Abstract
Introduction
Bronchoscopy is the main method in the diagnosis of various lung diseases. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the most modern bronchoscopic technique useful in diagnosis and staging of lung cancer (LC).
Objective
The aim of the study was to assess the yield of bronchoscopy in patients with suspected various respiratory diseases including LC. In particular, we examined the efficiency of different biopsy techniques in the diagnosis of LC in correlation with its localisation and pathomorphological type.
Patients and methods
The results of pathomorphological examinations from 5279 bronchoscopies performed in 2016–2018 were analysed. The material was collected with EBUS-TBNA, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endobronchial forceps biopsy. Clinical and demographic factors were analysed using the Fisher χ2 test.
Results
5279 patients were diagnosed due to various respiratory symptoms. LC was confirmed in 36.42% of patients. 40.81% of patients had no definitive pathomorphological diagnosis. Among patients with LC, the most frequent diagnosis was non-small cell LC: squamous cell lung cancer (SCC)—32.07% and adenocarcinoma (AC)—30.61%, then small cell LC—25.83% and not otherwise specified non-small cell lung cancer (NSCLC-NOS)—11.49%. Diagnosis of SCC was obtained significantly more often (χ2=43.143, p<0.000001) by forceps biopsy (41.09%) than by EBUS-TBNA/EUS-FNA (26.62%). On the contrary, diagnosis of AC or NSCLC-NOS was significantly more often (χ2=20.394, p<0.000007, and χ2=3.902, p<0.05, respectively) observed in EBUS-TBNA/EUS-FNA (34.31% and 12.6%) than in endobronchial biopsies (24.52% and 9.64%).
Conclusions
The use of bronchoscopy in the diagnosis of various lung diseases is vital but also has many limitations. Effectiveness of EBUS-TBNA and endobronchial forceps biopsy in the diagnosis of lung cancer is strongly affected by tumour localisation and type of cancer.
Keywords: bronchoscopy, respiratory tract tumours, respiratory medicine (see thoracic medicine)
Strengths and limitations of this study.
A total of 5279 patients were enrolled in the study group, which makes it one of the largest studies in the world with assessment of bronchoscopy effectiveness in routine clinical practice.
We analysed bronchoscopies performed in 2016–2018 in a few polish medical centres across the country, in a diversified population which ensures a high level of generalisability.
The study is an important contribution to the epidemiological data of advanced lung cancer in Poland.
Bronchoscopies were performed by eight different bronchoscopists. This fact may cause human-dependent variation in results.
Results of pathomorphological assessment of the material obtained during single bronchoscopy were analysed. Patients without diagnosed disease in the bronchoscopic material underwent further diagnostics using other methods or in other centres. Therefore, we were unable to report a definitive diagnosis in patients with inconclusive results of diagnostic procedures.
Introduction
Epidemiological analyses indicate that lung cancer (LC) is the most common cause of cancer-related deaths. It is usually diagnosed in an unresectable, advanced stage.1 2 LC is the most common cancer in men and the third most common in women. In 2018, there were more than two million new cases of LC worldwide.3
In the USA from 2004 to 2009, a total of 1 096 276 LC cases were diagnosed and reported. This American study investigated the histological type of LC and demographic characteristics of the patients. The incidence of individual types of LC was as follows: small cell lung cancer (SCLC)—14.9%, squamous cell lung cancer (SCC)—21.9%, adenocarcinoma (AC)—37.1% and large cell carcinoma (LCC)—3.2% of cases.4
It is difficult to obtain accurate epidemiological data on the occurrence of individual pathomorphological types of advanced LC in Poland. To date, no epidemiological studies have been conducted on a sufficiently large group of patients with advanced LC to obtain reliable results. Statistics on pathomorphological diagnoses of LC in material from bronchoscopy have not been conducted so far. In Poland, the main source of such data is the National Lung Cancer Registry, which only contains details about patients undergoing surgery in earlier stages of the disease. There where 17 783 patients diagnosed and operated on in Polish thoracic surgery centres and registered in the National Lung Cancer Registry in the years 2014–2018. This group includes 48.7% of patients with AC, 40.8% of patients with SCC, 6.45% of patients with LCC, 2.1% of patients with adenosquamous cell carcinoma, 1.05% of patients with SCLC and 0.9% of patients with not otherwise specified non-small cell lung cancer (NSCLC-NOS). This material included a low percentage of patients with SCLC (usually an inoperable type of LC) and patients with NSCLC-NOS (large surgical material is easier for pathomorphological examination, which is a factor of reducing misdiagnosis). According to International Association for the Study of Lung Cancer recommendations, LCC should be diagnosed only in surgical materials extracted from the entire resected tumour.5 Therefore, this type of cancer was recorded in surgical pathology in patients with non-advanced non-small cell lung cancer (NSCLC) and almost absent in patients with advanced LC diagnosed with tumour biopsy.2
Due to different locations of tumour types in the lungs or metastatic lymph nodes, varied approaches are required in cancer diagnosis. The peripheral location is characteristic for AC, while squamous and small cell carcinomas most often are located centrally. Bronchoscopy is an appropriate method for detecting LC and the endobronchial ultrasound-guided with transbronchial needle aspiration (EBUS-TBNA) procedure has an essential role in the investigation of LC. If the tumour is centrally located and infiltrated the bronchus, the most optimal procedure seems to be endobronchial biopsy using a brush or forceps. In contrast, AC frequently metastasizes to the mediastinal lymph nodes, which may be available on EBUS-TBNA or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) procedures.
