ABSTRACT
Bacteriophages are considered the most abundant entities on earth. However, there are merely seven sequenced double-stranded RNA (dsRNA) phages, compared to thousands of sequenced double-stranded DNA (dsDNA) phages. Interestingly, dsRNA viruses are quite common in fungi and usually have a lifestyle of commensalism or mutualism. Thus, the classical protocol of using double-layer agar plates to characterize phage plaques might be significantly biased in the isolation of dsRNA phages beyond strictly lytic lifestyles. Thus, we applied a protocol for isolating fungal viruses to identify RNA phages in bacteria and successfully isolated a novel dsRNA phage, phiNY, from Microvirgula aerodenitrificans. phiNY has a genome consisting of three dsRNA segments, and its genome sequence has no nucleotide sequence similarity with any other phage. Although phiNY encodes a lytic protein of glycoside hydrolase, and phage particles are consistently released during bacterial growth, phiNY replication did not block bacterial growth, nor did it form any plaques on agar plates. More strikingly, the phiNY-infected strain grew faster than the phiNY-negative strain, indicating a mutualistic parasitic lifestyle. Thus, this study not only reveals a new mutualistic parasitic dsRNA phage but also implies that other virus isolation methods would be valuable to identify phages with nonlytic lifestyles.
IMPORTANCE Viruses with dsRNA genomes are quite diverse and infect organisms in all three domains of life. Although dsRNA viruses that infect humans, plants, and fungi are quite common, dsRNA viruses that infect bacteria, known as bacteriophages, are quite understudied, and only seven dsRNA phages have been sequenced so far. One possible explanation for the rare isolation of dsRNA phages might be the protocol of the double-layer agar plate assay. Phages without strictly lytic lifestyles might not form plaques. Thus, we applied the protocol of isolating fungal viruses to identify RNA phages inside bacteria and successfully isolated a novel dsRNA phage, phiNY, with a mutualistic parasitic lifestyle. This study implies that dsRNA phages without strictly lytic lifestyles might be common in nature and deserve more investigations.
KEYWORDS: double-stranded RNA bacteriophage, mutualistic parasitic lifestyle, Cystoviridae family, Microvirgula aerodenitrificans, bacteriophage, dsRNA phage
INTRODUCTION
Bacteriophages (phages) are bacterial viruses that play important roles in bacterial biology, diversity, and evolution (1–4). However, the majority of knowledge about them is generated based on the study of double-stranded DNA (dsDNA) phages, while double-stranded RNA (dsRNA) phages remain under investigation (5). RNA phages are separated into only two families, namely, Cystoviridae with seven sequenced dsRNA phages (see Table S1 in the supplemental material) and Leviviridae with 38 single-stranded RNA (ssRNA) phages (6, 7). Recently, a metagenomic sequencing study revealed 122 RNA phages with partial genome sequences, implying that more unrecognized RNA bacteriophages have yet to be discovered (8).
One reason for the lack of dsRNA phages might be the phage isolation method of employing double-layer agar plates, which can isolate phages with a lytic lifestyle. In fungi, dsRNA viruses are quite common, and some of these dsRNA viruses have a latent infection lifestyle, coexisting inside fungi and having minor or moderate impacts on the host (9–11). Thus, we infer that RNA phages with a lifestyle of commensalism or mutualism might not be selected by the formation of plaques on agar; rather, they should be isolated within the bacterium.
RESULTS AND DISCUSSION
In this study, we attempted to isolate RNA bacteriophages inside bacteria using CF11 cellulose, which is a common method to characterize RNA fungi viruses (12, 13). The CF11 cellulose column has been used previously to purify dsRNA from phi6-infected bacteria (14); however, to our knowledge, it has never been used to isolate RNA bacteriophages from bacterial samples. We first randomly selected dozens of colonies from fermented sour soup samples in Guizhou Province, China. Luckily, we successfully isolated and identified three dsRNA segments from one colony (Fig. 1A), indicating the potential presence of dsRNA bacteriophages inside this bacterium.
