Abstract
Cardiovascular involvement is uncommon in pediatric patients with hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC-HUS). In this case report we presented a case of 17-month-old toddler who had a sporadic type of STEC-HUS complicated by acute myocarditis. The patient was successfully treated by a single dose of eculizumab after six doses of therapeutic plasma exchange (TPE) were inefficient to prevent the cardiac complication. Hepatotoxicity was observed after a single dose of eculizumab. Hepatic and cholestatic enzyme levels slowly returned to normal within 6 months. To the best of our knowledge, this is the first case of myocarditis/cardiomyopathy treated with eculizumab in STEC-HUS. This case illustrates the need for vigilance regarding myocardial involvement and eculizumab-induced hepatotoxicity in STEC-HUS.
Keywords: hemolytic uremic syndrome, myocarditis, eculizumab, therapeutic plasma exchange
Introduction
Hemolytic uremic syndrome (HUS) is characterized by nonimmune hemolytic anemia, acute renal failure, and thrombocytopenia. It is commonly caused by an infection known as Shiga toxin-producing Escherichia coli (STEC). It can lead to significant morbidity and mortality in the course of the disease. Acute kidney injury and complications involving other organ systems in STEC-HUS are primarily due to thrombotic microangiopathy (TMA). Endothelial damage, TMA, and microthrombi formation can occur in all of the small vessels of the organs, especially in the renal vessels. Gastrointestinal, neurological, pancreatic, hepatic, and cardiac dysfunction are extrarenal manifestations of STEC-HUS. 1 2
In this case report, we presented a 17-month-old child who had sporadic type STEC-HUS associated with acute myocarditis. The patient was successfully treated with six sessions of therapeutic plasma exchange (TPE) followed by a single dose of eculizumab (a human anti-C5 monoclonal antibody). Intriguingly, hepatotoxicity was observed after this single dose of eculizumab.
Case Report
Video 1 Transthoracic echocardiography demonstrating the dilatation of the left ventricle, moderate mitral regurgitation, and severe systolic dysfunction.
Video 2 Transthoracic echocardiography revealing a gradual improvement of EF, and a significant reduction in the amount of pericardial effusion. EF, ejection fraction.
A 17-month-old female was initially admitted to another hospital with a 3-day history of vomiting, nonbloody diarrhea, fatigue, anorexia, and decreased oral intake. Due to anemia, thrombocytopenia, and deterioration in kidney function tests, HUS was considered. She was referred to our institution's pediatric intensive care unit (PICU). Physical examination on the first day of admission into our PICU revealed a generally unwell child with lethargy (Glasgow coma scale, 13), tachypnea (42/min), and tachycardia (172/min). Oxygen saturation (98% in room air), blood pressure (87/51 mm Hg), and body temperature (36.7°C) were all normal. She had moderate edema and mild prolonged capillary refill time (3 seconds). Blood gas evaluation revealed pH, 7.28; pCO 2 , 31 mm Hg; and bicarbonate, 11.8 mmol/L with normal lactate levels. A complete blood count revealed a white blood cell (WBC) level of 12,000/mm 3 , a hemoglobin level of 7.2 g/dL, a hematocrit level of 19.1%, and a platelet level of 35,000/mm 3 . There were schistocytes on the peripheral blood smear, and the direct antiglobulin test was negative. Prothrombin time was 13 seconds, activated partial thromboplastin time was 42.8 seconds. A d-dimer level of 18,700 μU/L (range: 80–500 μU/L) and a fibrinogen level of 185 mg/dL was observed. The other test results were as follows: serum urea, 67 mg/dL; creatinine 0.69, mg/dL; uric acid, 8.3 mg/dL; lactate dehydrogenase, 1,275 U/L (range: 120–300 U/L), and C-reactive protein, 0.5 mg/dL (range: 0–0.5 mg/dL). Serum electrolytes were within normal limits, and the haptoglobin level was undetectable (<7.44 mg/dL, normal range: 30–200). von Willebrand's factor-cleaving metalloproteinase (ADAMTS13; 74%), vitamin B12 (259 pg/mL), and homocysteine (16.1 µmol/L) levels were detected within the normal range. A normal C3 level of 83 mg/dL (normal range: 49–109 mg/dL), and a low C4 level of 7.2 mg/dL (normal range: 14–42 mg/dL) were noted. Abdominal ultrasonography was consistent with bilateral grade-1 renal parenchymal disease. Proteinuria (++ + ) and hemoglobinuria (++ + ) with a density of 1 013 were detected in the urine analysis. The stool examination was negative for adenovirus, rotavirus, and norovirus, and there was no growth of Salmonella , Shigella , Campylobacter , and Aeromonas in the stool culture. (Our institution does not currently have shigatoxin [Stx] testing availability, thus polymerase chain reaction (PCR) analysis was sent to a remote laboratory for processing). TPE was started for a possible diagnosis of complement-mediated thrombotic microangiopathy. Furosemide infusion (0.2 mg/kg/h) was initiated for oliguria (0.7 mL/kg/h). Her serum creatinine levels peaked at 0.8 mg/dL in the first 48 hours, and then started to decrease slowly. There was significant improvement in the urine output after the administration of furosemide infusion and daily TPE obviating the need for continuous renal replacement therapy.
