Abstract
Background:
Hidradenitis suppurativa (HS) is a chronic debilitating disease with a relapsing and remitting course. Due to delay in diagnosis, patients are often referred when the disease is very severe. Management strategies vary across multiple guidelines.
Aims:
The aim of this study was to analyze the demographic and clinical characteristics of patients with HS among our outpatient attendees and to study the outcomes of various treatments offered.
Methodology:
This was a retrospective cohort study analyzing case files and photographic records of all patients diagnosed with HS, presenting to our tertiary care institute over 18 months.
Results:
A total of 22 patients (10 males and 12 females) of HS were studied with majority having Hurley stage 2 and 3 diseases. The most common site affected was axilla. Overweight and obese patients were 45.4% and 18.1%, respectively. Rifampicin–clindamycin combination or doxycycline was the first line therapy offered. Adalimumab was given in only two patients but could not be continued for long term due to financial issues. Surgery was performed in six patients. Procedures included wide local excision and deroofing which is left to heal by secondary intention. Least number of remissions and most satisfactory improvement was seen with a combination of antibiotics and surgery compared to medical treatment alone.
Limitations:
Retrospective nature and a single center study were the major drawbacks.
Conclusion:
Patients undergoing procedural intervention in addition to pharmacotherapy have best overall outcomes and involvement of a multidisciplinary team plays a key role, however a larger follow-up study is required.
Keywords: Hidradenitis suppurativa, India, treatment modalities
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, recurrent, debilitating inflammatory disease characterized by episodes of inflammatory nodules, pustules, abscesses, and scars occurring in apocrine gland rich areas. The diagnosis is usually delayed due to misdiagnosis as simple pyoderma.[1] Although management guidelines for HS exists, there is large heterogeneity among them. In this study, we exemplify the clinico-demographic characteristics of patients with HS among our outpatient attendees and demonstrate outcomes of various treatments offered.
Methodology
This was a retrospective study analyzing case records and photographs of patients with HS, attending dermatology OPD of our tertiary care institute between June 2018 and December 2019. Diagnosis of HS was made clinically and Hurley staging performed [Figure 1a–c]. Data obtained included patient's demographic data age, gender, body mass index (BMI), history of smoking and clinical characteristics like age at onset, associations like pilonidal sinus, acne, scalp folliculitis, polycystic ovarian syndrome (PCOS), details about past and current treatments and response and side effects to therapy.
Figure 1.
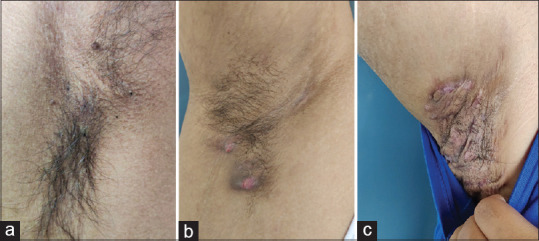
Hurley stage. (a) Hurley stage I - localized formation of single or multiple abscesses without sinus tracts or scarring. (b) Hurley stage II - single or multiple widely separated recurrent abscesses with sinus tract formation and scarring. (c) Hurley stage III - diffuse or near-diffuse involvement, interconnected tracts, and abscesses across the entire area
Statistical analysis
Descriptive statistics were applied to variables of demographic data and disease characteristics. Continuous data are presented as mean for normally distributed variables. Categorical data are presented as numbers and frequencies.
Results
Clinical parameters
During study period, 22 patients (10 males and 12 females) of HS were assessed. The mean age at disease onset and mean disease duration was 26.2 ± 8.46 years and 35 ± 24.2 months, respectively. Among our cohort, 36.6% had normal BMI, 45.4% were overweight and 18.1% obese. A positive family history was observed in 2 patients [Table 1].
Table 1.
