Abstract
Background
Patient and public involvement (PPI) in research is well-established in the UK. However, it can be challenging to introduce PPI to research communities where there is limited prior knowledge, experience or appreciation of PPI. We aimed to explore current PPI practices, experiences and ethical and operational challenges with PPI within our own research community in Austria, to inform strategies for supporting PPI in Austria going forward.
Methods
We surveyed scientists at 21 research institutes of the Ludwig Boltzmann Gesellschaft (LBG) and representatives of 32 medical and university research ethics committees in Austria using online questionnaires. We analysed quantitative data using descriptive statistics, and we collated textual responses to open questions. We combined survey data with anecdotal evidence from our personal experience to summarise current challenges around implementing PPI in Austria.
Results
Nineteen scientists from nine research institutes indicated generally positive attitudes towards PPI. However, the majority reported they rarely or never involved patients and members of the public in roles of consultation, collaboration or control in research. Six of eight ethics committees were unfamiliar with PPI. We discern five current challenges to implementing PPI in Austria: lack of knowledge and skills for PPI among scientists, scepticism about the usefulness of PPI, conflation of PPI with qualitative research, uncertainty about ethical requirements for PPI and uncertainty about publishing PPI activities.
Discussion
We suggest that the provision of guidance about ethical requirements of PPI is a strategic priority. To address this, and following on from a recently introduced PPI training and grant scheme by the LBG, our surveys have initiated a dialogue with ethics committees and have informed the development of a checklist for ethical aspects of PPI.
Conclusion
Our experiences may provide useful examples to others who seek to introduce or strengthen PPI practices within their own research communities.
Keywords: citizen science, community participation, open innovation in science, patient and public involvement, service user involvement, surveys and questionnaires
Background
Patient and public involvement (PPI) in research refers to the active involvement of members of the public in research processes and activities, with the aim that research is carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them.1 The rationale for PPI includes a moral/ethical dimension, based on the argument that those who have lived experience of the phenomenon being researched (eg, a health condition) should also have a voice in related research; a methodological dimension, claiming that PPI leads to greater relevance and credibility of research funding proposals and improved study designs, for example, with respect to acceptability of study procedures to research participants; and a political dimension, based on citizens’ rights and proposed advantages of alliances between researchers, patients and the public.2 Typical examples for PPI activities are involvement of patients and members of the public in the setting of research priorities, as co-applicants on research grant applications, as members of study steering or advisory groups, and as co-researchers.1
PPI has largely originated in the UK, where it was introduced during the 1990s and has been supported at the highest level of national research governance. The National Institute for Health Research (NIHR), the UK’s largest publicly funded health research funder, has made PPI a requirement for research grant applications.3 NIHR-sponsored national advisory organisation INVOLVE4 and other centres and support networks5 offer expertise to researchers for the implementation and advancement of PPI in healthcare research, and regulator Health Research Authority (HRA) publishes clear regulatory and ethical guidance on PPI for researchers.6
Other recent international developments are also promoting the inclusion of patients’ voices in research. The US Food and Drug Administration Center for Drug Evaluation and Research is working on a series of guidance documents to support stakeholders (patients, researchers, medical product developers and others) in collecting and submitting patient experience data for medical product development and regulatory decision-making. The first document in this series, ‘Collecting Comprehensive and Representative Input’, was published in 2020.7 In Europe, a multistakeholder public–private partnership, the European Patients' Academy on Therapeutic Innovation (EUPATI), was established by the IMI-EUPATI project (2012–2017). This programme provides education and training to increase the capacity and capability of patients and patient representatives to understand and meaningfully contribute to medicines research and development.8 EUPATI National Platforms mirror the EUPATI partnership at national level and are currently set up in 22 European countries, including Austria.