Metastasis to mediastinal lymph nodes is typical for each of type of LC. We distinguish compartments of the mediastinal lymph nodes: superior mediastinal nodes (stations 2, 3 and 4), aortic nodes (stations 5 and 6), inferior mediastinal nodes (stations 7, 8 and 9), hilar and interlobar lymph nodes (stations 10 and 11) and peripheral lymph nodes (station 12 for lobar nodes, station 13 for segmental nodes and station 14 for subsegmental nodes). EBUS-TBNA is most often used in the diagnosis of superior mediastinal nodes, station 7 for inferior mediastinal nodes and stations 10, 11 and 12 for lymph nodes. EUS-FNA is preferred in the diagnosis of superior mediastinal nodes and stations 7, 8 and 9 for inferior mediastinal nodes. Moreover EUS-FNA is used for sampling subphrenic lymph nodes and metastases in the liver and left adrenal gland. Both methods—EBUS-TBNA and EUS-FNA—are preferred for mediastinal lymph node assessment for evaluation of N-stage in patients with NSCLC.6 These two methods could be used in sampling visible tumours localised in central airways but also peripherally to the main bronchi, that is, in lobar or even segmental bronchi.
LC staging is initially assessed through imaging studies. Normal mediastinum lymph nodes are defined below 10 mm in CT. Such a size of these lymph nodes suggests the N0 clinical stage. Currently, sampling for N0 nodes is not recommended, while surgery is the primary treatment method for N0/N1 stage of LC. However, everyday practice shows that it is worth collecting non-enlarged nodes for pathomorphological examination because cancer cells are often found in such nodes.7 8
The sensitivity of detecting LC using different bronchoscopy methods varies from 34% to 88%, depending on the size and location of the tumour and preliminary diagnosis of the patients.9 Meta-analysis of 18 studies which included a total of 1201 patients with LC was performed for assessment of sensitivity and specificity of ultrasound-guided fine needle aspiration (FNA) in mediastinal staging of LC. Authors showed sensitivity of 83% (range 45%–100%) and specificity of 97% (range 88%–100%) of these methods.10 In eight studies limited to patients with enlarged mediastinal lymph nodes seen on CT, sensitivity was 90% (95% CI 84% to 94%) and specificity was 97% (95% CI 95% to 98%). In patients without enlarged mediastinal lymph nodes visible on CT, the overall sensitivity was 58% (95% CI 39% to 75%). Therefore, the use of EBUS-TBNA increases the accuracy in the estimation of the stage of LC and may radically influence the further treatment of the patient and the selection of the treatment methods. EUS-FNA enables confirming the presence of distant metastases, which has a decisive impact on therapeutic decisions. However, all false-negative results delay cancer diagnosis and force the repetition of diagnostic procedures including surgery. Earlier detection of LC gives patients the chance for better treatment.11
Aim
The objective of our study was a descriptive analysis of lung diseases diagnoses, especially LC, established by various bronchoscopic procedures. We devoted special attention to the possibility of diagnosis of individual pathomorphological types of LC with various techniques used for collecting materials during bronchoscopy.
Material and methods
In our observational cross-sectional study, we analysed the results of pathomorphological examination carried out on the material obtained during 5279 bronchoscopies performed in 2016–2018. Those bronchoscopies were performed in three Polish pulmonology departments. The study included 1892 women and 3387 men with a median age of 65 years.
The study was retrospective and relied fully on the analysis of documents gathered, thus eliminating the need for collaboration between patients and researchers. No patients were enrolled specifically to carry out this study.
