The Mexican chicken bug (Haematosiphon inodorus), a blood-sucking ectoparasite, may present a new threat to some birds. Golden eagle nestlings parasitized by H. inodorus are in poorer physiological condition and grow more slowly than non-parasitized nestlings. Parasitism increases the probability that nestlings will fledge early, often leading to death.
Keywords: telomere, parasitism, Mexican chicken bug, Haematosiphon inodorus, corticosterone, Aquila chrysaetos
Abstract
Haematophagous ectoparasites can directly affect the health of young animals by depleting blood volume and reducing energetic resources available for growth and development. Less is known about the effects of ectoparasitism on stress physiology (i.e. glucocorticoid hormones) or animal behaviour. Mexican chicken bugs (Haematosiphon inodorus; Hemiptera: Cimicidae) are blood-sucking ectoparasites that live in nesting material or nest substrate and feed on nestling birds. Over the past 50 years, the range of H. inodorus has expanded, suggesting that new hosts or populations may be vulnerable. We studied the physiological and behavioural effects of H. inodorus on golden eagle (Aquila chrysaetos) nestlings in southwestern Idaho. We estimated the level of H. inodorus infestation at each nest and measured nestling mass, haematocrit, corticosterone concentrations, telomere lengths and recorded early fledging and mortality events. At nests with the highest levels of infestation, nestlings had significantly lower mass and haematocrit. In addition, highly parasitized nestlings had corticosterone concentrations twice as high on average (42.9 ng/ml) than non-parasitized nestlings (20.2 ng/ml). Telomeres of highly parasitized female nestlings significantly shortened as eagles aged, but we found no effect of parasitism on the telomeres of male nestlings. Finally, in nests with higher infestation levels, eagle nestlings were 20 times more likely to die, often because they left the nest before they could fly. These results suggest that H. inodorus may limit local golden eagle populations by decreasing productivity. For eagles that survived infestation, chronically elevated glucocorticoids and shortened telomeres may adversely affect cognitive function or survival in this otherwise long-lived species. Emerging threats from ectoparasites should be an important management consideration for protected species, like golden eagles.
Introduction
Parasites can have deleterious effects on animals from diverse life histories and geographic locations (Møller et al., 2009). Parasite virulence varies widely among systems, but as the ranges of parasites shift in response to changes in landscape and climate, naive hosts that lack physiological and behavioural defence mechanisms will be exposed to novel challenges (Cumming and Van Vuuren, 2006; Møller et al., 2013; Cable et al., 2017). Haematophagous ectoparasites survive on blood meals from their hosts and can negatively affect individual survival, particularly of young animals (Merino and Potti, 1995). For altricial bird species, nestlings are particularly vulnerable to the negative effects of haematophagous ectoparasites because they are unable to escape infested nests during a time of high energetic demands, rapid structural growth and maturation of physiological systems (Dube et al., 2018). Understanding how ectoparasites, particularly novel species, influence nestling condition, development and physiology is important for the conservation and management of avian species because costs incurred during growth and development may have long-term negative effects on host survival and population abundance (Møller, 1993; Brown et al., 1995).
The costs of parasitism are important to hosts when they lower reproductive success or survival (Newton, 1998). Haematophagous ectoparasites siphon nutritional resources from hosts by consuming blood meals (Lehmann, 1993) and severe infestations can adversely affect nestling growth, induce anaemia and lead to haemorrhages and muscle weakness (Brown and Brown, 1986; Chapman and George, 1991; Justice-Allen et al., 2016). Further, exposure to ectoparasitism may elicit a physiological stress response leading to elevated corticosterone (Quillfeldt et al., 2004; Eggert et al., 2010). Chronically elevated corticosterone can have significant costs for developing birds, such as reduced growth efficiency (Müller et al., 2009), compromised immune defence (Stier et al., 2009) and decreased cognitive ability (Kitaysky et al., 2003). Finally, the effects of ectoparasitism on nestlings may be reflected in telomeres, the repetitive DNA sequences on the ends of chromosomes. Telomeres can be prematurely shortened by parasitic infections, which could lead to cellular senescence, altered tissue function and adverse effects on organismal health (Asghar et al., 2016). Further, relationships between parasitism and telomere length may be sex specific because of endocrine–immune interactions (Sudyka et al., 2019). Shorter telomeres in early life, as well as faster rates of telomere shortening over time, have been shown to predict longevity (Bize et al., 2009; Heidinger et al., 2012; Tricola et al., 2018) and reproductive success (Pauliny et al., 2006; Eastwood et al., 2019) in different avian species. Therefore, telomere lengths may provide additional information on the effects of ectoparasitism on nestling fitness and survival.
The Mexican chicken bug (Haematosiphon inodorus; Hemiptera: Cimicidae) is an avian haematophagous ectoparasite that affects North American raptor species (Lee, 1955; Platt, 1975; Grubb et al., 1986). Mexican chicken bugs live in nest material and surrounding substrate and require blood meals during every stage of life (Usinger, 1966). McFadzen et al. (1996) reported on the northern range expansion of H. inodorus into southwestern Idaho, but little is known about the distribution and abundance of H. inodorus in relation to nesting raptors in North America. Although few studies have considered the ecological impact of H. inodorus on raptors, McFadzen and Marzluff (1996) found that prairie falcon (Falco mexicanus) nestlings infested by H. inodorus in southwestern Idaho had lower mass and haematocrit levels and experienced mortality as a result of high infestation levels. This finding is similar to what has been reported in other avian species parasitized by cimicids (Brown and Brown, 1986).
Golden eagles (Aquila chrysaetos) are a widespread, but uncommon, large-bodied raptor currently facing threats across their North American range (Kochert and Steenhof, 2002). High densities of golden eagles nest along the cliffs and rocky outcrops near the Snake River in southwestern Idaho (Steenhof et al., 1997). Golden eagle nests often contain a diverse assemblage of ectoparasites (Katzner et al., 2020), and the presence of H. inodorus has been documented in eagle nests in southwestern Idaho (McFadzen et al., 1996), but the physiological consequences of H. inodorus infestation on eagle nestlings have not been studied. Understanding how H. inodorus affect golden eagle nestlings is important for the conservation and management of the species, which faces threats across its range from urbanization and human population growth (Kochert and Steenhof, 2002). Our objective was to assess the effect of H. inodorus infestation on nestling eagle health. Specifically, we examined whether the level of H. inodorus infestation affected nestling mass, haematocrit levels, corticosterone concentrations and telomere lengths. In addition, we assessed the relationship between H. inodorus infestation and the probability of nestlings departing nests before they could fly, often resulting in mortality, or dying in the nest.