Promoting PPI in Austria
In Austria, the independent non-profit research organisation Ludwig Boltzmann Gesellschaft (LBG) champions PPI in clinical and healthcare research as part of its Open Innovation in Science (OIS) strategy. OIS is an umbrella term that describes the ‘opening up’ of the scientific process through various strategies, including citizen science, open access to scientific outputs and data, open innovation approaches from business and industry and PPI.9 To promote PPI, the LBG OIS Center initiated a multistakeholder process in 2019, co-developing a patient and public involvement and engagement (PPIE) ‘how to’ guide with researchers from various disciplines, patient organisations and citizen scientists.10 11 This laid the foundation for a national PPI funding programme introduced in 2020 (ppie.lbg.ac.at) which supports researchers to implement PPI activities with up to €60 000 over 12 months. The call is embedded in continuous consultation and training on PPI, and peer support for researchers and members of the public to foster mutual learning. With these measures, the LBG OIS Center functions as a national point of contact and competence centre, aiming to embed meaningful PPI practices in the Austrian research landscape and offering support to researchers on an individual level.
In another OIS initiative, the LBG established two new Ludwig Boltzmann Institutes for Digital Health which commenced work in 2019. Both institutes were tasked with incorporating PPI throughout their programmes of research.12 Initial experiences made by researchers at these two institutes (STK, EK, MK-P, ES and AS-H among others), however, have surfaced challenges in implementing PPI practices, including lack of awareness and knowledge about the PPI concept in the local scientific communities, lack of appreciation of the value of involving patients as ‘experts by experience’ and fear of violating research ethics if PPI activities are carried out without formal ethical approval. We therefore undertook a scoping exercise and conducted surveys among researchers and representatives of research ethics committees in Austria. The aim was to explore current PPI practices, experiences and ethical and operational challenges with PPI, to gauge in how far our personal experiences might be reflected within our wider research community and to draw insights which may inform strategies for supporting PPI in research in Austria going forward.
Methods
Study design
In summer 2020, we conducted two online surveys to scope current PPI practices, experiences and ethical and operational challenges with PPI. In the design and conduct of the surveys we followed standard ethical research guidelines.
Data collection
Survey invitations were distributed by email and contained the access link, researcher contact details and information about the study purpose and publication of anonymised data. The surveys were open for 3 weeks, and reminders were emailed twice.
The first survey was distributed among postdoctoral researchers, principal investigators and OIS managers at 21 LBG-funded research institutes and groups. The questionnaire consisted of 10 items which were structured according to three aspects (three roles a patient or member of the public may take on in relation to research): participation (ie, entering a study as a study ‘subject’), engagement/dissemination (ie, engaging with information about research activities and findings) and involvement (ie, making an active contribution to research processes and activities).1 9 We formulated questions with Likert-scale response options to explore how frequently respondents undertook certain activities such as involving patients and members of the public in the conceptualisation of research proposals. Additionally, we formulated semantic differential scale items to gauge respondents’ attitudes towards PPI, a multiple-choice item about ethical aspects, and two open questions about ethical aspects and general challenges around PPI.
The second survey addressed representatives (primary contact persons) from 23 medical and 9 university research ethics committees in Austria. This was a short questionnaire consisting of three multiple-choice items with optional free text answers. We asked whether the committee was familiar with the concept of PPI, how the committee dealt with queries regarding PPI and whether the committee was interested in joining a national PPI working group.
Analysis
We conducted descriptive statistical analyses for quantitative survey data and collated textual responses to open questions. Using survey data to contextualise our personal experiences, we articulated five current challenges to implementing PPI practices in Austria.
Patient and public involvement
Members of the public were not involved in the design and conduct of the surveys, because the immediate barriers to PPI we encountered in our work seemed to relate to awareness, knowledge and perceptions among researchers. Members of the public have been involved in the design and concept of the PPIE programme and funding model in 2019.