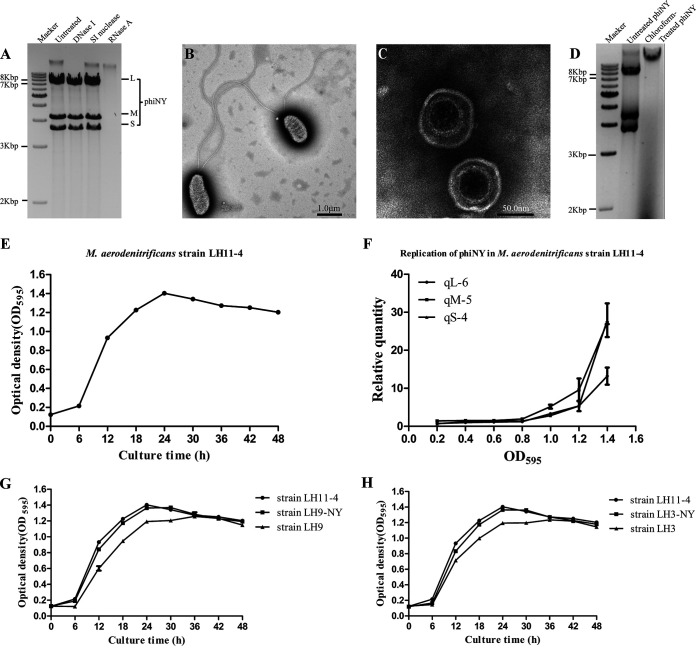
FIG 1.
Biological characterization of bacteriophage phiNY and the host bacterium Microvirgula aerodenitrificans strain LH11-4. (A) Agarose gel electrophoretic profile of the bacteriophage phiNY genome. (B, C) Transmission electron micrographs of the host bacterium, M. aerodenitrificans strain LH11-4, and bacteriophage phiNY. (D) The presence of double-stranded RNAs (dsRNAs) was detected from the phiNY-reinfected colony, but not the chloroform-treated phiNY-infected colony. (E) Growth curves of M. aerodenitrificans strain LH11-4 with phiNY (optical density at 595 nm [OD595], mean ± standard deviation [SD] of three biological replicates). (F) Quantification of phiNY by reverse transcription–quantitative real-time PCR (RT-qPCR) from the precipitate of M. aerodenitrificans strain LH11-4 (mean ± SD of three biological replicates). (G) Growth curves of M. aerodenitrificans strain LH11-4 with phiNY, phiNY-negative strain LH9, and the phiNY-reinfected strain LH9-NY (OD595, mean ± SD of three biological replicates). (H) Growth curves of M. aerodenitrificans strain LH11-4 with phiNY, phiNY-negative strain LH3, and the phiNY-reinfected strain LH3-NY (OD595, mean ± SD of three biological replicates).
The host bacterium was classified as Microvirgula aerodenitrificans by 16S rRNA gene amplification and sequencing and was named strain LH11-4 (Fig. 1B; see also Table S2 in the supplemental material). The phage particles, which were collected from the supernatant of the bacterial culture, were enveloped, tailless, spherical, and approximately 80 nm in diameter. The phage isolated in this study was named phiNY (Fig. 1C).
Phage particles can be detected in the supernatant using transmission electron microscopy (TEM) (Fig. 1C) and indicate the release of phages from the bacteria. However, phiNY did not form any plaque on the host, and the replication of phiNY is consistent with the growth of bacterium LH11-4 but did not inhibit the growth of the host. (Fig. 1E and F).
To further test the lifestyle relationship between phiNY and the host, we randomly isolated 93 colonies from L11-4 to select a phiNY-negative strain. We isolated and identified 9 colonies (Fig. 2) that were negative. Two phiNY-negative strains (LH9 and LH3) could be reinfected with phiNY and maintained for generations, while others were phiNY-resistant strains that could not be reinfected. Moreover, the chloroform-treated phiNY could not infect LH3 (Fig. 1D), indicating that phiNY is sensitive to organic solvents and has a lipid envelope.
FIG 2.