Materials and methods
Study area
Our study area was located in southwestern Idaho along the Snake River Canyon in, and adjacent to, the Morley Nelson Snake River Birds of Prey National Conservation Area (NCA; Fig. 1). Steep basalt cliffs in the Snake River Canyon provide ample ledges for golden eagles to construct large stick nests. Golden eagle nesting territories in southwestern Idaho often contain multiple alternative nests (Millsap et al., 2015. The number of nests per territory can range from 1 to 18 with some pairs using the same nest every year, whereas other pairs switch nests within their territory from year to year (Kochert and Steenhof, 2012). The surrounding uplands in the NCA consist of native shrub-steppe and salt-desert communities characterized by sagebrush-dominated shrublands, native perennial grasses, disturbed grasslands and rangeland dominated by exotic annual grasses, irrigated agricultural land and rural and suburban development (U.S. Department of the Interior, 1996).
Figure 1.
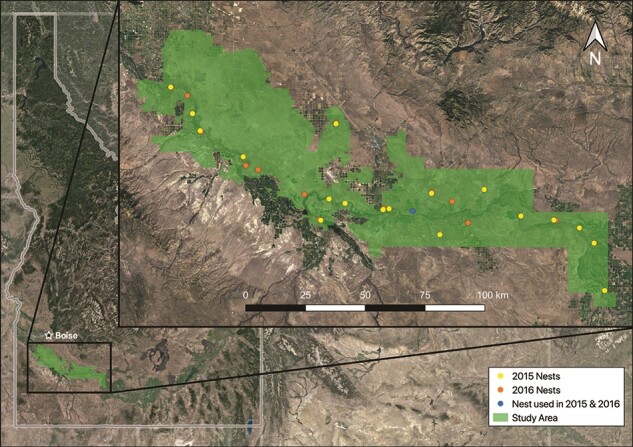
Location of study area (represented by green polygon) in southwestern Idaho, USA. Yellow circles represent golden eagle nests sampled in 2015; orange circles, golden eagle nests sampled in 2016; and the single blue circle, a nest used in both 2015 and 2016.
Field techniques
In 2015 and 2016, we monitored 35 historical golden eagle nesting territories to determine occupancy and breeding attempts following the methods described in Steenhof et al. (1997). We made regular nest observations from >400 metres and visually assessed nestling age based on feather development (Driscoll, 2010). We began visiting nests every 8–10 days when nestlings were able to thermoregulate (ca. 21 days old; Katzner et al., 2020) until nestlings were ~51 days old (typically 3 or 4 visits). After nestlings reached 51 days, we monitored nestlings from remote observation points every 3–5 days to determine survival and age at fledging. If fledging was not directly observed, fledging age was estimated as the average between the last date the nestling was observed in the nest and the first date the nestling was not observed in the nest. If a nestling left the nest before they were 51 days old (80% of average fledging age; Steenhof et al., 2017), we categorized that nestling as an ‘early’ fledgling because typically these birds were not able to fly, and we searched the area underneath the nest for recently fledged young or carcasses. We continued to monitor recent fledglings until they moved away from the immediate nest area (~400 metres), at which point determining their location and fate became increasingly difficult.
During each nest visit, we visually assessed the abundance of adult and nymph H. inodorus present on the nest surface and on nestlings for 4–8 minutes and grouped infestation levels into three categorical ranks: (1) no infestation, no bugs observed in the nest material or on nestlings; (2) low infestation. 1–100 bugs observed in total; or (3) high infestation, >100 bugs observed. In a related study, these infestation categories correlated with quantitative estimates of H. inodorus density made from pit fall traps placed in the nest (Dudek, 2017). We recorded nestling mass using a Pesola spring scale during each visit and categorized the fullness of nestlings’ crops into quartiles. We collected blood samples from the brachial vein when nestlings were ~28 and 49 days old with a 25-gauge needle and syringe. We collected blood for haematocrit and genetic sexing in both years and collected blood for corticosterone in 2015 and telomeres in 2016. During blood-collection visits, we used a cloth sack to transport nestlings from the nest to the top of the cliff where we worked in teams to collect blood. In 2015, we recorded the duration of time between our first contact with nestlings and the completion of blood sampling to account for the researcher-induced stress response on corticosterone in statistical models (Romero and Reed, 2005) and attempted to collect samples within 20 minutes. At some nests we were unable to collect blood within 20 minutes and we did not measure corticosterone. Blood samples for corticosterone assays were collected into 0.8 ml lithium heparin mini centrifuge tubes. For haematocrit samples, blood was aliquoted into two heparinized 75 mm capillary tubes per nestling. In 2016, we used the packed blood cells from haematocrit samples to measure telomere lengths. All blood samples were kept on ice in the field until processing at Boise State University on the same day of sampling. We centrifuged haematocrit samples for 6 minutes at 11000 × g and measured the proportion of packed blood cells relative to whole blood. Haematocrit levels were represented by the average of the two samples. For the corticosterone samples, we centrifuged blood samples at 6048 × g for 10 minutes to separate plasma from the cellular fraction of whole blood and stored plasma samples and cells separately at −20°C. We stored whole blood cells in lysis buffer for telomere analysis in 2016 and to determine nestling sex with single-nucleotide polymorphism genotyping (Doyle et al., 2016) at Purdue University (West Lafayette, IN, USA; 2015 samples) or by Avian Biotech International (Tallahassee, FL, USA; 2016 samples). All field methods followed protocols approved by the Boise State University Institutional Animal Care and Use Committee (Protocol #006-AC14-007).
Corticosterone assay
We used enzyme-linked immunosorbent assays (ELISA, Cayman Chemicals) to estimate corticosterone concentrations in nestling plasma. We divided each plasma sample into 30-μl duplicates then twice extracted corticosterone using 5 ml of diethyl ether. We poured off the lipophilic supernatant and evaporated it under a stream of nitrogen gas in a warm water bath. Following kit instructions, we reconstituted extracted samples with 100 μl of buffer, vortexed and divided into 50-μl aliquots that were added to 96-well plates coated with mouse monoclonal antibody. We added corticosterone-specific acetylcholinesterase tracer and rabbit corticosterone antiserum to the 96-well plates and then incubated plates on an orbital shaker for 2 hours. We then rinsed plates to remove any non-bound corticosterone and developed them in a dark chamber with Ellman’s reagent for 1 hour. We read plates at 405 nm with a Biotek EL800 plate reader. We validated the corticosterone assay by comparing the slopes of a plasma dilution curve to the assay standard curve slope. Plasma dilution curves consisted of different ratios of pooled eagle plasma to buffer. There was no significant difference between the slopes of the plasma dilution and assay standard curve. We calculated the concentration of corticosterone in samples by comparing results to a standard curve established with known concentrations. We determined extraction efficiency by analysing a standard corticosterone sample and calculated inter-assay variation from repeated values of a pooled sample. We corrected all values for assay extraction efficiency (mean ± SD) 88.0 ± 9.4%. Inter-assay variation averaged 5.4%, and average intra-assay variation was 2.7%.