Despite stabilization of kidney function, the general condition of the patient deteriorated on the day 6 of PICU admission. Mild respiratory insufficiency, altered mental status, and lethargy were noted; therefore, oxygen therapy via high-flow nasal cannula was started. Shortly after, the patient had a sudden cardiac arrest. After 2 minutes of cardiopulmonary resuscitation (CPR), the patient had return of spontaneous circulation. She was intubated, and invasive mechanical ventilatory support was promptly initiated. Electrocardiogram (ECG) did not reveal any signs of ischemia or arrhythmia. A transthoracic echocardiographic examination demonstrated that the left ventricle was dilated with moderate mitral regurgitation and severe systolic dysfunction (ejection fraction [EF]: 40%, shorting fraction [SF]: 20%). A small pericardial effusion was noted ( Video 1 ). Troponin I and pro-B-type natriuretic peptide (pro-BNP) levels were measured to be 62 IU/mL (normal range is below 0.1 IU/mL) and 3,270 pg/mL (normal: 31–675 pg/mL), respectively. Intravenous milrinone infusion (0.75 µg/kg/min) was administered and furosemide infusion was continued. Eculizumab (300 mg) was administered intravenously after the termination of TPE due to the patient's condition of HUS associated with cardiac involvement. Daily echocardiographic examinations revealed a gradual improvement of EF, and there was a significant reduction in the amount of pericardial effusion ( Video 2 ). Also, troponin I and pro-BNP levels gradually normalized.
Hepatic tests (AST, ALT, GGT, and ALP) were normal during the first 7 days of hospitalization. After eculizumab administration, the AST, ALT, GGT, and ALP levels were increased notably ( Fig. 1 ). In the advanced laboratory tests for cholestatic liver disease, ammonia, α-fetoprotein, factor V, factor VII, immunoglobulin (Ig)-G, IgM, IgA, and thyroid function tests were all within normal limits. Serological tests for hepatotropic viruses (hepatitis A, B, and C; Epstein–Barr virus; coxsackie B virus; cytomegalovirus; human immunodeficiency virus; toxoplasma; rubella; herpes simplex virus; parvovirus B19; and influenza), antinuclear antibody (ANA), anti-dsDNA, antimitochondrial antibody (AMA), antismooth muscle antibody (ASMA), and antiliver kidney microsomal type-1 antibody were negative. Abdominal ultrasonography was normal. Ursodeoxycholic acid (30 mg/kg/day, administered enterally) and N-acetylcysteine infusion (150 mg/kg/day) were administered intravenously.
Fig. 1.