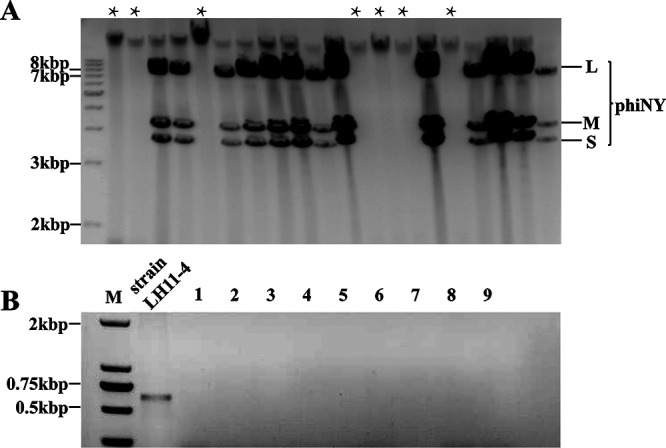
Investigation of carrier rate of phiNY in M. aerodenitrificans strain LH11-4. (A) The phiNY-negative colonies were detected using CF11 cellulose extraction followed by gel electrophoresis. (B) Confirmation of the absence of the phiNY genome in 9 colonies by RT-PCR.
More strikingly, phiNY-reinfected strains LH9-NY and LH3-NY and the parent strain LH11-4 grew faster than phiNY-negative strains LH9 and LH3, indicating that phiNY improves the growth of bacterial host (Fig. 1G and H), in contrast to other lytic dsRNA phages. This phenomenon is partly due to the poor lytic efficiency of phage phiNY, which enables the growth of the host. On the other hand, phiNY might encode a protein that improves the growth rate of the host, which needs further investigation.
The genome of bacteriophage phiNY was revealed by next-generation sequencing followed by 5′/3′ rapid amplification of cDNA ends (RACE) PCR using virus-specific primers (see Table S3 in the supplemental material) and was found to consist of segments L (7,135 bp), M (3,229 bp), and S (2,657 bp) (GenBank accession numbers MW471133, MW471134, and MW471135, respectively). The genome size was similar to that of other dsRNA bacteriophages (see Table S4 in the supplemental material). However, BLAST analysis showed that phiNY had no nucleotide sequence similarity with any other phage.
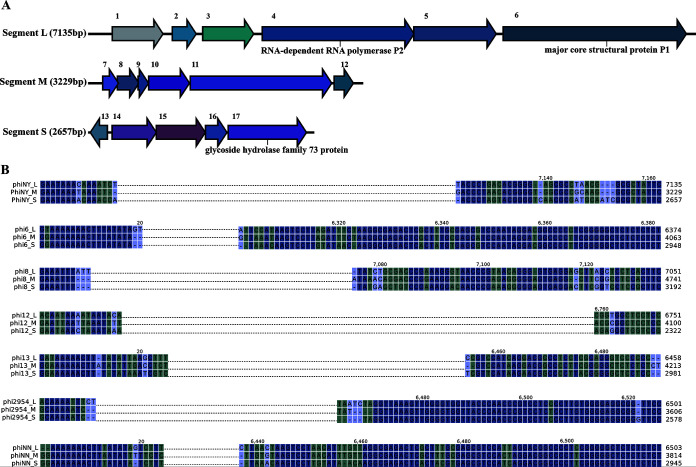
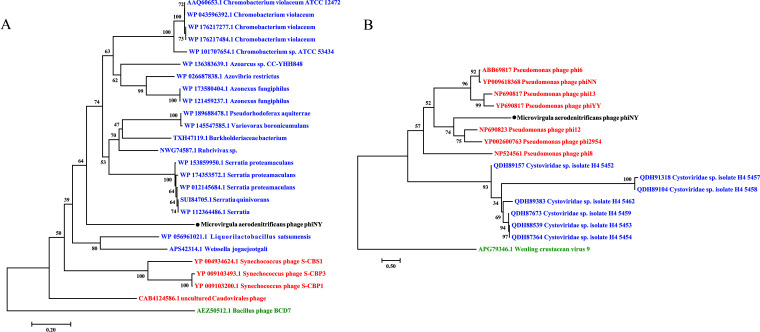
Seventeen open reading frames (ORFs) were annotated in phiNY using RAST (15). Based on amino acid similarity, only 3 ORFs were similar to functionally characterized genes, whereas 14 ORFs encode hypothetical proteins (Fig. 3A; see also Table S5 in the supplemental material). ORF4 encodes a predicted RNA-dependent RNA polymerase, P2, which might play an important role in the replication and transcription of the phage genome (16). ORF6 encodes the predicted major core structural protein, P1. In addition, ORF17 in segment S encodes a predicted glycoside hydrolase family 73 protein. The phylogenetic tree also shows that ORF17 of phiNY is evolutionarily related to the glycoside hydrolase family 73 protein from bacteria and phages, suggesting that ORF17 of phiNY might be the lysozyme in phiNY and thus might play a key role in phage infection and host lysis (Fig. 4A). In addition, we found that phiNY and other dsRNA phages have different conserved sequences at the 5′ and 3′ termini (Fig. 3B).