Telomere assay
We extracted DNA using the Zymo Quick-DNA Microprep Plus Kit (#D4074) according to the manufacture’s protocol. We assessed DNA purity by 260/280 nm absorbance ratio (NanoDrop). We quantified DNA by absorbance at 260 nm, and the concentration was normalized to 2 ng/μl in an elution buffer containing 10 mM Tris-HCl pH 8.5 and 0.1 mM EDTA (Zymo). We performed Quantitative PCR (qPCR) using a Roche LC96. We performed PCR reactions in 20-μl volumes containing ~8 ng DNA, 10 μl of 2x Biotium Fast Plus EvaGreen® qPCR Master Mix and 10 pmol each of forward and reverse primers (500 nM final primer concentration). A standard curve for each primer pair was included in triplicate on each plate. We prepared the standard curve by seven serial dilutions (1∶4) of DNA. We used the standard curve to determined DNA concentrations of each sample using both the reference and telomere primers. We quantified telomere lengths as the ratio of the DNA concentration determined with the telomere primers divided by the average of the concentrations determined from the two reference primers (Hudon et al., 2020; Table 1). Telomere primers are previously published (Cawthon, 2002). For all primers, we used a two-step thermal cycling profile of 95°C for 2 minutes, followed by 40 cycles of 95°C for 5 seconds and 55°C for 30 seconds, with signal acquisition at the end of the 55°C step, followed by a melt curve generated by increasing temperatures at the end of the thermal cycling. We ran all samples in triplicate along with three negative controls per primer pair. If the variation between technical replicates (Ct standard deviation) was greater than 0.5, we repeated the assay for that sample.
Table 1.
Reference gene and telomere primer sequences used to estimate the length of telomere-DNA sequences of golden eagle nestlings in southwestern Idaho, USA, in 2016
| Name | Sequence |
|---|---|
| tel 1b | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT |
| tel 2b | CAGCCGAAAGGCCCTTGGCAGGAGGGCTGCTGGTGGTCTACCCTT |
| UCE.28-F | AAATACCACCCAACAGTTT |
| UCE.28-R | AAGCCCTATACAGATGGAT |
| UCE.239-F | TCAGATGTTCAGCCTATT |
| UCE.239-R | AATACCATGTTAATTATCCTCAA |
Statistical analysis
We used linear mixed models (LMMs) to examine the effects of H. inodorus infestation on nestling mass, haematocrit, corticosterone concentrations, telomere length and the probability that nestlings would fledge early or die. We included nest identity as a random variable in all models and nestling identity as a random variable in the nestling mass, haematocrit, corticosterone concentrations and telomere length models. Sampling year was included as a random variable in the nestling mass, haematocrit and the probability that nestlings would fledge early or die models that were sampled in 2015 and 2016, but not for the corticosterone or telomere length models that were only measured in 1 year. We treated H. inodorus infestation level as a category to account for possible non-linear effects of infestation of physiology, with the exception of the model to explain whether nestlings fledged early or died where H. inodorus infestation level was treated as a numerical rank. To examine effects on nestling mass and haematocrit, we used the following fixed effects: H. inodorus infestation level at the time of sampling, age (in days, linear and polynomial), sex, the interaction between infestation level and age and the interaction between infestation level and sex. Before modelling, we adjusted mass for crop contents using a sex- and age-specific crop size equation to estimate the mass of crop contents (Collopy, 1984) and then subtracted crop mass from measured body mass. We used a pairwise comparison to evaluate the mean differences between each infestation level. We used an LMM to predict the effect of age, sex, time of day, handling time (minutes), infestation level at the time of sampling and the interaction between infestation level and handling time on nestling corticosterone concentrations. We used an LMM to examine whether age, sex, infestation level at the time of sampling or interactions among these factors, explained telomere length. Finally, we created a generalized LMM with a binomial distribution to predict the probability that nestlings in highly infested nests fledged early or died. We used the infestation level when nestlings were approximately 28 days old (mean 28.5 ± 3.1 days) for this analysis because we did not have infestation levels at the time nestlings died or fledged early. No nestlings died or left the nest before 28 days, so the infestation level at 28 days could be applied across all birds in our study. Finally, we compared whether there were differences in mass, haematocrit and telomere length for nestlings that fledged early or died and nestlings that fledged successfully at >51 days with an LMM that included nestling identity, nest and year (for mass and haematocrit) as a random effect. We did not make this comparison for CORT because we had a low sample size for the fledged early or died group. All analyses were performed in R (version 4.0.0, R Core Team, 2020) and linear models were created using functions lmer and glmer in the package lme4 (Bates et al., 2015). Interactions were removed if they were not significant. Pairwise comparisons were conducted using the function lsmeans in the package lsmeans (Lenth, 2016). Descriptive statistics are reported as mean ± standard deviation and confidence intervals (CIs) are reported at 95%.
Results
We measured H. inodorus infestation levels at occupied nests in 19 and 16 golden eagle territories in 2015 and 2016, respectively. Ten territories were sampled in both years (different nests in each year) and one nest was used 2 years in a row and thus was sampled in both years. Of the 35 nests sampled, 23% (n = 8) experienced no H. inodorus infestation, 46% (n = 16) experienced low levels of infestation and 31% (n = 11) experienced high levels of infestation.
We measured nestling mass from 31 eagle nestlings in 2015 and 26 nestlings in 2016. There was a significant interaction between age and H. inodorus infestation rank on mass (χ2 = 13.50, P < 0.01; Fig. 2) and females were significantly heavier than males (χ2 = 73.71, P < 0.01). Nestlings in the no-infestation and low-infestation groups tended to gain mass as they grew (all 95% CI for effect of age positive) with the rate of mass gain tapering as they reached mature sizes (Fig. 2); however, nestlings in the low-infestation group had significantly lower rate of mass gain compared to the no infestation group (Table A1). Nestlings in the high-infestation group had a lower rate of mass gain compared to the no-infestation group (Table A1), and in females, the 95% CIs for effect of age overlapped zero suggesting that female nestlings may not gain mass during growth in this group (Fig. 2). There was no interaction between age, the level of H. inodorus infestation and sex (interaction term χ2 = 0.27, P = 0.87).
Figure 2.