Various diseases of the respiratory system were indication for bronchoscopy: 3127 (59.2%) patients had suspicion of chest tumour in CT; 882 (16.7%) patients demonstrated hilar lymphadenopathy; 205 (3.9%) patients had suspicion of sarcoidosis; and 20 (0.4%) patients had suspicion of pulmonary fibrosis. Other indications for bronchoscopy occurred in 1045 (19.8%) patients (eg, suspicion of tuberculosis, chronic cough, haemoptysis, etc). In patients with suspected cancer, samples of the tissue were acquired through bronchoscopy, and the technique was chosen in compliance with tumour or the metastatic lymph node’s location as it is described in the introduction. Forceps biopsies were performed using Olympus BF-1T180 and Pentax EB-1970K bronchoscopes; EBUS-TBNA was performed using Olympus BF-UC180F and Pentax EB-1970UK bronchoscopes (22-gauge needles); and EUS-FNA was performed using Olympus GF-UCT180 endoscope. Premedication for bronchoscopy was under local or general anaesthesia, depending on the situation.
Samples underwent pathomorphological examination, which included H&E staining, mucicarmine staining and immunohistochemistry (IHC) examination, such as staining of thyroid transcription factor 1 and p63/p40. Samples diagnosed with non-squamous NSCLC were in-depth reported and underwent molecular testing for the presence of EGFR gene mutation by real-time PCR technique technique, ALK gene rearrangement and PD-L1 expression by IHC. PD-L1 expression was also assessed in patients with SCC. LCC of the lung according to the 2015 WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart cannot be diagnosed in small specimens and aspiration biopsy materials. The diagnosis of LCC can only be made in the postoperative material. Therefore, there were no patients diagnosed with LCC in our study. Such patients were included in the group of patients diagnosed with NSCLC-NOS. Chromogranin and synaptophysin were used in IHC examination of neuroendocrine tumours (SCLC or NSCLC-NOS). All the centres participating in the study used these same procedures described previously.
After receiving the diagnosis, we selected a population of patients with LC and divided them into groups of patients with different cancer types detectable with bronchoscopic procedure (SCC, AC, LCC, NSCLC-NOS and SCLC). Then, we assessed the prevalence of different types of LC in the materials obtained with various bronchoscopic procedures.
Clinical and demographic factors were analysed using Pearson’s χ2 test. P values below 0.05 were considered significant. The percentages reflect the relative number of all LCs diagnosed with a particular procedure. We analysed only the results of the first-time bronchoscopy, which could be non-diagnostic. The evaluation of relative diagnostic yield (sensitivity) of different bronchoscopic procedures could not be done because, in our study, it was not possible to verify the final diagnosis of patients in the materials collected during the next bronchoscopy or another procedure (this applies mainly to patients with lung tumour or hilar lymphadenopathy). The following diagnostic procedures were carried out in various clinical centres throughout Poland. Therefore, we were unable to verify the diagnoses obtained later.
Patient and public involvement
Patients were not involved in research.
Results
In 3565 (67.5%) patients, EBUS-TBNA and transoesophageal EUS-FNA were performed and cytological material was archived in a cellblock. In 1346 (25.5%) patients, EBUS-TBNA (without EUS-FNA) was the only diagnostic procedure. In the remaining patients, EBUS-TBNA was supplemented by EUS-FNA. There were no patients in whom EUS-FNA would be the only diagnostic method. A total of 1714 (32.5%) patients had non-ultrasound-guided bronchoscopy with the forceps biopsy of endobronchial lesions allowing obtainment of a small histological specimen.
LC was confirmed in a group of 1923 (36.42%) patients, including 1280 men and 643 women. Reactive lymph nodes were found in 16.06% of the patients; sarcoidosis was diagnosed in 4.13%, idiopathic pulmonary fibrosis in 0.19% and metastases to the lungs from other organs in 2.39% of the patients. Of these patients, 40.81% had no definitive diagnosis (figure 1). LC was confirmed in 51% of patients with suspected tumour in CT, while in the group of patients with hilar lymphadenopathy, LC was diagnosed in only 10.1% of cases.
Figure 1.