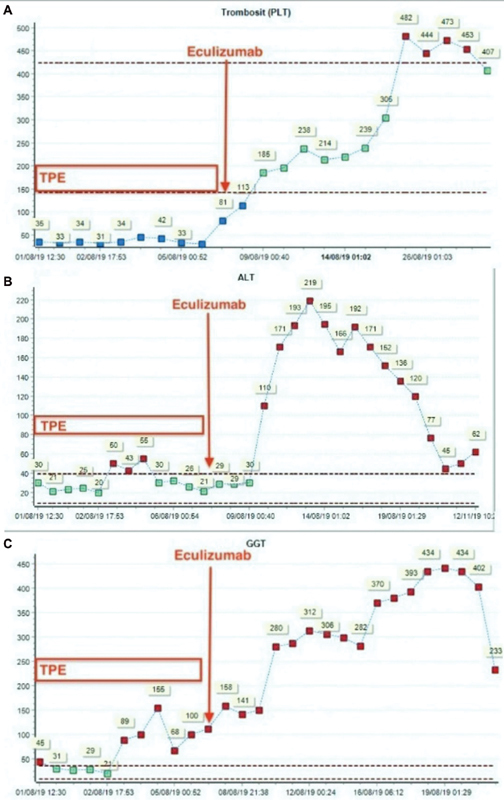
The notable increase in the levels of trombosit (PLT), alanine transaminase (ALT), gamma glutamyltransferase (GGT) after the administration of eculizumab. TPE, therapeutic plasma exchange.
On the day 8 of PICU admission, Shiga toxin-producing and enteroaggregative E. coli (EAEC) were detected in the stool: STEC-HUS serotype 0157:H7 and EAEC serotype O104:H4 were both positive according to National Public Health Laboratory investigation by Multiplex Qualitative reverse transcriptase PCR. Therefore, the diagnosis of STEC-HUS was confirmed. Due to this result and the associated adverse effect of hepatotoxicity, eculizumab treatment was terminated. The patient was extubated on the day 10 of PICU admission. Following extubation, cardiac magnetic resonance imaging was done, which demonstrated subendocardial gadolinium enhancement of the left ventricle consistent with acute myocarditis. Her general improvement continued and 4 days later she was transferred to a regular pediatric ward. She stayed in the regular pediatric ward for a week and was discharged from the hospital after returning to her normal state of health. She was followed by our outpatient clinic for 6 months and continued the treatment with oral ursodeoxycholic acid due to her decreasing cholestasis laboratory findings AST: 63 U/L; ALT: 77 IU/L; GGT: 402 U/L; ALP: 527 U/L; TB: 0.75 mg/dL; and DB: 0.33 mg/dL. After 6 months, oral ursodeoxycholic acid treatment terminated once the laboratory findings of cholestasis normalized.
Discussion
Myocardial involvement is uncommon in pediatric patients with STEC-HUS. Recent literature indicates that 3 to 5% of patients with STEC-HUS present ischemic cardiomyopathy/myocarditis at the acute phase of STEC-HUS. 2 3 The fact that serum troponin level and echocardiography were not documented before eight day of hospitalization should be acknowledged, as it may be that left ventricular dysfunction and myocardial ischemia were, in fact, present subclinically earlier than detected. TMA of the coronary vessels is the main underlying histopathological finding, and it leads to myocardial ischemia/infarction/dysfunction, myocarditis, congestive heart failure, arrhythmias, and pericardial effusion/tamponade. 2 3 4 5 6 Histopathologically proven, myocarditis has also been reported in association with HUS. 6 7 Abu-Arafeh et al 6 reported a case of a 13-year-old adolescent with HUS and documented E. coli O157:H7 who had sudden cardiac arrest and subsequently died due to the development of hypotension, bradycardia, and ventricular arrhythmias. Histopathological examination of the myocardium on autopsy demonstrated inflammation consisting of monocytic and lymphocytic infiltrates. Ray et al 7 also reported two children with myocarditis due to coxsackie B virus infection in association with HUS. However, in our case, we could not detect any concurrent viral etiology.
We thought TMA and coronary thrombosis led to left ventricular dysfunction in our patient. She was treated with milrinone infusion, and eculizumab was initiated for this life-threatening cardiac involvement secondary to HUS. The fact that the cardiac failure happened after 8 days of TPE suggests that TPE was ineffective in treating the cardiac component. Still, the occurrence of a life-threatening cardiomyopathy/myocarditis 6 days after admission and at the time when kidney function was improving is unusual. Mauras et al recently reported in a quite similar case. 15 In our patient, acute myocarditis was accepted to be the primary diagnosis due to her rapid recovery and the notable amelioration in her pro-BNP levels. Cardiac magnetic resonance imaging findings were also consistent with acute myocarditis.