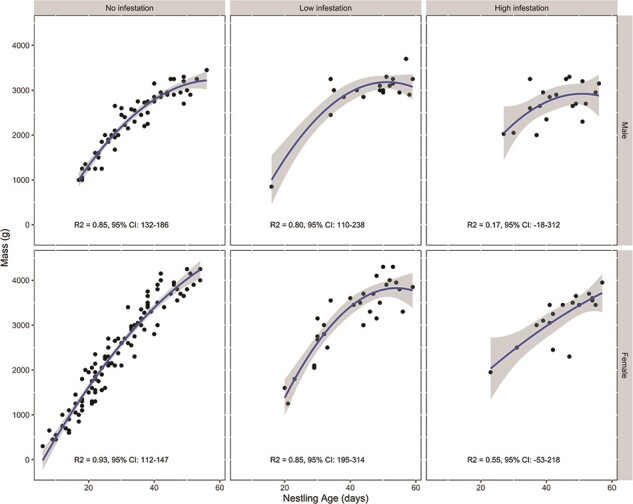
Golden eagle nestling mass (black circles), predicted mass (blue line) and associated 95% CIs (solid grey area) measured from nestlings experiencing different levels of H. inodorus infestation in nests in southwestern Idaho, USA, in 2015 and 2016. The R2 and 95% CIs for the effect of age on mass is shown at the bottom of each grid cell. Nestlings in the low- and high-infestation group gained mass more slowly as they developed compared to nestlings in the no-infestation group (χ2 = 13.5, P < 0.01).
We measured haematocrit from 24 nestlings in 2015 and 26 nestlings in 2016. Mean nestling haematocrit over both seasons was 0.29 ± 0.06 (range, 0.12–0.54). Nestlings from nests with high levels of H. inodorus infestation had significantly lower haematocrit than nestlings from non-infested nests or nests with low levels of infestation (χ2 = 19.2, P < 0.01; Fig. 3; Table A2). Haematocrit did not vary by nestling age (χ2 = 1.05, P = 0.30) or sex (χ2 < 0.1, P = 0.97).
Figure 3.
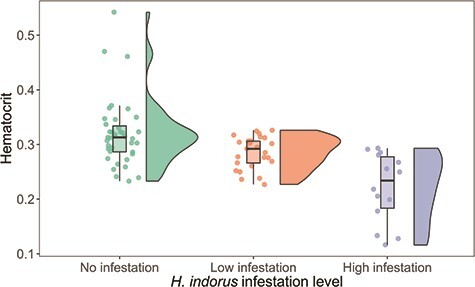
Haematocrit measured from golden eagle nestlings experiencing different levels of H. inodorus infestation in nests in southwestern Idaho, USA, in 2015 and 2016. Bold lines within boxes represent the median, upper and lower limits of the box are the first and third quartiles, whiskers contain 1.5 times the interquartile range and the coloured circles represent each measured value. The width of the shaded area in the violin plot represents the distribution of haematocrit values. Nestling haematocrit decreased in highly infested nests (χ2 = 25.1, P < 0.01). Asterisk indicates a statistically different group.
Circulating corticosterone concentrations were measured from 26 nestlings in 2015. Nestling corticosterone concentrations averaged 22.29 ± 15.17 ng/ml (range, 3.70–80.65 ng/ml). Corticosterone concentrations were significantly higher for nestlings that experienced high H. inodorus infestation compared to nestlings from nests with either no infestation or low infestation (χ2 = 26.7, P < 0.01; Fig. 4; Table A3). Nestling handling time was 9.7 ± 3.2 minutes (range, 4.0–19.0). Nestling corticosterone concentrations increased with handling time (β = 2.53, 95% CI = 1.23, 3.78, χ2 = 17.5, P < 0.01), but there was no significant interaction between handling time and infestation level (χ2 = 2.6, P = 0.28), suggesting that infestation category did not influence the acute stress response. We found no effect of nestling age (χ2 = 0.4, P = 0.51), sex (χ2 < 0.1, P = 0.90) or time of day (χ2 = 0.3, P = 0.60) on corticosterone concentrations.
Figure 4.
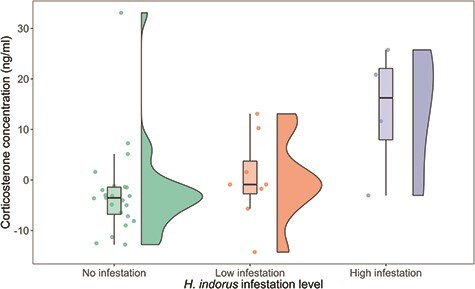
Residual corticosterone concentrations from a model that included handling time (minutes) for golden eagle nestlings experiencing different levels of H. inodorus infestation in nests in southwestern Idaho, USA, in 2015. Bold lines within boxes represent the median, upper and lower limits of the box are the first and third quartiles, whiskers contain 1.5 times the interquartile range and the coloured circles represent each measured value. The width of the shaded area in the violin plot represents the distribution of residual corticosterone concentration. Nestling corticosterone concentrations (ng/ml) increased in highly infested nests (χ2 = 21.1, P < 0.01). Asterisk indicates a statistically different group.
We quantified telomere lengths for 26 nestlings in 2016. Telomere lengths ranged from 0.11 to 1.22. We found a significant interaction between sex, age and infestation level on telomere lengths (χ2 = 8.9, P < 0.01). Telomeres of female nestlings significantly shortened with age in highly infested nests, whereas telomeres of female nestlings did not change in nests with either no infestation or low infestation nests. Male nestling telomere length did not change with age or infestation level (Fig. 5; Table A4).
Figure 5.
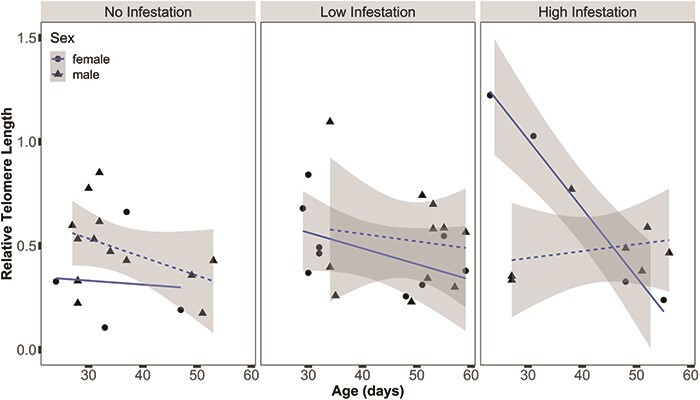
Observed golden eagle nestling telomere lengths (female, black circles; male, black triangles), predicted length (female, solid blue line; male, dotted blue line) and associated 95% CIs (solid grey area) measured from nestlings experiencing different levels of H. inodorus infestation in nests in southwestern Idaho, USA, in 2016. Female nestling telomere lengths decreased in highly infested nests (χ2 = 8.9, P = 0.01). For females in the high-infestation group, R2 = 0.98; for all other groups with no relationship over time, R2 < 0.1.