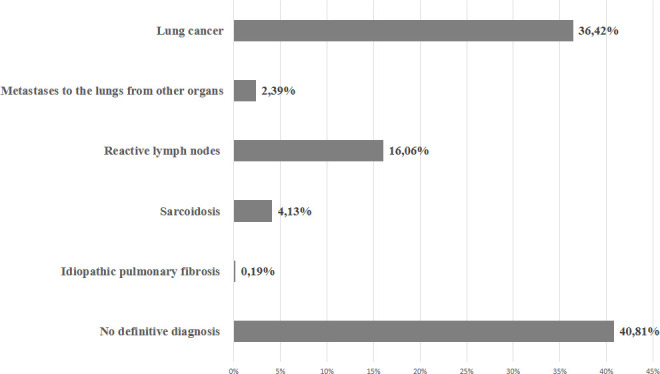
Results of pathomorphological examination carried out on material obtained from 5279 bronchoscopies (entire study population).
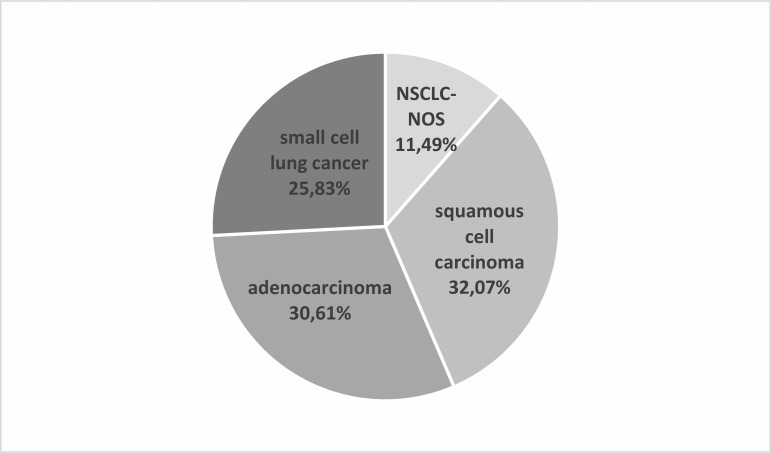
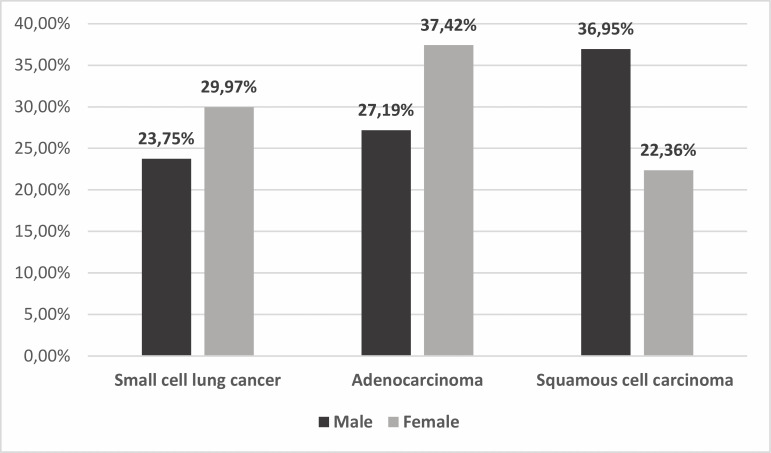
Among those with LC, squamous cell carcinoma (32.07%) was most often diagnosed, then AC (30.61%), SCLC (25.83%) and NSCLC-NOS (11.49%) (figure 2). SCLC and AC were significantly more frequent (χ2=8.649, p=0.0033, and χ2=21.128, p<0.000005, respectively) in women (29.97% and 37.42% of women with LC) than in men (23.75% and 27.19% of male patients with LC). Squamous cell carcinoma appeared significantly more often (χ2=41.881, p<0.000001) among male (36.95%) than among female (22.36%) patients with LC (figure 3). SCC was significantly more often (χ2=4.17, p=0.041) diagnosed in the group of patients older than 65 years than in younger patients. Other pathomorphological types of LC occurred with similar frequency in these two age groups.
Figure 2.
Incidence of individual pathomorphological types of LC in the entire study group of patients with LC. LC, lung cancer; NSCLC-NOS, not otherwise specified non-small cell lung cancer.
Figure 3.
Incidence of individual pathomorphological types of LC according to the gender of patients with LC. LC, lung cancer.
Endobronchial biopsies significantly more often (χ2=7.566, p=0.0059) provided material for the diagnosis of LC than the EBUS-TBNA and EUS-FNA procedures. A total of 42.35% of endobronchial biopsies and 33.6% of TBNA and FNA provided material sufficient to diagnose LC. Fine needle biopsy of lymph nodes enabled the diagnosis of LC in 29.2% of cases, fine needle biopsy of lung tumour in 66.6% of cases and forceps biopsy of bronchial mucosa lesions in 48.2% of cases. These differences were statistically significant.