Results
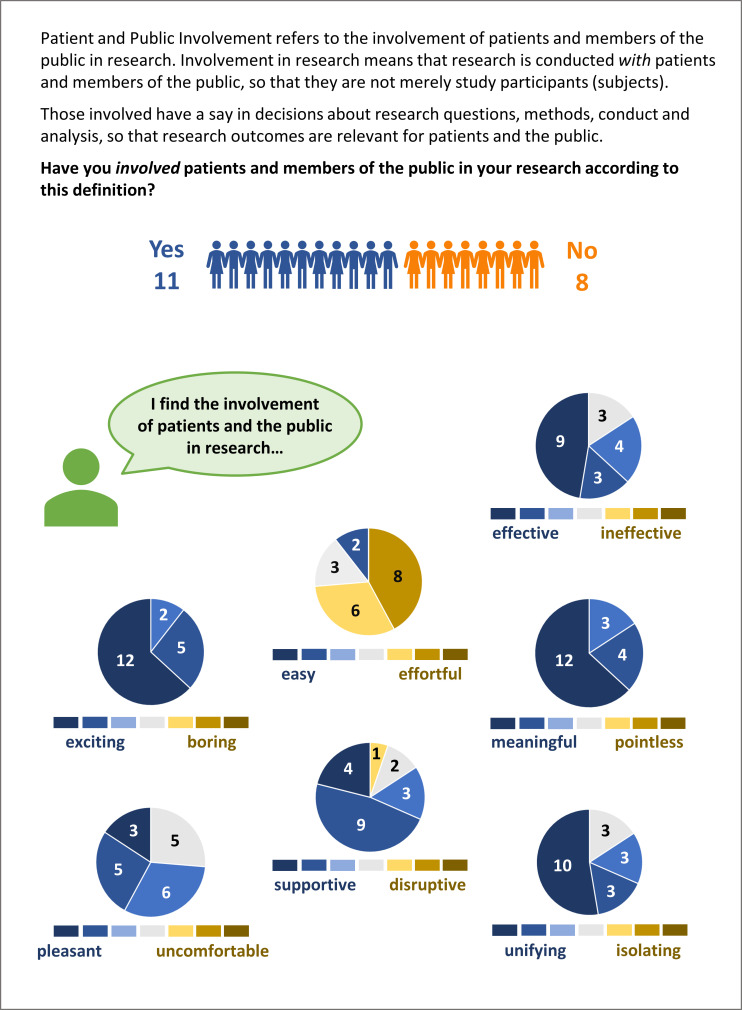
In our first survey, 19 scientists from nine different institutes/groups from disciplines across natural sciences, technical sciences, humanities, social sciences and health sciences indicated generally positive attitudes towards the involvement of patients and the public in research (figure 1). Eleven had previously conducted PPI activities, and eight had not (self-report). Respondents were generally active in disseminating research findings to patients or the public, via traditional media, social media, popular science events and other channels, which represents engagement/dissemination and not involvement. Two-thirds indicated they rarely or never involved patients or members of the public in consultant roles. Three-quarters indicated they rarely or never involved patients or the public in the development and conduct of research studies. And almost all indicated they rarely or never involved patients or members of the public in research lead or study oversight roles. With respect to ethical aspects of PPI (especially when individuals are invited because of their ‘patient’ roles), those respondents who had prior experience with PPI indicated that they tend to seek guidance from ethics committees, but not submit formal ethics applications for PPI activities.
Figure 1.
Attitudes towards patient and public involvement among 19 researchers from nine different institutes/groups of the Ludwig Boltzmann Gesellschaft. Respondents rated seven attitudinal dimensions on 7-point semantic differential scales. Pie charts describe frequencies of ratings.