We used data from 29 nestlings in 2015 and 25 nestlings in 2016 to assess effect of H. inodorus on the probability of nestlings leaving the nest before 51 days of age or dying in, or below, the nest. Nestlings in our study left the nest at 62 ± 8 days (range, 42–74 days) on average. About a quarter of the nestlings, 22% (12/54), died in the nest prior to fledging, left the nest before 51 days old (45 ± 3 days) or fell out of the nest and died shortly after reaching fledging age. Three nestlings were found dead in the nest prior to fledging, seven nestlings fledged early, five of which were found dead below the nest and two ‘fledge-age’ nestlings (aged 55 days and 56 days) fell out of the nest and were found dead below the nest. We found that H. inodorus infestation level at a nest was positively associated with the probability that nestlings left the nest early or died (β = 25.8, 95% CI = 7.6, 44.2; χ2 = 7.76, P < 0.01; Table 2, Fig. 6). There was no significant difference in mass between nestlings that left the nest early or died (Fig. A1; χ2 = 0.7, P = 0.41). Nestlings that left the nest early or died had lower haematocrit than nestlings that lived and fledged at a later age (Fig. A2; χ2 = 9.9, P < 0.01). Corticosterone concentrations of nestlings that left the nest early or died were higher (64.2 ± 11.2 ng/ml, n = 2) compared to corticosterone concentrations of nestlings that lived and fledged at a later age (19.7 ± 11.3 ng/ml, n = 25). There was no significant difference in telomere lengths between eagles that lived and eagles that died or left the nest <51 days (Fig. A3; χ2 = 0.2, P = 0.64).
Table 2.
The count and percentage of golden eagle nestlings that fledged from the nest before 51 days or died for each rank of H. inodorus infestation in southwestern Idaho, USA, in 2015 and 2016
| Nestlings that fledged early or died in the nest | |||
|---|---|---|---|
| Infestation level | Total number of nestlings | Number | Percentage |
| No infestation | 42 | 3 | 7% |
| Low infestation | 7 | 6 | 86% |
| High infestation | 5 | 5 | 100% |
Figure 6.

A golden eagle nestling, ~56 days old, falling to its death from a nest highly infested with H. inodorus in southwestern Idaho, USA in 2015. This photo was captured by a motion-activated camera installed on the cliff wall adjacent to the nest.
Discussion
Ectoparasitism by H. inodorus had a detrimental effect on golden eagle nestling health and survival, suggesting H. inodorus may be an emerging or understudied threat to golden eagles in southwestern Idaho. The effects of higher levels of H. inodorus infestation on nestling mass and haematocrit suggest that H. inodorus ectoparasitism created an energetically expensive cost to nestling development and physical condition. Additionally, corticosterone concentrations were higher in nestlings exposed to high levels of H. inodorus infestation and telomere lengths of female nestlings were significantly shorter in highly infested nests, suggesting that H. inodorus ectoparasitism was physiologically stressful. Finally, H. inodorus infestations were positively associated with early fledging, which can lead to death for young birds leaving cliff nests before they are capable of flight, and mortality prior to fledging. We found 22% of eagle nestlings in our study fledged early or died prior to fledging in nests with high levels of H. inodorus infestation. Taken together, these results indicate that H. inodorus infestation contributed to reduced productivity through nestling mortality and may have lasting effects on young eagles that survive the nestling phase.
High ectoparasite infestations can negatively affect fitness-related traits in birds (Møller, 1990; Hurtrez-Boussès et al., 1997; Heeb et al., 2000). There is a positive relationship between nestling mass (body condition) and juvenile survival (Arizaga et al., 2015; Jones et al., 2017), so events that birds experience as nestlings may have long reaching effects. Similar to other studies (e.g. Whitworth and Bennett, 1992; Hurtrez-Boussès et al., 1997), we found that nestling haematocrit decreased in highly infested nests, suggesting a direct effect of ectoparasitism on nestling condition. The reported haematocrit range for healthy raptors is 0.35–0.55 (Monks and Forbes, 2007); however, nestlings typically have lower haematocrit values than adults (reviewed in Fair et al., 2007). In our study, nestling haematocrit ranged from 0.12 to 0.54 and high levels of H. inodorus ectoparasitism were associated with the lowest haematocrit values.
Anaemia and metabolic stress can cause immunosuppression and increase the susceptibility of birds to pathogenic agents (Folstad and Karter, 1992). During development, nestling birds make trade-offs between growth, survival and immune function (Brommer, 2004). Although H. inodorus is not a known vector of any pathogen, the closely related swallow bug (Oeciacus vicarius) is a vector for the Buggy Creek virus (Togaviridae: Alphavirus) and transmits the pathogen to cliff swallows (Petrochelidon pyrrhonota; Fassbinder-Orth et al., 2013). Additionally, Justice-Allen et al. (2016) reported that parasitism by Argasid ticks caused paresis and ataxia in bald eagle (Haliaeetus leucocephalus) nestlings. Avian tick paralysis is caused by neurotoxins associated with many tick species (Luttrell et al., 1996). Although ectoparasitism decreased the physiological condition of nestlings, we noted no other abnormal effects of H. inodorus ectoparasitism on eagle nestlings and repeated biting did not appear to cause additional symptoms. Further study is needed to evaluate the potential role of H. inodorus as a vector of arboviruses and other diseases.
Our study is the first to report circulating corticosterone concentrations for golden eagles and we validated the use of ELISA assays on eagle plasma. Our results show that the intensity of H. inodorus infestation influences corticosterone concentrations in eagle nestlings and suggest that glucocorticoids act as a mechanism to overcome the effects of ectoparasitism. Ectoparasitism has been linked to elevated corticosterone concentrations in some bird species (Quillfeldt et al., 2004; Raouf et al., 2006), but not others (Lobato et al., 2008; Eggert et al., 2010; Pryor and Casto, 2015), suggesting that an adrenal response to ectoparasitism may depend on the ability of the host species to cope with the parasitism event (St. Juliana et al., 2014). Interspecific differences in stress response to ectoparasitism demonstrate the importance of investigating corticosterone response in raptor species. The pattern of exploitation by the ectoparasite and the history of association between the host and ectoparasite may explain differences in host responses. Host species that co-evolve with parasite species may exhibit less of a stress response due to acquired physiological defence mechanisms (Khokhlova et al., 2008). Conversely, relatively new host–parasite relationships may allow parasites to exert a comparatively strong negative effect on their hosts (Koop et al., 2013). Therefore, the stress response elicited by golden eagle nestlings in our study to H. inodorus infestation may be related to the relatively recent arrival of H. inodorus in southwestern Idaho.