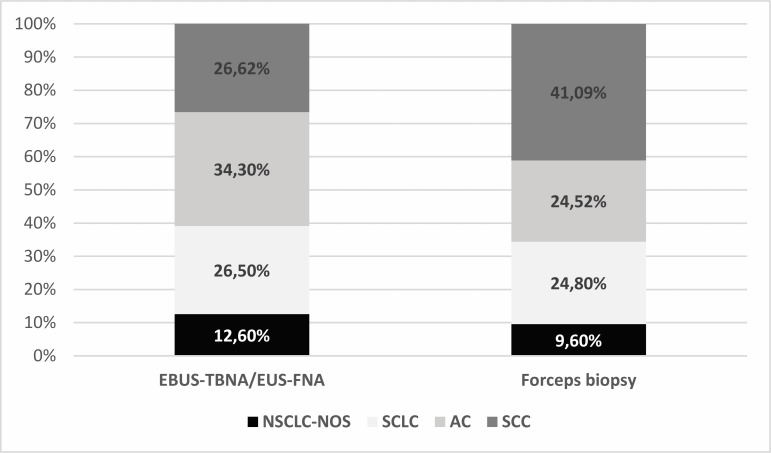
Among patients with LC, TBNA or FNA compared with endobronchial biopsies gave a similar result (χ2=0.656, p=0.418) in the detection of SCLC (26.5% vs 24.8%). On the other hand, the diagnosis of AC and NSCLC-NOS was obtained significantly more frequently (χ2=20.394, p=0.000006, and χ2=3.902, p=0.0482) in EBUS-TBNA and EUS-FNA compared with endobronchial biopsies (34.3% vs 24.52% and 12.6% vs 9.6%, respectively). SCC, among other LC types detected by bronchoscopy, was diagnosed in 41.77% in materials obtained by forceps biopsy and only in 26.62% in materials with EBUS-TBNA or EUS-FNA (χ2=43.143, p<0.000001), which was directly related to the more frequent central location and bronchial infiltration of this type of tumour. CT showed that in 79% of patients with SCC, the tumour was centrally located in the large bronchi. The analysis of bronchoscopic images showed that tumour deformed the bronchial mucosa or showed endobronchial growth in 67% of patients with SCC. (figure 4). Table 1 shows the results of bronchoscopy procedures in the diagnosis of individual pathomorphological types of LC, depending on the place of collection of the material.
Figure 4.
Percentage of patients with different types of LC detected in materials collected with different bronchoscopic techniques. Frequency of different types of LC was calculated in the whole group of patients undergoing a given bronchoscopic procedure (100%), which resulted in the diagnosis of LC. AC, adenocarcinoma; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; LC, lung cancer; NSCLC-NOS, not otherwise specified non-small cell lung cancer; SCC, squamous cell lung cancer; SCLC, small cell lung cancer.
Table 1.
Results of varied techniques during bronchoscopy in the diagnosis of individual pathomorphological types of LC depending on the place of collection of the material and the nodal station
| Material | SCLC | Adenocarcinoma | Squamous cell carcinoma | NOS | Total LC |
| EBUS-TBNA/EUS-FNA of lymph nodes | 250 (27.7%) | 331 (36.7%) | 209 (23.1%) | 114 (12.5%) | 904 (100%) |
| EBUS-TBNA/EUS-FNA of tumour | 88 (24.7%) | 94 (25.8%) | 135 (37.2%) | 44 (12.3%) | 361 (100%) |
| EBUS-TBNA/EUS-FNA metastases to adrenal gland | 3 (42.9%) | 1 (14.3%) | 2 (28.6%) | 1 (14.3%) | 7 (100%) |
| Forceps biopsy of tumour | 156 (22%) | 163 (25%) | 270 (41.5%) | 62 (9.5%) | 651 (100%) |
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; LC, lung cancer; NOS, not otherwise specified; SCLC, small cell lung cancer.
Discussion
Our study on the results of EBUS-TBNA/EUS-FNA and forceps biopsy in obtaining materials for the diagnosis of various lung diseases is among the largest worldwide. The study points to numerous problems arising from the use of these techniques in routine clinical practice. We are aware of the many limitations of our study. First, we cannot determine the sensitivity and specificity of our methods because it was not possible to determine the final diagnosis in such a large group of patients (5279 cases). Second, we could not distinguish between material collected by EBUS-TBNA and EUS-FNA. We do not know how many patients with LC were diagnosed only in the material from EBUS-TBNA or only in the material from EUS-FNA, or in both types of these materials. We also do not know the number of biopsies performed during one bronchoscopy. These data are missing from the results of the pathomorphological examination that we analysed. Third, diagnosis of LCC was not possible in small specimens (LCC was probably qualified to the NSCLC-NOS group). A limitation of our study was also the lack of detailed clinical and radiological characteristics of all patients who underwent bronchoscopy procedures.
