenitors at bone fracture sites: from a rat model to a first-in-man study. Stem Cells 34:2956–2966. [DOI] [PubMed] [Google Scholar]
- 138.Jungbluth P, Hakimi AR, Grassmann JP, et al. 2013. The early phase influence of bone marrow concentrate on metaphyseal bone healing. Injury 44:1285–1294. [DOI] [PubMed] [Google Scholar]
- 139.Lin CY, Chang YH, Sung LY, et al. 2014. Long-term tracking of segmental bone healing mediated by genetically engineered adipose-derived stem cells: focuses on bone remodeling and potential side effects. Tissue Eng Part A 20:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu JZ, Qin H, Wang XQ, et al. 2009. Repair of large segmental bone defects using bone marrow stromal cells with demineralized bone matrix. Orthop Surg 1:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burchardt H 1987. Biology of bone transplantation. Orthop Clin North Am 18:187–196. [PubMed] [Google Scholar]
- 142.Johnson RG. 2014. Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine (Phila Pa 1976) 39:695–700. [DOI] [PubMed] [Google Scholar]
- 143.Goodrich LR, Chen AC, Werpy NM, et al. 2016. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am 98:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