Chronically elevated corticosterone caused by ectoparasitism could be deleterious to the survival of eagle nestlings by reducing immune function (Wingfield et al., 1997) and cognitive capabilities (Kitaysky et al., 2003). Corticosterone has been found to stimulate activity in numerous avian species including dispersal in juvenile western screech-owls (Megascops kennicottii; Belthoff and Dufty, 1998), pre-migratory restlessness in red knots (Calidris canutus; Piersma et al., 2000) and migratory flights of bar-tailed godwits (Limosa lapponica; Landys-Ciannelli et al., 2002), and nestling corticosterone concentrations have been shown to increase prior to fledging (Heath, 1997; Corbel and Groscolas, 2008). Given the positive effect that corticosterone has on movement (Breuner et al., 1998), it is possible that increased corticosterone concentrations, resulting from high H. inodorus infestation, may have facilitated fledging attempts before nestlings were able to fly. A controlled study that accounts for confounding effects of reduced provisioning near the time of fledging (Bustamanté and Hiraldo, 1990), energetic deficits caused by parasites and other stressors would be necessary to test this hypothesis. Unfortunately, we did not have these conditions or the sample size to evaluate this relationship.
In our study, we recorded nine instances of early or unsuccessful fledging from highly infested nests, 78% of which resulted in mortality. Early fledging from nests with high H. inodorus infestations has been documented in prairie falcons (McFadzen and Marzluff, 1996) and golden eagles (Morales-Yañez and Rodríquez-Estrella, 2019). Increased movements within a cliff nest’s limited area may increase the risk for individuals experiencing deteriorating health conditions. At one nest in 2015, a 56-day-old nestling, previously observed to be in poor physical condition, was documented falling off the edge of a highly infested nest to its death. Furthermore, decreased cognitive ability as a result of high corticosterone concentrations can reduce foraging ability of recently fledged birds, which could increase the likelihood of post-fledging mortality due to starvation (Kitaysky et al., 2003). Although we do not know the long-term fate of all nestlings in our study, we observed that some nestlings from highly infested nests survived to fledge and disperse from their natal territory. The long-term effects of low mass and haematocrit on the survival of fledgling eagles are unknown and warrant further study.
We found that the telomeres of female nestlings, but not males, shortened with age when exposed to high infestations of ectoparasites. Sex-specific effects of parasites on telomere dynamics has also been observed in Eurasian blue tits (Cyanistes caeruleus) and may reflect hormonal mechanisms that mediate the response to parasitic infections in male birds (Klein, 2004; Sudyka et al., 2019). Alternatively, eagles exhibit reverse sexual-size dimorphism and the high cost of infestation on growth rates (mass gain) may have been more severe for larger female nestlings.
Given the costs of ectoparasitism to nestling condition, development and survival, it is important to understand the physiological response to ectoparasite infestation. When avian populations are stressed by habitat reduction or alteration, the individual response to haematophagous ectoparasites may compound negative population-level effects (Loye and Carroll, 1998). North American golden eagle populations, like many wildlife populations worldwide, continue to face threats from habitat loss and the reduction of their historical prey populations (Kochert and Steenhof, 2002). Our study was not an experimental study and therefore we cannot rule out that H. inodorus infestation levels are correlated with other factors that may explain the physiological effects we observed. Increased monitoring and study of the effects of ectoparasitism on the condition and survival of young animals through experimental studies, especially in systems experiencing shifts in parasite distributions and the introductions of novel parasite species associated with climate change, will be important for future conservation efforts of imperilled species.
Funding
This work was supported by the U.S. Fish and Wildlife Service Western Golden Eagle Team (Project numbers F15AP00200 and F16AC00242), Bureau of Land Management (Project number L14AC00342), Boise State University Raptor Research Center, a National Science Foundation Research Experiences for Undergraduates award (DBI-1263167 to M.T.H.) and an National Science Foundation Track 2 EPSCoR Program award (OIA-1826801).
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgements
We thank Mike Kochert and Karen Steenhof for advice and guidance in designing this project and sharing research experience in our study areas. We acknowledge the USGS Snake River Field Station for providing historical golden eagle nesting data from the NCA and surrounding areas. We thank Ian Robertson for insights about ectoparasite biology. We thank Teresa Ely, Caitlin Davis, Erin Arnold, Jordan Harrison, Steve Crane and Joe Weldon for assistance and support in the field. Mike Kochert and two anonymous reviewers provided helpful comments on an earlier draft.
REFERENCES
- Arizaga J, Herrero A, Aldalur A, Cuadrado JF, Oro D (2015) Effect of pre-fledging body condition on juvenile survival in yellow-legged gulls Larus michahellis. Acta Ornithol 50: 139–147. [Google Scholar]
- Asghar M, Palinauskas V, Zaghdoudi-Allan N, Valkiūnas G, Mukhin A, Planonova E, Färnert A, Bensch S, Hasselquist D (2016) Parallel telomere shortening in multiple body tissues owing to malaria infection. Proc R Soc B 283: 1–7. 10.1098/rspb.2016.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- Belthoff JR, Dufty AM (1998) Corticosterone, body condition and locomotor activity: a model for dispersal in screech-owls. Anim Behav 55: 405–415. [DOI] [PubMed] [Google Scholar]
- Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B 276: 1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC (1998) Non-invasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen Comp Endocrinol 111: 386–394. [DOI] [PubMed] [Google Scholar]
- Brommer JE (2004) Immunocompetence and its costs during development: an experimental study in blue tit nestlings. Proc R Soc Lond B 271: 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Brown MB (1986) Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67: 1206–1218. [Google Scholar]