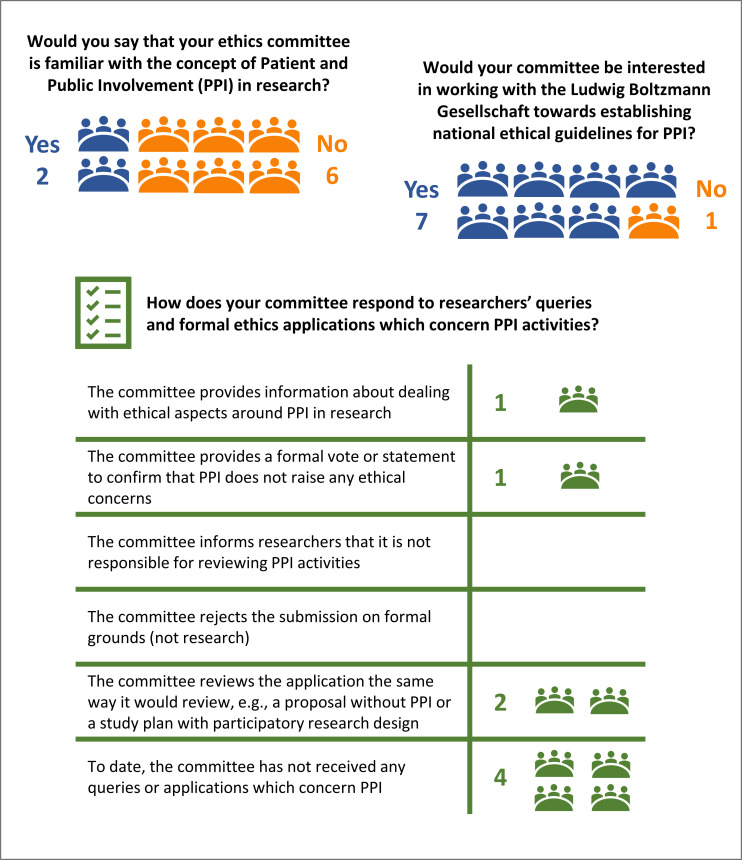
In our second survey, we received responses from 8 of 32 research ethics committees. Two respondents stated that committee members were familiar with PPI, and six respondents stated that they were not. The two committees familiar with PPI reported that they offered information to researchers about PPI practices and provided statements in support of PPI activities. Seven respondents expressed interest in joining a national working group, with the aim to foster PPI by coordinating research governance and ethical practices (figure 2).
Figure 2.
Responses (frequencies) from representatives of eight research ethics committees in Austria.
Points for attention
Our survey findings bring into focus and contextualise, and to some extent corroborate, the anecdotal evidence from our personal experience of introducing PPI practices at our Ludwig Boltzmann Institutes for Digital Health. Based on this we observe five challenges or ‘points for attention’:
While some researchers have considerable knowledge and experience of PPI, it appears that a large segment of the research community in Austria has limited awareness and knowledge of the PPI concept, let alone the necessary skills and experience for successfully conducting PPI.
We have encountered scepticism towards the usefulness and impact of PPI among LBG researchers and Austrian ethics committee members. Critics may ask for convincing evidence, especially when weighing up resources required for good quality PPI against expected outcomes.
There can be a conflation of PPI activities with qualitative research. Particularly among quantitative researchers, PPI conversations with individuals or groups can be misunderstood as qualitative data collection.
We have noticed uncertainty and sometimes considerable concern among clinical researchers who are unfamiliar with PPI about ethical aspects of PPI. This is grounded in the (valid) ethical imperative that patient information for clinical research purposes must not be collected before ethical approval has been granted, but it neglects the difference between patients’ enrolment as study participants versus patients’ involvement as PPI contributors. Especially PPI at the study conceptualisation and design stage, which takes place before a research ethics application is submitted, can create anxiety and fear of unethical conduct.
Lastly, there is uncertainty among researchers whether information collected through PPI activities should or could be published in peer-reviewed scientific articles.
Discussion
Our online surveys showed that respondents have differing levels of experience with PPI, from very limited experience to actively and competently involving members of the public in several phases of the research cycle. In our first survey, responses outline a trend whereby the implementation of PPI activities decreases with increasing degree of involvement (from consultation to collaboration to control).13 The sharing of decision-making and control over the research is particularly rare. This snapshot encourages us to further promote PPI on individual level (ie, offering training and facilitating exchange among researchers), and to introduce support structures on institutional and national level. In our second survey, most representatives from research ethics committees were unfamiliar with the PPI concept, but interested in discussing its ethical aspects. Acknowledging the potential for self-selection and social desirability bias in these surveys and limitations due to a low response rate from research ethics committees, our findings nevertheless indicate that awareness and knowledge of PPI should be addressed, and clear guidance on research governance and ethical requirements should be provided for the Austrian healthcare research community. To some extent, this requires a cultural shift and consensus building within the scientific community and among relevant stakeholders, such as funders, universities, research institutes and regional and federal medical research ethics committees.