Fresh frozen plasma (FFP), TPE, and eculizumab have all been used to treat severe forms of STEC-HUS, without demonstrated efficacy. 8 9 We initiated TPE after she was admitted to the PICU for possible diagnosis of complement-mediated HUS because of nonbloody diarrhea, low C4 level. While eculizumab is not a standard-of-care treatment for STEC-HUS, it has been used in some cases of severe STEC-HUS. 10 11 In this case, eculizumab treatment was applied for acute myocarditis that emerged despite daily TPE treatment. The improvement of kidney function during the first week of hospitalization may just be the spontaneous evolution of the STEC-HUS, favored by the careful volume monitoring and hydration.
Another intriguing development, in this case, was that the patient had a low level of C4. The diagnosis of atypical HUS in children is a diagnosis of exclusion either by history or exclusion of STEC-HUS using a rapid test of Stx in stools, mandatory in any child presenting HUS. Usually, STEC-HUS patients do not have a low C4 level. Furthermore, low C4 does not necessarily favor a diagnosis of atypical HUS, a disease of the alternative pathway of complement with normal C4 levels in the majority of cases, even those with low C3 (the latter is inconstant because the complement overactivation is mostly at the vascular endothelial cell surface, not in the circulation). We think that the reason behind the patient's persistently low C4 concentration was that her complement pathway was active at the time; lending rationale to our claim that eculizumab is a treatment option for STEC-HUS patients. 8 Unfortunately, we could not definitively diagnose STEC-HUS immediately because STEC in her stool examination was sent to the external center for further analysis, since PCR analysis could not be done in our hospital, and we had to wait for 8 days to receive the results. We performed daily TPE in the early period and continued until eculizumab was supplied. Renal function and hematological parameters gradually improved with TPE. At our patient's age of 17 months, both STEC-HUS and atypical HUS overlap epidemiologically (with STEC-HUS far more frequent than atypical HUS). The treatment of STEC-HUS today remains exclusively supportive, with no benefit from plasma infusions or TPE, which are no longer recommended. The efficiency of eculizumab in STEC-HUS is unproven by case reports or small retrospective series, but this treatment remains proposed in patients with life-threatening neurological or cardiac complications. We are hopeful that the results of two prospective trials of eculizumab in children with STEC-HUS can elucidate the benefit of this treatment.
Hepatotoxicity in association with eculizumab has been reported in the literature. 12 13 14 Hayes et al reported elevated aminotransferases in 7 of 11 children treated with eculizumab. 12 One patient was discontinued because of excessive elevation of liver enzymes, jaundice, and clinical symptoms of tender hepatomegaly after eculizumab infusion. Oruc et al 13 have observed hepatotoxicity following eculizumab treatment in an adult HUS patient with no preexisting liver disease. The mechanism of hepatotoxicity associated with biologic drugs like eculizumab is not fully understood. Antibody-mediated injury and blockage of C5 (which is useful in hepatic defense and regeneration) are some explanations for the underlying mechanism. In our patient, hepatic and cholestatic enzymes were increased after a single dose of eculizumab. Ursodeoxycholic acid and N-acetylcysteine infusion were administered. Because of hepatotoxicity and a diagnosis of STEC-HUS with subsequent recovery of acute kidney injury and acute myocarditis, eculizumab treatment was discontinued. Hepatic and cholestatic enzymes returned to normal after six months in our patient.
Conclusion
To the best of our knowledge, this is the first case reported in the literature about acute fulminant myocarditis and eculizumab-induced hepatotoxicity in a toddler diagnosed with STEC-HUS. Eculizumab treatment can be useful in terms of convalescing STEC-HUS associated acute myocarditis; however, hepatotoxicity may also develop in the treatment process. This case illustrates the need for vigilance when dealing with myocardial involvement and eculizumab-induced hepatotoxicity in patients with STEC-HUS.
Funding Statement
Funding None.
Conflict of Interest None declared.
Authors' Contributions
All authors participated in creating content for the manuscript, editing, and provided final approval for submission. No undisclosed authors contributed to the manuscript.
Ethical Approval
Permission was granted by the parents and patient to publish the case report.