FIG 3.
Bioinformatics analysis of phiNY. (A) Genome maps of L, M, and S segments in the dsRNA phage phiNY. The predicted open reading frames (ORFs) are numbered, and the annotations are shown above. (B) Alignment of 5′ and 3′ termini of the L, M, and S segments in 7 dsRNA phages. Based on the alignment results, we identified similar sequences at the 5′ and 3′ termini of 7 dsRNA phages.
FIG 4.
Phylogenetic relationship of phiNY. (A) Phylogenetic relationships between glycoside hydrolases and lysozymes from bacteria and phages, which have similarity with ORF17 of phage phiNY. The name of the phage discovered in this study, phiNY, is shown in black. The names of bacterium reference sequences and phage reference sequences, including both the GenBank accession number and species name, are shown in blue and red, respectively. The cell wall hydrolase of Bacillus phage BCD7 is selected as an outgroup and is shown in green. (B) Phylogenetic analysis of RNA-dependent RNA polymerases from phiNY and other dsRNA phages. The phylogenetic tree is constructed based on RNA-dependent RNA polymerase protein alignment. The name of the phage discovered in this study, phiNY, is shown in black. The names of reference sequences in the genus Cystovirus and unclassified Cystoviridae species, including both the GenBank accession number and the phage species name, are shown in red and blue, respectively. Wenling crustacean virus 9 of Bunyavirales, which also has a segmented genome, is selected as an outgroup and is shown in green.
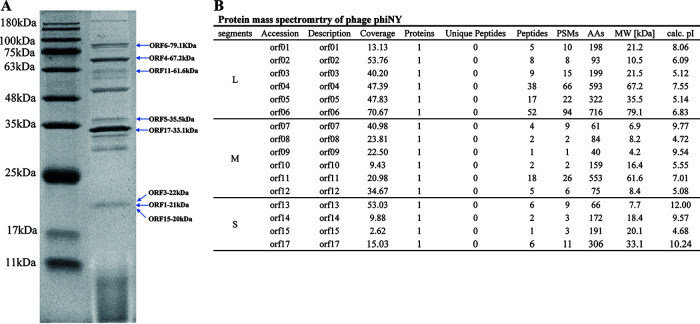
Structural proteins of phiNY were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by high-performance liquid chromatography–mass spectrometry (HPLC-MS). Structural proteins were detected with molecular weights of 10 to 100 kDa (Fig. 5). Sixteen ORFs were successfully detected by HPLC-MS, including the predicted major core structural protein P1. Most of the identified proteins are functionally unknown and merit further study.
FIG 5.
(A) SDS-PAGE of phiNY structural proteins. Molecular mass markers are shown on the left. (B) Protein mass spectrometry results for phiNY.
A phylogenetic tree was constructed using the RNA-dependent RNA polymerase proteins from phiNY and other dsRNA phages and indicated the distant relationships between phiNY and the other dsRNA phages, demonstrating that phiNY is a unique dsRNA phage (Fig. 4B).