However, we found that advanced SCLC may be more common in Poland than previously thought. This tumour is characterised by rapid growth and metastases; therefore, more often it could be diagnosed in advanced stages using bronchoscopic techniques. SCLC diagnosis in EBUS-TBNA/EUS-FNA of lymph nodes and endobronchial biopsy occurs at the same frequency. Furthermore, the difference in the percentage of patients with squamous cell carcinoma and AC diagnosed with endobronchial biopsies and TBNA or FNA is noteworthy. In our study, most patients with AC were diagnosed with EBUS-TBNA or EUS-FNA of lymph nodes, while patients with squamous cell carcinoma were diagnosed more often based on examination of material from endobronchial biopsy (forceps biopsy). Patients in cohort with squamous cell carcinoma were more likely to have endobronchial disease accessible by forceps. Therefore, we could not ascertain that endobronchial biopsy is more effective for diagnosis of SCC as there was no comparison to EBUS-TBNA and EUS-FNA for those patients. Thus, we point to the problem that results of bronchoscopic procedures depend on the location of the primary tumour and the presence of metastases in the lymph nodes.
Schmid-Bindert et al showed how often they detected different pathomorphological types of LC using various bronchoscopic methods. Small biopsies were collected by three different methods: forceps biopsy (44.6%), EBUS-TBNA (32.7%) and CT-guided core biopsy (22.8%). Thirty-eight per cent of adenocarcinoma, 51% of squamous cell carcinoma and 11% of NSCLC-NOS were diagnosed using forceps biopsy. EBUS-TBNA results were as follows: 45% of AC, 30% of squamous cell carcinoma and 24% of NSCLC-NOS.12
Many authors emphasise that the diagnosis of NSCLC-NOS is the most common in the case of material obtained from EBUS-TBNA. Esterbrook et al found that NSCLC-NOS rate was 20.8% in EBUS-TBNA samples. Similar results were achieved by Navani et al. In a group of 774 patients with known or suspected LC, 23% of the patients had a final diagnosis of NSCLC-NOS.13 14 Our study confirmed the high percentage of patients with NSCLC-NOS diagnosed with EBUS-TBNA procedures. Endobronchial biopsy was less likely to provide a diagnosis of NSCLC-NOS.
Chin et al reported that EBUS-TBNA is the most sensitive diagnostic method for SCLC detection because it allows sampling of specimens from mediastinal as well as submucosal lesions. They also mentioned that the quality of specimens obtained by needle aspiration is better than by forceps biopsies, which may contain crushed artefacts.15 In addition, other studies noticed that the sensitivity of EBUS-TBNA for SCLC detection was higher than that for NSCLC diagnosis.16 17 Our findings confirmed the aforementioned statements. The majority of SCLC cases were diagnosed with EBUS-TBNA. Three patients with SCLC were diagnosed from metastatic lesions in the adrenal gland using transoesophageal EUS-FNA.
Many authors raise the problem that forceps biopsy has low sensitivity in the diagnosis of LC. Forceps biopsy has a diagnostic yield ranging between 65% and 82%. In a preliminary study by Pasko et al conducted in 212 patients with LC suspicion, authors compared sensitivity and accuracy of routine bronchoscopy techniques: endobronchial biopsy, EBUS-TBNA, and combination of EBUS-TBNA and EUS-FNA. Sensitivity and accuracy of endobronchial biopsy versus EBUS-TBNA versus combination of transbronchial biopsies were 43% vs 44.3% vs 93.7% and 93.8% vs 94.7% and 94.8%, respectively. This demonstrates high usefulness of the combination of EBUS-TBNA and EUS-FNA in the diagnosis of LC.18 Verma et al demonstrated sensitivity of EBUS-TBNA in cancer diagnosis of 91.4% in a small group of 37 patients with lesions located adjacent to the trachea or lesions located adjacent to the main bronchi.19 Tournoy et al indicated that EBUS-TBNA has a sensitivity of 82% and low negative predictive value (23%).20 Similar results were obtained by Zhao et al for lesions located near the central airways.21 Oki et al showed that the combined endoscopic method with EBUS-TBNA and EUS-FNA with a single bronchoscope gives better results in staging of NSCLC than each technique alone. However, they mentioned that a significant number of patients had false-negative EBUS-TBNA and EUS-FNA results. Moreover, Oki et al suggested that a very important issue is bronchoscopists’ experience, which may cause differences in the results.22 On the other hand, Wallace et al showed a EBUS-TBNA sensitivity of only 69% in a group of 150 patients with LC suspicion.23
Despite the relatively low negative predictive value of EBUS-TBNA, there is an indication to perform other procedures (eg, surgical procedures) for final diagnosis in a significant group of patients. In our study, 56.9% of patients who underwent bronchoscopy did not receive a definitive diagnosis of the diseases, and they have been subjected to other diagnostic procedures or observations. We showed the results of all performed bronchoscopies and three different methods of material collection (endobronchial biopsy and combination of EBUS-TBNA and EUS-FNA). Moreover, unselected and heterogeneous patients were recruited in three different hospitals that employed a total of eight bronchoscopists. In most studies, the evaluation of the usefulness of EBUS-TBNA and EUS-FNA for detecting malignancy was conducted in selected patients with high clinical suspicion of the tumour. Small, preselected groups of patients with high risk of LC could be the reason for the low negative predictive value of bronchoscopic procedures in these studies. Thus, these observations may not reflected the real clinical situation.