- Brown CR, Brown MB, Rannala B (1995) Ectoparasites reduce long-term survival of their avian host. Proc R Soc Lond 262: 313–319. [Google Scholar]
- Bustamanté J, Hiraldo F (1990) Factors influencing family rupture and parent-offspring conflict in the black kite Milvus migrans. Ibis 132: 58–67. [Google Scholar]
- Cable J, Barber I, Boag B, Ellison AR, Morgan ER, Murray K, Pascoe EL, Sait SM, Wilson AJ, Booth M (2017) Global change, parasite transmission and disease control: lessons from ecology. Phil Trans R Soc B 372: 1–17. 10.1098/rstb.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30: e47. 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, George J (1991) The effects of ectoparasites on cliff swallow growth and survival. In JE Loye, M Zuk, eds, Bird-Parasite Interactions: Ecology, Evolution and Behavior. Oxford University Press, Oxford, United Kingdom, pp. 69–92. [Google Scholar]
- Collopy MW (1984) Parental care and feeding ecology of golden eagle nestlings. Auk 101: 753–760. [Google Scholar]
- Corbel H, Groscolas R (2008) A role for corticosterone and food restriction in the fledging of nestling white storks. Horm Behav 53: 557–566. [DOI] [PubMed] [Google Scholar]
- Cumming GS, Van Vuuren DP (2006) Will climate change affect ectoparasite species ranges? Glob Ecol Biogeogr 15: 486–497. [Google Scholar]
- Doyle JM, Katzner TE, Roemer GW, Cain IIIJW, Millsap BA, McIntyre CL, Sonsthagen SA, Fernandez NB, Wheeler M, Bulut Z et al. (2016) Genetic structure and viability selection in the golden eagle (Aquila chrysaetos), a vagile raptor with a Holarctic distribution. Conserv Genet 17: 1307–1322. [Google Scholar]
- Driscoll DE (2010) Protocol for Golden Eagle Occupancy, Reproduction, and Prey Population Assessment. American Eagle Research Institute, Apache Junction, Arizona, USA, p. 55. [Google Scholar]
- Dube WC, Hund AK, Turbek SP, Safran RJ (2018) Microclimate and host body condition influence mite population growth in a wild bird-ectoparasite system. Int J Parasitol Parasites Wildl 7: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek BM (2017) The role of disease and ectoparasites in the ecology of nestling golden eagles. Theses and Dissertations. Boise State University. 10.18122/B2BH8X. [DOI]
- Eastwood JR, Hall ML, Teunissen N, Kingma SA, Aranzamendi NH, Fan M, Roast M, Verhulst S, Peters A (2019) Early-life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Mol Ecol 28: 1127–1137. [DOI] [PubMed] [Google Scholar]
- Eggert LM, Jodic PG, O’Reilly KM (2010) Stress response of brown pelican nestlings to ectoparasite infestation. Gen Comp Endocrinol 166: 33–38. [DOI] [PubMed] [Google Scholar]
- Fair J, Whitaker S, Pearson B (2007) Sources of variation in haematocrit in birds. Ibis 149: 535–552. [Google Scholar]
- Fassbinder-Orth CA, Barak VA, Brown CR (2013) Immune responses of a native and an invasive bird to Buggy Creek Virus (Togaviridae: Alphavirus) and its arthropod vector, the swallow bug (Oeciacus vicarius). PLoS One 8: 1–7. 10.1371/journal.pone.0058045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139: 603–622. [Google Scholar]
- Grubb TW, Eakle L, Tuggle BN (1986) Haematosiphon inodorus (Hemiptera: Cimicidae) in a nest of a bald eagle (Haliaeetus leucocephalus) in Arizona. J Wildl Dis 22: 125–127. [DOI] [PubMed] [Google Scholar]
- Heath JA (1997) Corticosterone levels during nest departure of juvenile American kestrels. Condor 99: 806–811. [Google Scholar]
- Heeb P, Kölliker M, Richner H (2000) Bird-ectoparasite interactions, nest humidity, and ectoparasite community structure. Ecology 81: 958–968. [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109: 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon SF, Hurtado EP, Beck JD, Burden SJ, Bendixsen DP, Callery KR, Forbey JS, Waits LP, Miller RA, Nielsen ÓK et al. (2020) Primers to highly conserved elements optimized for qPCR-based telomere length measurements in vertebrates. Mol Ecol Resour 21: 59–67. [DOI] [PubMed] [Google Scholar]
- Hurtrez-Boussès S, Perret P, Renaud F, Blondel J (1997) High blowfly parasitic loads affect breeding success in a Mediterranean population of blue tits. Oecologia 112: 514–517. [DOI] [PubMed] [Google Scholar]
- Jones TM, Ward MP, Benson TJ, Brawn JD (2017) Variation in nestling body condition and wing development predict cause-specific mortality in fledgling dickcissels. J Avian Biol 48: 439–447. [Google Scholar]
- Justice-Allen A, Orr K, Schuler K, McCarty K, Jacobson K, Meteyer C (2016) Bald eagle nestling mortality associated with Argas radiatus and Argas ricei tick infestation and successful management with nest removal in Arizona, USA. J Wildl Dis 52: 940–944. [DOI] [PubMed] [Google Scholar]
- Khokhlova IS, Ghazaryan L, Krasnov BR, Degen AA (2008) Effects of parasite specificity and previous infestation of hosts on the feeding and reproductive success of rodent-infesting fleas. Func Ecol 22: 530–536. [Google Scholar]
- Kitaysky A, Kitaiskaia E, Piatt J, Wingfield JC (2003) Benefits and costs of increased levels of corticosterone in seabird chicks. Horm Behav 43: 140–149. [DOI] [PubMed] [Google Scholar]
- Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26: 247–264. [DOI] [PubMed] [Google Scholar]
- Kochert MN, Steenhof K (2002) Golden eagles in the U.S. and Canada: status, trends, and conservation challenges. J Raptor Res 36: 32–40. [Google Scholar]
- Katzner TE, Kochert MN, Steenhof K, McIntyre CL, Craig EH, Miller TA. (2020) Golden eagle (Aquila chrysaetos), version 2.0. In PG Rodewald, BK Keeney, eds. Birds of the World. Cornell Lab of Ornithology, Ithaca, NY, USA. 10.2173/bow.goleag.02 (date last accessed, 15 January 2021). [DOI] [Google Scholar]
- Kochert MN, Steenhof K (2012) Frequency of nest use by golden eagles in southwestern Idaho. J Raptor Res 46: 239–247. [Google Scholar]
- Koop JAH, Owen JP, Knutie SA, Aguilar MA, Clayton DH (2013) Experimental demonstration of a parasite-induced immune response in wild birds: Darwin’s finches and introduced nest flies. Ecol Evol 3: 2514–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys-Ciannelli MM, Ramenofsky M, Piersma T, Jukema J, Wingfield JC (2002) Baseline and stress-induced plasma corticosterone during long-distance migration in the bar-tailed godwit, Limosa lapponica. Physiol Biochem Zool 75: 101–110. [DOI] [PubMed] [Google Scholar]
- Lee RD (1955) The biology of the Mexican chicken bug, Haematosiphon inodorus (Dugès). Pan-Pac Entomol 31: 47–61. [Google Scholar]