In summary, we applied a method to characterize fungus viruses to isolate phages from bacteria and successfully selected a novel dsRNA phage, phiNY, from Microvirgula aerodenitrificans. phiNY replicates in a way that promotes the growth rate of the bacterium. Bioinformatic analysis reveals that phiNY had no nucleotide sequence similarity with other phages, implying genetic diversity of dsRNA phages, which might be widely distributed in microbial communities and could be very difficult to detect by metagenomic studies due to their poor sequence similarities. Thus, this study not only reveals a new dsRNA phage with a mutualistic parasitic lifestyle but also proves the importance of other virus-characterizing methods in isolating new phages.
MATERIALS AND METHODS
Isolation and purification of dsRNA phage phiNY.
Fermented sour soup, which is a traditional fermented condiment of Miao nationalities in Guizhou, China, was collected (without fixative or preservative) in a volunteer’s home and transported to the research facility at ambient temperature, avoiding exposure to heat, and stored at 4°C until processed. Single colonies were picked by inoculations of 10-fold dilutions from the fermented sour soup samples.
Then, we tried to isolate RNA bacteriophages using CF11 cellulose (Sigma, USA) as previously described (12) with minor modification. Briefly, the pelleted bacterial cells were centrifuged from 5- to 10-ml liquid cultures and ground in liquid nitrogen. The powder obtained was suspended in 0.4 ml extraction buffer (0.1 M NaCl, 50 mM Tris-HCl [pH 8], 0.5 mM EDTA [pH 8], 1% SDS, 25 mM glycine, and 0.1% 2-mercaptoethanol) and 0.4 ml phenol-chloroform (1:1 [wt/wt]). The suspension was vortexed for 5 min, followed by centrifugation at 4°C for 10 min at 12,000 rpm. The collected aqueous phase was adjusted to a final concentration of 19% ethanol and 0.06 g/ml CF11 cellulose (Sigma), and then chilled on ice for 30 min. dsRNA was washed twice by adding elution buffer (0.1 M NaCl, 50 mM Tris-HCl [pH 8], and 0.5 mM EDTA [pH 8]). dsRNA was precipitated with 3 M NaOAc (pH 5.5) and cold absolute ethanol at −20°C for 60 min. Finally, dsRNA was dissolved in 25 μl double-distilled water (ddH2O).
We successfully isolated the dsRNA bacteriophage phiNY from a colony from fermented sour soup samples in Guizhou Province. The dsRNA bacteriophage phiNY was then freed of contaminating DNA and single-stranded RNA by treatment with RNase-free DNase I and S1 nuclease (TaKaRa, Dalian, China) (Fig. 1A).
16S rRNA gene sequencing.
The host bacterium M. aerodenitrificans LH11-4 was cultured in Luria-Bertani (LB) medium at 28°C. Cultures were preserved in glycerol (25%) at −80°C for long-term storage. The genomic DNA of purified M. aerodenitrificans strain LH11-4 was prepared using a bacterial genomic DNA extraction kit (Tiangen Biotech, Beijing, China). The primers 27F and 1492R (see Table S3 in the supplemental material) were used to amplify the 16S rRNA gene of the isolated strain LH11-4 (see Table S2 in the supplemental material). The thermocycling conditions were as follows: 94°C for 5 min; 30 cycles of 94°C for 30 sec, 55°C for 45 sec, and 72°C for 1 min; and a final extension at 72°C for 5 min. Amplified cDNA products were then cloned into the pMD18-T vector (TaKaRa, Dalian, China) and sent for Sanger sequencing.
Transmission electron microscopy.
The dsRNA bacteriophage phiNY particles were extracted from the liquid cultures of M. aerodenitrificans strain LH11-4 after inoculation in LB broth at 28°C. Briefly, the supernatants of liquid cultures were centrifuged twice at 12,000 rpm for 10 min at 4°C and filtered twice through a 0.22-μm filter membrane. phiNY was purified by precipitation with polyethylene glycol (PEG) 8000, followed by sucrose density gradients (20 to 40%) and centrifugation at 30,000 rpm for 2 h at 4°C. In addition, the M. aerodenitrificans strain LH11-4 after inoculation was centrifuged at 2,500 rpm for 15 min. The morphology of phage phiNY and the M. aerodenitrificans strain LH11-4 was examined after negative staining with 2% phosphotungstic acid and using a JEM-2100 transmission electron microscope (Jeol, Japan).