In studies where population was heterogenic regarding the disease (LC, sarcoidosis and tuberculosis), EBUS-TBNA had diagnostic value only in 60%–75% of patients.24 Lange et al showed diagnostic results of EBUS-TBNA in only 61.4% of unselected patients undergoing routine diagnostic procedures.25 Fournier et al examined 185 patients with extrathoracic malignancy and mediastinal lymphadenopathy in real-life practice. Pathomorphological types of malignancy were successfully identified using EBUS-TBNA in only 93 patients (50.3%). The diagnostic sensitivity, specificity, negative predictive value and positive predictive value were 68.4%, 100%, 53.3% and 100%, respectively.26 Murthi et al conducted research comparing the accuracy of EBUS-TBNA to surgery in diagnosis of hilar and mediastinal pathologies. EBUS-TBNA for all pathologies had an accuracy of 81.2% and a sensitivity of 55.1%.27
Conclusions
Comparing all these data, we could conclude that bronchoscopy is vital but not an ideal technique in the routine diagnosis of respiratory diseases. Our study showed that 41% of bronchoscopy materials were insufficient to perform reliable pathomorphological examination. This mainly concerned patients with suspected lung tumour or lymphadenopathy. The use of brush biopsy, forceps biopsy, bronchoaspirate analysis EBUS-TBNA and EUS-FNA simultaneously, if desired, was of the highest diagnostic value. However, sometimes this fails and bronchoscopy must be repeated, or thoracic procedures (eg, mediastinoscopy or thoracoscopy) must be performed. We found that the EBUS-TBNA value in daily clinical practice differed from that in clinical trials. Therefore, precise estimation of the frequency of individual pathomorphological types of LC, based on material obtained bronchoscopically, is not possible. However, EBUS-TBNA plays an essential role in staging of invasive LC. Therefore, its value in the diagnosis of LC is not limited to demonstrating the presence of cancer type but, above all, to determining the extent of the disease and qualification for appropriate treatment. It seems that the incidence of LC with a typical peripheral localisation (AC) may be underestimated in comparison to the incidence of LC with a typical central localisation (squamous cell carcinoma and SCLC), when bronchoscopy is used as the primary diagnostic method.
Supplementary Material
Acknowledgments
The authors thank Professor Tadeusz Orłowski and Professor Maciej Krzakowski for their helpful comments that improved the research and manuscript.
Footnotes
JBła and MłgFąk contributed equally.