Addressing points for attention
With respect to addressing the five challenges we formulate above, we offer the following considerations:
Researchers’ limited awareness, knowledge and skills for PPI have been highlighted as main stumbling blocks in the active involvement of members of the public in research.14 Signposting researchers to international scientific communities in which PPI is an established and valued practice (also considering other descriptors which are used internationally to describe an approach that is similar to PPI in spirit, such as ‘community engagement’15 or ‘patient-focused drug development’16) could emphasise the importance of PPI and increase motivation for researchers to acquire adequate knowledge and skills for PPI.
Demands for evidence of the usefulness of PPI are not straightforward to answer, as expected impacts of PPI are multifaceted, for example, benefiting research processes and outcomes, but also bringing about positive personal outcomes for PPI contributors and researchers.17 A growing literature demonstrates these positive outcomes of PPI, although this evidence is also limited by methodological complexities.18 A recent meta-analysis of seven randomised controlled trials demonstrated that PPI interventions modestly but significantly increased participant enrolment (OR 1.16, 95% CI 1.01 to 1.34).19 Such high-level evidence will speak to proponents of the traditional hierarchy of evidence paradigm. However, the value of evidence from qualitative and mixed methods reviews in describing nuanced and multifaceted impacts of PPI should not be neglected18 and should be considered for future research. PPI grant schemes should require that proposals incorporate processes for evaluating the impact of PPI.
The conflation of PPI activities with qualitative research has also been reported by others.20 There is a need to raise awareness and understanding of patients’ different roles, that is, patients as PPI contributors versus patients as research participants. Research institutions and ethics committees should provide guidance and training to support researchers in recognising these differences and in implementing PPI activities appropriately. Moreover, power differentials between researchers and PPI contributors need to be addressed,21 for example, by providing PPI contributors with adequate training opportunities and compensation (monetary or other) for their time.
Authoritative guidance at national level to address ethical concerns about PPI among healthcare researchers is needed. This should state unequivocally that PPI activities principally do not require formal review and approval by a research ethics committee, including PPI at the conceptualisation and design stage of a research proposal. In the UK, for example, such guidance is provided by the HRA:
Do I need HRA ethical approval before I work with patients and the public? No. You do not need to submit an application to a Research Ethics Committee in order to involve the public in the planning or the design stage of research, even if the people involved are NHS [National Health Service] patients.22
At an international level and endorsed by the WHO, the International Ethical Guidelines for Health-related Research Involving Humans (Guideline 7: Community Engagement) provide a similarly helpful resource.15 Although this does not clarify ethical requirements for PPI as explicitly as in the above example, the same message can be inferred:
Researchers and research ethics committees should be cognizant of the point at which the process of community engagement becomes a stage of formative research that itself requires ethics review. (CIOMS,15 p26)
Uncertainty about publishing PPI in peer-reviewed articles could be addressed by distinguishing three publication scenarios: the description of PPI in the methods section of scientific articles (this has recently been encouraged by the BMJ, signalling the importance attributed to PPI by a world-leading medical journal),14 the publication of PPI activities as research studies ‘in their own right’ and the publication of research studies about PPI.
Further developments
Following on from our survey findings, we identified the need for guidance about ethical requirements of PPI as a priority. As in the UK, formal ethical approval of PPI activities is currently not required by Austrian law if members of the public act as PPI contributors and not as study participants. In Austria, there is only a legal obligation for review by ethics committees in the case of clinical trial of drugs or medical devices and in the application of new medical methods and applied medical research to humans. National consensus and explicit guidance on this point would further raise awareness of researchers applying participatory research designs and PPI in its different forms—from consultation to collaboration to control—and their different ethical requirements.