Investigation carrier rate of phiNY in M. aerodenitrificans strain LH11-4.
A total of 93 bacterial colonies were used for the investigate the carrier rate of phiNY in M. aerodenitrificans strain LH11-4 (Fig. 2A). Single colonies were picked when M. aerodenitrificans strain LH11-4 was grown on LB plates at 28°C for 2 days. Then, the phiNY genome was extracted using CF11 cellulose (Sigma) from 93 colonies using the procedure described by Peyambari and Roossinck (12). We successfully isolated nine colonies without phiNY, and the absence of phiNY was checked using reverse transcription-PCR (RT-PCR) with sequence-specific primers (Table S3; Fig. 2B). The thermocycling conditions were as follows: 94°C for 4 min; 30 cycles of 94°C for 30 sec, 55°C for 45 sec, and 72°C for 1 min; and a final extension at 72°C for 5 min.
Infection of M. aerodenitrificans strain LH3 with phiNY.
Early-logarithmic-phase culture of M. aerodenitrificans strain LH3 or LH9 was mixed with purified phiNY and incubated at room temperature for 1.5 h. The mixed cultures were plated on the LB plates at 28°C for 2 days. Then, the presence of phiNY in the colonies was detected using CF11 cellulose (Sigma) followed by gel electrophoresis. The respective phiNY-infected M. aerodenitrificans strains were named LH9-NY and LH3-NY.
Chloroform-treated phiNY.
Chloroform treatment was performed by vigorously shaking a mixture of 0.5 ml purified phiNY and 0.5 ml chloroform for 1 min. The mixture was centrifuged at 12,000 rpm for 5 min. The chloroform-treated phiNY was collected from the aqueous phase. M. aerodenitrificans strain LH3 (optical density at 595 nm [OD595] = 0.5) was mixed with the chloroform-treated phiNY and incubated at room temperature for 1.5 h. The mixed cultures were plated on the LB plates. Then, single colonies were picked when M. aerodenitrificans strain LH3 was grown on LB plates at 28°C for 2 days, and the presence of phiNY was determined using CF11 cellulose followed by gel electrophoresis.
Growth curve experiment.
A total of 1 ml bacterial culture (OD595 = 0.6) was added to 100-ml liquid cultures (n =3). Incubation was continued for 48 h. Samples were taken at different time points to measure the OD595. The growth curve was drawn using GraphPad Prism 5.0 software (Fig. 1E, G, and H).
Reverse transcription–quantitative real-time PCR.
The gene-specific primers for reverse transcription–quantitative real-time PCR (RT-qPCR) (Table S3) were designed to target segments L, M, and S of PhiNY. In addition, the 16S rRNA housekeeping gene primers to detect the reference gene for normalization are also listed in Table S3. Briefly, a total of 3 ml M. aerodenitrificans strain LH11-4 with phiNY was added to 300 ml of LB broth, and the mixture was cultured in an incubator (200 rpm, 28°C). Samples (each 1.5 ml) were taken at the different OD595 (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, and 1.4). All of the samples were centrifuged (12,000 rpm, 20 min), and the pelleted cells were collected. The total RNA of the pelleted cells was isolated and purified using a GeneJet RNA purification kit (Thermo Scientific, Shanghai, China) following the manufacturer’s instructions. Then, the first-strand cDNA was synthesized using the Hifair II 1st Strand cDNA Synthesis SuperMix for qPCR kit (Yeasen Biotechnology, Shanghai, China). Finally, the samples (OD595 = 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, and 1.4) were detected with SYBR green real-time PCR (quantitative PCR [qPCR]) in triplicate on the QuantStudio 3 real-time PCR system (Applied Biosystems, Thermo, China), and the relative gene expression was analyzed using the comparative threshold cycle (2−ΔΔCT) method (17). The curve of relative DNA quantities based on the OD595 of 0.1 was drawn in GraphPad Prism 5.0 software.