References
- 1.Canpolat N. Hemolytic uremic syndrome. Turk Pediatri Ars. 2015;50(02):73–82. doi: 10.5152/tpa.2015.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid M, Andreoli S. Extrarenal manifestations of the hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC HUS) . Pediatr Nephrol. 2019;34(12):2495–2507. doi: 10.1007/s00467-018-4105-1. [DOI] [PubMed] [Google Scholar]
- 3.Thayu M, Chandler W L, Jelacic S, Gordon C A, Rosenthal G L, Tarr P I. Cardiac ischemia during hemolytic uremic syndrome. Pediatr Nephrol. 2003;18(03):286–289. doi: 10.1007/s00467-002-1039-3. [DOI] [PubMed] [Google Scholar]
- 4.Veien M, Kamperis K, Rittig S. HUS-induced cardiac and circulatory failure is reversible using cardiopulmonary bypass as rescue. Pediatr Nephrol. 2017;32(11):2155–2158. doi: 10.1007/s00467-017-3736-y. [DOI] [PubMed] [Google Scholar]
- 5.Palanca Arias D, López Ramón M, Jiménez Montañés L. Biomarkers detect involvement of acute myocardial injury in a paediatric haemolytic-uraemic syndrome patient. Cardiol Young. 2016;26(05):983–986. doi: 10.1017/S1047951115002759. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Arafeh I, Gray E, Youngson G, Auchterlonie I, Russell G. Myocarditis and haemolytic uraemic syndrome. Arch Dis Child. 1995;72(01):46–47. doi: 10.1136/adc.72.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray C G, Portman J N, Stamm S J, Hickman R O. Hemolytic-uremic syndrome and myocarditis. Association with coxsackievirus B infection. Am J Dis Child. 1971;122(05):418–420. doi: 10.1001/archpedi.1971.02110050088010. [DOI] [PubMed] [Google Scholar]
- 8.Buelli S, Zoja C, Remuzzi G, Morigi M. Complement activation contributes to the pathophysiology of shiga toxin-associated hemolytic uremic syndrome. Microorganisms. 2019;7(01):E15. doi: 10.3390/microorganisms7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loos S, Oh J, Kemper M J. Eculizumab in STEC-HUS: need for a proper randomized controlled trial. Pediatr Nephrol. 2018;33(08):1277–1281. doi: 10.1007/s00467-018-3972-9. [DOI] [PubMed] [Google Scholar]
- 10.Mahat U, Matar R B, Rotz S J. Use of complement monoclonal antibody eculizumab in Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome: a review of current evidence . Pediatr Blood Cancer. 2019;66(11):e27913. doi: 10.1002/pbc.27913. [DOI] [PubMed] [Google Scholar]
- 11.Percheron L, Gramada R, Tellier S et al. Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol. 2018;33(08):1385–1394. doi: 10.1007/s00467-018-3903-9. [DOI] [PubMed] [Google Scholar]
- 12.Hayes W, Tschumi S, Ling S C, Feber J, Kirschfink M, Licht C. Eculizumab hepatotoxicity in pediatric aHUS. Pediatr Nephrol. 2015;30(05):775–781. doi: 10.1007/s00467-014-2990-5. [DOI] [PubMed] [Google Scholar]
- 13.Oruc A, Ayar Y, Vuruskan B A et al. Hepatotoxicity associated with eculizumab in a patient with atypical hemolytic uremic syndrome. Nefrologia. 2018;38(04):448–450. doi: 10.1016/j.nefro.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Yesilbas O, Sevketoglu E, Petmezci M T et al. Infant onset severe complement-mediated hemolytic uremic syndrome complicated by secondary sclerosing cholangitis. J Clin Apher. 2018;33(05):619–623. doi: 10.1002/jca.21651. [DOI] [PubMed] [Google Scholar]
- 15.Mauras M, Bacchetta J, Duncan A. Escherichia coli-associated hemolytic uremic syndrome and severe chronic hepatocellular cholestasis: complication or side effect of eculizumab? Pediatr Nephrol. 2019;34(07):1289–1293. doi: 10.1007/s00467-019-04234-6. [DOI] [PubMed] [Google Scholar]