To initiate this national consensus and promote change on a structural level, we invited a dialogue with Austrian research ethics committees about PPI. To date, five committees have joined an informal working group coordinated by the LBG OIS Center and have supported the development of a checklist for ethical aspects of PPI.23 We view this as a crucial step to inform about PPI and its ethical challenges, to align our vision and to address the conflation of PPI with qualitative research by outlining differences and ethical considerations around PPI also in ethics applications. The checklist is based on existing ethics guidelines in research10 and on the GRIPP-2 statement for reporting PPI in research publications.24 The checklist could serve as best practice example and standard operating procedure for Austrian ethics committees in dealing with PPI. Applying the checklist to their own work, applicants may be asked to, for example, describe the role of patients and members of the public in their project, distinguish between study participation and involvement and highlight possible ethical issues. This could support quality assurance and implementation of standards for PPI and give researchers an opportunity to self-evaluate their ethical considerations around PPI.
Conclusion
This initiative for scoping PPI practices within the LBG research community in Austria has led to a wider discussion in the organisation and dialogue with stakeholders, including research ethics committees. With our recently published checklist we have made progress towards providing ethical guidance for PPI in the Austrian research context, but we suggest that addressing consensus on governance and ethics of PPI remains a top strategic priority at a national structural level. Further strategic priorities are the ongoing provision of support at individual and institutional/organisational levels through PPI training opportunities and grant schemes to raise awareness and foster researchers’ knowledge and skills, and the building of the evidence base for PPI in the Austrian context through impact evaluations and formal research about PPI. It will be opportune and important to increase the involvement of patients and members of the public in the decision-making and delivery of these strategic measures. We envisage that the LBG OIS Center will continue to lead this work in collaboration with researchers, ethics committees, patients and members of the public, with the aim to achieve authentic and beneficial implementation of PPI in the Austrian research community.
Supplementary Material
Footnotes
Twitter: @RaphaelaKaisler
Contributors: In May 2020, the authors formed a working group to exchange experiences of introducing patient and public involvement at their respective organisations. REK, STK, EK, MK-P, ES and AS-H conceived and planned the formative surveys of Ludwig Boltzmann Institutes and research ethics committees in Austria. REK, STK, EK, MK-P, ES and AS-H contributed to drafting the online questionnaires. The surveys were administered by REK and analysed by REK and STK. REK, STK, EK, MK-P, ES and AS-H contributed to the interpretation of survey findings. REK, STK, EK, MK-P, ES and AS-H contributed to the conceptualisation of this article. REK, STK, MK-P and ES wrote the first manuscript draft. All authors critically reviewed the manuscript for intellectual content, approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Hayes H, Buckland S, Tarpey M. Briefing notes for researchers: public involvement in NHS, public health and social care research. Eastleigh, England: INVOLVE, 2012. [Google Scholar]
- 2.Greenhalgh T, Hinton L, Finlay T, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect 2019;22:785–801. 10.1111/hex.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson P, Mathie E, Keenan J, et al. Research with patient and public invOlvement: a realist evaluation – the RAPPORT study. Health Serv Deliv Res 2015;3:1–176https://www.journalslibrary.nihr.ac.uk/hsdr/hsdr03380/#/abstract 10.3310/hsdr03380 [DOI] [PubMed] [Google Scholar]
- 4.Involve. Available: https://www.invo.org.uk/ [Accessed 01 Mar 2021].
- 5.National Institute of Health Research . Involve patients. Available: https://www.nihr.ac.uk/health-and-care-professionals/engagement-and-participation-in-research/involve-patients.htm [Accessed 24 Feb 2021].
- 6.Health Research Authority (HRA) . Public involvement. Available: https://www.hra.nhs.uk/planning-and-improving-research/best-practice/public-involvement/ [Accessed 11 Nov 2020].