Proteomic analysis of phage structural proteins.
Proteomic analysis was performed as previously described (18). Briefly, purified particles were denatured with heat and loaded onto a 15% (wt/vol) polyacrylamide gel. Proteins were stained with Coomassie brilliant blue R250 dye and washed with methanol-acetic acid-H2O. The protein bands that included all the structural proteins were excised from the gel for high-performance liquid chromatography–mass spectrometry (HPLC-MS) analysis. HPLC-MS data were processed using Agilent Spectrum Mill proteomics software to allocate each protein to the corresponding gene.
Bioinformatics analysis.
We digested nucleic acids of phiNY by using RNaseA, DNase I, and S1 nuclease (all from TaKaRa) and performed agarose gel electrophoresis to determine the type of nucleic acid present. The NEBNext Ultra II directional RNA library prep kit (NEB) for Illumina was used for library preparation. Whole-genome sequencing of phiNY was performed using the MiSeq platform (Illumina, San Diego, CA). The genome sequence was assembled using SPAdes v3.13.0 software (19). Clones for the terminal sequences of phiNY were generated by T4 RNA ligase oligonucleotide-mediated amplification with sequence-specific primers (Table S3), as described by Lambden et al. (20, 21). Genome annotation was accomplished and amino acid sequences were derived using the online tool RAST (http://www.rast.nmpdr.org) (15). To manually verify the predicted ORFs, we performed a BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and Conserved Domain analysis (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (22). The alignment analysis of segments L, M, and S was performed with CLC Genomics Workbench 12.0.2 (Qiagen, Hilden, Germany).
The rooted phylogenetic tree based on RNA-dependent RNA polymerase was constructed using the maximum-likelihood method with a bootstrap assessment of ClustalW in MEGA7 software, based on 1,000 replicates and a random seed. The genome and amino acid sequences of RNA-dependent RNA polymerase used to build the tree were downloaded from the NCBI database. The rooted phylogenetic tree based on glycoside hydrolase was constructed using the neighbor-joining method in MAGA7.0 software, based on 1,000 replicates. We constructed a multiple-sequence alignment of their amino acid sequences using MAFFT 7.475 (23). For sequence selection of the phylogenetic tree shown in Fig. 4, ORF17 was compared with the nonredundant (nr) database and virus database (taxonomy identifier 10239) by NCBI BLASTp search, and the sequence with the highest similarity was selected from the results. The tree was embellished using Evolview (24).
Data availability.
The phiNY genomic sequence was deposited in GenBank. The accession numbers for segments L, M, and S are MW471133, MW471134, and MW471135, respectively.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant 31760015 to Tingting Zhang and grants 81672001 and 81621005 to Yigang Tong), by the Guizhou Provincial Natural Science Foundation (grant ZK [2021]081 to Tingting Zhang), and by the Fundamental Research Funds for Central Universities (grant BUCTRC201917 to Yigang Tong).
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Yigang Tong, Email: Tong.yigang@gmail.com.
Shuai Le, Email: leshuai2004@tmmu.edu.cn.
Tingting Zhang, Email: ztt-gd@163.com.