Contributors: Conception and design of the study: JBł, MF, PK and JP. Administrative support: PK, JP, AP, JBu, AS, JS, MM, PK, RK and JM. Provision of study material or patients: JP, AP, JBł, JBu AS, JS, MM, PK, RK and JM. Collection and assembly of data: JBł, MF, PK, JP, AP, JBu, AS, JS, MM, PK and RK. Data analysis and interpretation: JBł, MF and PK. Manuscript writing: JBł, MF and PK. All authors gave final approval of the published manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol of the study was approved by the committee of ethics and research at the Medical University of Lublin (KE-0254/5/2018).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Burke AP, et al. Introduction to the 2015 World Health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 2015;10:1240–2. 10.1097/JTO.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Houston KA, Henley SJ, Li J, et al. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer 2014;86:22–8. 10.1016/j.lungcan.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zugazagoitia J, Enguita AB, Nuñez JA, et al. The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: current concepts and future prospects. J Thorac Dis 2014;6:S526–36. 10.3978/j.issn.2072-1439.2014.01.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of thoracic surgeons (ESTs). Eur Respir J 2015;46:40–60. 10.1183/09031936.00064515 [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010;37:1168–74. 10.1016/j.ejcts.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe S-ichi, Asamura H, Suzuki K, et al. Problems in diagnosis and surgical management of clinical N1 non-small cell lung cancer. Ann Thorac Surg 2005;79:1682–5. 10.1016/j.athoracsur.2004.11.025 [DOI] [PubMed] [Google Scholar]
- 9.Rivera MP, Wahidi MM, Mehta AC. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:142–65. 10.1378/chest.12-2353 [DOI] [PubMed] [Google Scholar]
- 10.Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and metaanalysis. Chest 2007;131:539–48. 10.1378/chest.06-1437 [DOI] [PubMed] [Google Scholar]
- 11.Sutedja TG, Codrington H, Risse EK, et al. Autofluorescence bronchoscopy improves staging of radiographically occult lung cancer and has an impact on therapeutic strategy. Chest 2001;120:1327–32. 10.1378/chest.120.4.1327 [DOI] [PubMed] [Google Scholar]
- 12.Schmid-Bindert G, Wang Y, Jiang H, et al. EBUS-TBNA provides highest RNA yield for multiple biomarker testing from routinely obtained small biopsies in non-small cell lung cancer patients - a comparative study of three different minimal invasive sampling methods. PLoS One 2013;8:77948. 10.1371/journal.pone.0077948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navani N, Brown JM, Nankivell M. Suitability of EBUS-TBNA specimens for subtyping and genotyping of NSCLC: a multi-centre study of 774 patients. Am J Respir Crit Care Med 2012;185:1316–22. 10.1164/rccm.201202-0294OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esterbrook G, Anathhanam S, Plant PK. Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer 2013;80:30–4. 10.1016/j.lungcan.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 15.Chin R, Cappellari JO, McCain TW, et al. Increasing use of bronchoscopic needle aspiration to diagnose small cell lung cancer. Mayo Clin Proc 2000;75:796–801. 10.4065/75.8.796 [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Villar A, Botana M, Leiro V, et al. Validity and reliability of transbronchial needle aspiration for diagnosing mediastinal adenopathies. BMC Pulm Med 2010;10:24. 10.1186/1471-2466-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharafkhaneh A, Baaklini W, Gorin AB, et al. Yield of transbronchial needle aspiration in diagnosis of mediastinal lesions. Chest 2003;124:2131–5. 10.1378/chest.124.6.2131 [DOI] [PubMed] [Google Scholar]
- 18.Pasko E, Serafin-Bromblik J, Piekorz W. Combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscopes routine videobronchoscopy for diagnosis of advanced lung cancer – a prospective study. Eur Respir J 2034;2016:48. [Google Scholar]
- 19.Verma A, Jeon K, Koh W-J, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of central lung parenchymal lesions. Yonsei Med J 2013;54:672–8. 10.3349/ymj.2013.54.3.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45–9. 10.1016/j.lungcan.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Xie Z, Zhou Z-L, et al. Diagnostic value of endobronchial ultrasound-guided transbronchial needle aspiration in intrapulmonary lesions. Chin Med J 2013;126:4312–5. [PubMed] [Google Scholar]
- 22.Oki M, Saka H, Ando M, et al. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2014;148:1169–77. 10.1016/j.jtcvs.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 23.Wallace MB, Pascual JMS, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540–6. 10.1001/jama.299.5.540 [DOI] [PubMed] [Google Scholar]
- 24.Madan K, Mohan A, Ayub II, et al. Initial experience with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) from a tuberculosis endemic population. J Bronchology Interv Pulmonol 2014;21:208–14. 10.1097/LBR.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 25.Lange TJ, Kunzendorf F, Pfeifer M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in routine care - plenty of benign results and follow-up tests. Int J Clin Pract 2012;66:438–45. 10.1111/j.1742-1241.2012.02907.x [DOI] [PubMed] [Google Scholar]
- 26.Fournier C, Hermant C, Gounant V, et al. Diagnostic of mediastinal lymphadenopathy in extrathoracic cancer: a place for EBUS-TBNA in real life practice? Respir Med Res 2019;75:1–4. 10.1016/j.resmer.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Murthi M, Donna E, Arias S, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in real life. Front Med 2020;7:118. 10.3389/fmed.2020.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.