- 7.Center for Drug Evaluation and Research (CDER) . Patient-focused drug development: collecting comprehensive and representative input. guidance for industry, food and drug administration staff, and other stakeholders. silver spring, MD: U.S. food and drug administration 2020. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input [Accessed 26 Nov 2020].
- 8.European Patients’ Academy on Therapeutic Innovation (EUPATI) . EUPATI: patient engagement through education. Available: https://eupati.eu/ [Accessed 26 Nov 2020].
- 9.Beck S, Bergenholtz C, Bogers M, et al. The open innovation in science research field: a collaborative conceptualisation approach. Ind Innov 2020. 10.1080/13662716.2020.1792274 [DOI] [Google Scholar]
- 10.Kaisler RE, Missbach B. Patient and public involvement and engagement in research – a ‘how to’ guide for researchers, 2020. Zenodo [Accessed 06 Nov 2020]. [DOI] [PMC free article] [PubMed]
- 11.Kaisler RE, Missbach B. Co-creating a patient and public involvement and engagement 'how to' guide for researchers. Res Involv Engagem 2020;6:32. 10.1186/s40900-020-00208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch J, Leimueller G, Malfent L. Best open innovation in science practice for the establishment of interdisciplinary & inter-sectoral collaboration platforms for the implementation of PM [abstract]. 2nd ICPerMed Workshop – 'Best practice in personalised medicine' recognition 2019, personalised medicine for all citizens and patients within sustainable implementation. Madrid 2019;5https://www.icpermed.eu/en/icpermed-recognition-2019.php [Google Scholar]
- 13.Oliver SR, Rees RW, Clarke-Jones L, et al. A multidimensional conceptual framework for analysing public involvement in health services research. Health Expect 2008;11:72–84. 10.1111/j.1369-7625.2007.00476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicks P, Richards T, Denegri S, et al. Patients' roles and rights in research. BMJ 2018;362:k3193. 10.1136/bmj.k3193 [DOI] [PubMed] [Google Scholar]
- 15.Council for International Organizations of Medical Sciences (CIOMS) . International ethical guidelines for health-related research involving humans. 4th edn. Geneva Switzerland, 2016. [Google Scholar]
- 16.Center for Drug Evaluation and Research (CDER) . CDER patient-focused drug development. Silver spring, MD: U.S. food and drug administration. Available: https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development [Accessed 22 Nov 2020].
- 17.Baldwin JN, Napier S, Neville S, et al. Impacts of older people's patient and public involvement in health and social care research: a systematic review. Age Ageing 2018;47:801–9. 10.1093/ageing/afy092 [DOI] [PubMed] [Google Scholar]
- 18.Price A, Albarqouni L, Kirkpatrick J, et al. Patient and public involvement in the design of clinical trials: an overview of systematic reviews. J Eval Clin Pract 2018;24:240–53. 10.1111/jep.12805 [DOI] [PubMed] [Google Scholar]
- 19.Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ 2018;363:k4738. 10.1136/bmj.k4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liabo K, Boddy K, Burchmore H, et al. Clarifying the roles of patients in research. BMJ 2018;361:k1463. 10.1136/bmj.k1463 [DOI] [PubMed] [Google Scholar]
- 21.Di Lorito C, Godfrey M, Dunlop M, et al. Adding to the knowledge on patient and public involvement: reflections from an experience of co-research with carers of people with dementia. Health Expect 2020;23:691–706. 10.1111/hex.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Research Authority (HRA) . Public involvement. what do I need to do? Available: https://www.hra.nhs.uk/planning-and-improving-research/best-practice/public-involvement/what-do-i-need-do/ [Accessed 11 Nov 2020].
- 23.Kaisler RE. Checkliste für Forschungsvorhaben mit Bürger*innen-Einbindung [Checklist for research projects with patient and public involvement activities], 2021. Zenodo. Available: https://zenodo.org/record/4573970 [Accessed 04 Mar 2021].
- 24.Staniszewska S, Brett J, Simera I. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358:j3453. 10.1136/bmj.j3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.