Julie K. Pfeiffer, University of Texas Southwestern Medical Center
REFERENCES
- 1.Dion MB, Oechslin F, Moineau S. 2020. Phage diversity, genomics and phylogeny. Nat Rev Microbiol 18:125–138. 10.1038/s41579-019-0311-5. [DOI] [PubMed] [Google Scholar]
- 2.Olszak T, Latka A, Roszniowski B, Valvano MA, Drulis-Kawa Z. 2017. Phage life cycles behind bacterial biodiversity. Curr Med Chem 24:3987–4001. [DOI] [PubMed] [Google Scholar]
- 3.Stern A, Sorek R. 2011. The phage-host arms race: shaping the evolution of microbes. Bioessays 33:43–51. 10.1002/bies.201000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broniewski JM, Meaden S, Paterson S, Buckling A, Westra ER. 2020. The effect of phage genetic diversity on bacterial resistance evolution. ISME J 14:828–836. 10.1038/s41396-019-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callanan J, Stockdale SR, Shkoporov A, Draper LA, Ross RP, Hill C. 2018. RNA phage biology in a metagenomic era. Viruses 10 10.3390/v10070386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poranen MM, Mantynen S, Ictv Report C. 2017. ICTV virus taxonomy profile: Cystoviridae. J Gen Virol 98:2423–2424. 10.1099/jgv.0.000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Mushegian AR, Nibert ML, Sabanadzovic S, Sanfaçon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Orton RJ, Smith DB, Gorbalenya AE, Davison AJ. 2017. 50 Years of the International Committee on Taxonomy of Viruses: progress and prospects. Arch Virol 162:1441–1446. 10.1007/s00705-016-3215-y. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy SR, Janowski AB, Zhao G, Barouch D, Wang D. 2016. Hyperexpansion of RNA bacteriophage diversity. PLoS Biol 14:e1002409. 10.1371/journal.pbio.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghabrial SA, Caston JR, Jiang D, Nibert ML, Suzuki N. 2015. 50-Plus years of fungal viruses. Virology 479–480:356–368. 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Ghabrial SA, Suzuki N. 2009. Viruses of plant pathogenic fungi. Annu Rev Phytopathol 47:353–384. 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- 11.Hillman BI, Annisa A, Suzuki N. 2018. Viruses of plant-interacting fungi. Adv Virus Res 100:99–116. 10.1016/bs.aivir.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Peyambari M, Roossinck MJ. 2018. Characterizing mycoviruses. Methods Mol Biol 1848:13–24. 10.1007/978-1-4939-8724-5_2. [DOI] [PubMed] [Google Scholar]
- 13.Balijja A, Kvarnheden A, Turchetti T. 2008. A non-phenol-chloroform extraction of double-stranded RNA from plant and fungal tissues. J Virol Methods 152:32–37. 10.1016/j.jviromet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Coplin DL, Van Etten JL, Koski RK, Vidaver AK. 1975. Intermediates in the biosynthesis of double-stranded ribonucleic acids of bacteriophage phi6. Proc Natl Acad Sci U S A 72:849–853. 10.1073/pnas.72.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA, Stevens R, Vonstein V, Wattam AR, Xia F. 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makeyev EV, Grimes JM. 2004. RNA-dependent RNA polymerases of dsRNA bacteriophages. Virus Res 101:45–55. 10.1016/j.virusres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Arocho A, Chen B, Ladanyi M, Pan Q. 2006. Validation of the 2−ΔΔCT calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol 15:56–61. 10.1097/00019606-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Lu S, Shen W, Zhao X, Shen M, Tan Y, Li G, Li M, Wang J, Hu F, Le S. 2016. Characterization of the first double-stranded RNA bacteriophage infecting Pseudomonas aeruginosa. Sci Rep 6:38795. 10.1038/srep38795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Wei D, Jiang D, Fu Y, Li G, Ghabrial S, Peng Y. 2006. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J Gen Virol 87:241–249. 10.1099/vir.0.81522-0. [DOI] [PubMed] [Google Scholar]
- 21.Lambden PR, Cooke SJ, Caul EO, Clarke IN. 1992. Cloning of non-cultivatable human rotavirus by single primer amplification. J Virol 66:1817–1822. 10.1128/JVI.66.3.1817-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. 2020. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48:D265–D268. 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozewicki J, Li S, Amada KM, Standley DM, Katoh K. 2019. MAFFT-DASH: integrated protein sequence and structural alignment. Nucleic Acids Res 47:W5–W10. 10.1093/nar/gkz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH. 2019. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res 47:W270–W275. 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 to S5<br>. Download JVI.00399-21-s0001.pdf, PDF file, 0.2 MB (223.9KB, pdf)
Data Availability Statement
The phiNY genomic sequence was deposited in GenBank. The accession numbers for segments L, M, and S are MW471133, MW471134, and MW471135, respectively.