- Lehmann T (1993) Ectoparasites: direct impact on host fitness. Parasitol Today 9: 8–13. [DOI] [PubMed] [Google Scholar]
- Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69: 1–33. [Google Scholar]
- Lobato E, Merino S, Moreno J, Morales J, Tomás G, Martínez de la Puente J, Osorno JL, Kuchar A, Möstl E (2008) Corticosterone metabolites in blue tit and pied flycatcher droppings: effects of brood size, ectoparasites and temperature. Horm Behav 53: 295–305. [DOI] [PubMed] [Google Scholar]
- Loye J, Carroll S (1998) Ectoparasite behavior and its effects on avian nest site selection. Ann Entomol Soc Am 91: 159–163. [Google Scholar]
- Luttrell MP, Creekmore LH, Mertins JW (1996) Avian tick paralysis caused by Ixodes brunneus in the southeastern United States. J Wildl Dis 32: 133–136. [DOI] [PubMed] [Google Scholar]
- McFadzen ME, Vekasy MS, Morishita TY, Greve JH (1996) Northern range extension for Haematosiphon inodorus (Dugès) (Hemiptera: Cimicidae). Pan-Pac Entomol 72: 41–42. [Google Scholar]
- McFadzen ME, Marzluff JM (1996) Mortality of prairie falcons during the fledging-dependence period. Condor 98: 791–800. [Google Scholar]
- Merino S, Potti J (1995) Mites and blowflies decrease growth and survival in nestling pied flycatchers. Oikos 73: 95–103. [Google Scholar]
- Millsap BA, Grubb TG, Murphy RK, Swem T, Watson JW (2015) Conservation significance of alternative nests of golden eagles. Glob Ecol Conserv 3: 234–241. [Google Scholar]
- Møller AP (1990) Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71: 2345–2357. [Google Scholar]
- Møller AP (1993) Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol 62: 309–322. [Google Scholar]
- Møller AP, Arriero E, Lobato E, Merino S (2009) A meta-analysis of parasite virulence in nestling birds. Biol Rev 84: 567–588. [DOI] [PubMed] [Google Scholar]
- Møller AP, Merino S, Soler JJ, Antonov A, Badás EP, Calero-Torralbo MA, de Lope F, Eeva T, Figuerola J, Flensted-Jensen E et al. (2013) Assessing the effects of climate on host-parasite interactions: a comparative study of European birds and their parasites. PLoS One 8: 1–11. 10.1371/journal.pone.0082886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DJ, Forbes NA (2007) Physiology: hematological. In DM Bird, KL Bildstein, eds, Raptor Research and Management Techniques. Hancock House, Blaine, Washington, USA, pp. 278–285. [Google Scholar]
- Morales-Yañez I, Rodríquez-Estrella R (2019) Golden eagle nestlings infested by Mexican chicken bugs in Chihuahua, Mexico. J Rap Res 53: 111–113. [Google Scholar]
- Müller C, Jenni-Eiermann S, Jenni L (2009) Effects of a short period of elevated circulating corticosterone on postnatal growth in free-living Eurasian kestrels Falco tinnunculus. J Exp Biol 212: 1405–1412. [DOI] [PubMed] [Google Scholar]
- Newton I (1998) Population Limitation in Birds. Academic Press, San Diego, California, USA, p. 597. [Google Scholar]
- Pauliny A, Wagner RH, Augustin J, Szép T, Blomqvist D (2006) Age-independent telomere length predicts fitness in two bird species. Mol Ecol 15: 1681–1687. [DOI] [PubMed] [Google Scholar]
- Piersma T, Reneerkens J, Ramenofsky M (2000) Baseline corticosterone peaks in shorebirds with maximal energy stores for migration: a general preparatory mechanism for rapid behavioral and metabolic transitions? Gen Comp Endocrinol 120: 118–126. [DOI] [PubMed] [Google Scholar]
- Platt SW (1975) The Mexican chicken bug as a source of raptor mortality. Wilson Bull 87: 557. [Google Scholar]
- Pryor LJ, Casto JM (2015) Blood-feeding ectoparasites as developmental stressors: does corticosterone mediate effects of mite infestation on nestling growth, immunity and energy availability? J Exp Zool 323: 466–477. [DOI] [PubMed] [Google Scholar]
- Quillfeldt P, Masello JF, Möstl E (2004) Blood chemistry in relation to nutrition and ectoparasite load in Wilson’s storm-petrels Oceanites oceanicus. Polar Biol 27: 168–176. [Google Scholar]
- R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR (2006) Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav 71: 39–48. [Google Scholar]
- Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A Mol Integr Physiol 140: 73–79. [DOI] [PubMed] [Google Scholar]
- St. Juliana JR, Khokhlova IS, Wielebnowski N, Kotler BP, Krasnov BR (2014) Ectoparasitism and stress hormones: strategy of host exploitation, common host-parasite history and energetics matter. J Anim Ecol 83: 1113–1123. [DOI] [PubMed] [Google Scholar]
- Steenhof K, Kochert MN, McDonald TL (1997) Interactive effects of prey and weather on golden eagle reproduction. J Anim Ecol 66: 350–362. [Google Scholar]
- Steenhof K, Kochert MN, McIntyre CL, Brown JL (2017) Coming to terms about describing golden eagle reproduction. J Raptor Res 51: 378–391. [Google Scholar]
- Stier KS, Almasi B, Gasparini J, Piault R, Roulin A, Jenni L (2009) Effects of corticosterone on innate and humoral immune functions and oxidative stress in barn owl nestlings. J Exp Biol 212: 2085–2091. [DOI] [PubMed] [Google Scholar]
- Sudyka J, Podmokła E, Drobniak SM, Dubiec A, Arct A, Gustafsson L, Cichoń M (2019) Sex-specific effects of parasites on telomere dynamics in a short-lived passerine—the blue tit. Sci Nat 106: 6. 10.1007/s00114-019-1601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricola GM, Simons MJP, Atema E, Boughton RK, Brown JL, Dearborn DC, Divoky G, Eimes JA, Huntington CE, Kitaysky AS et al. (2018) The rate of telomere loss is related to maximum lifespan in birds. Phil Trans R Soc B 20160445: 373. 10.1098/rstb.2016.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usinger RL (1966) Monograph of Cimicidae. The Entomological Society of America, College Park, Maryland, USA. [Google Scholar]
- U.S. Department of the Interior (1996) The effects of military training and fire in the Snake River Birds of Prey National Conservation Area. BLM/IDARNG Research Project Final Report. U.S. Geological Survey, Snake River Field Station, Boise, Idaho, USA, p 130.
- Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J (1997) Environmental stress, field endocrinology, and conservation biology. In JR Clemmons, R Buchholz, eds, Behavioral Approaches to Conservation in the Wild. Cambridge University Press, Cambridge, United Kingdom, pp. 95–131. [Google Scholar]
- Whitworth TL, Bennett GF (1992) Pathogenicity larval of Protocalliphora (Diptera: Calliphoridae) parasitizing nestling birds. Can J Zool 70: 2184–2191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