Patient characteristics
| Clinical characteristics | Hurley Stage 1 (n=3, 13.6%) | Hurley Stage 2 (n=7, 31.8%) | Hurley Stage 3 (n=12,54.4%) | Total n=22, (%) | |
|---|---|---|---|---|---|
| Sex, (n) | Male | 1 | 2 | 7 | 10 (45.4) |
| Female | 2 | 5 | 5 | 12 (54.4) | |
| Age of onset (year), mean | 21 | 26.8 | 27.1 | 26.2+- 8.46 | |
| Disease duration (months), mean | 12 | 40 | 37.8 | 35+-24.2 | |
| BMI subgroups† | Normal | 2 | 2 | 4 | 8 (36.3) |
| Overweight | 0 | 3 | 7 | 10 (45.4) | |
| Obese | 1 | 2 | 1 | 4 (18.1) | |
| Associations with follicular occlusion syndrome | Severe Acne/Acne conglobata | 0 | 4 | 3 | 7 (31.8) |
| Pilonidal sinus | 0 | 3 | 4 | 7 (31.8) | |
| Scalp folliculitis | 0 | 1 | 1 | 2 (9) | |
| PCOS | Yes | 0 | 3 | 0 | 3 (13.6) |
| No | 2 | 2 | 5 | 9 | |
| Not applicable | 1 | 2 | 7 | 10 | |
| Smoking status | Current smoker | 0 | 1 | 2 | 3 (13.6) |
| Site of involvement | Axillary | 3 | 6 | 12 | 21 (95) |
| Inguinal | 0 | 4 | 7 | 11 (50) | |
| Intermammary | 0 | 2 | 4 | 6 (27.2) | |
| Retro auricular | 0 | 0 | 2 | 2 (9) | |
| Perianal | 0 | 0 | 5 | 5 (22.7) | |
| Abdominal | 0 | 1 | 0 | 1 (4.5) | |
†Body mass Index- Normal body weight was defined as BMI below 19-25 kg/m2, overweight if the BMI ranged between 25 and 29.9 kg/m2, obese if the BMI was above 30 kg/m2
Disease characteristics
Hurley stages I, II, and III were seen in 13.6%, 31.8%, and 54.4% patients, respectively, and this did not correlate with either gender or age. The most common site affected was axilla (95%) followed by inguinal area (40%). Associations [Figure 2a] like acne (including acne conglobata) were seen in 31.8% patients. Another 31.8% had current or past history of pilonidal sinus [Figure 2b]. Scalp folliculitis was seen in 9% of cases. Past history or current history of PCOS was seen in 13.6% of patients, two of whom were on metformin and cyproterone acetate. Follicular occlusion triad and tetrad (HS, acne conglobata, pilonidal sinus, dissecting cellulitis of scalp) was seen in two and one patient, respectively [Table 1].
Figure 2.
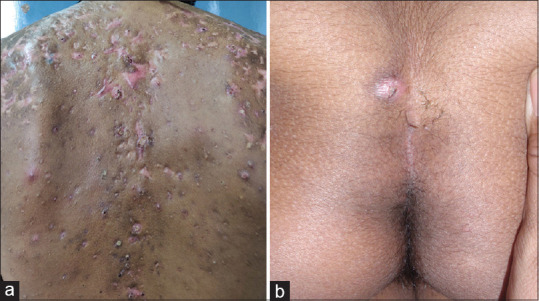
Associations with HS. (a) Acne conglobata in a patient with HS.(b) Pilonidal sinus in a patient with HS
Treatment offered
All patients had already received multiple short courses of oral antibiotics followed by incision and drainage of individual solitary lesions in past (19, 86.3%).
At our center routine pus culture was not done in all patients. The most common medical management prescribed by us was combination of rifampicin (600 mg) and clindamycin (300 mg twice daily) in 81.1% for an average duration of 4.2 months. The next common drug given was isotretinoin (20 mg, daily in72.7%) followed by either doxycycline or minocycline (63.6%). Average duration of isotretinoin therapy was 9.4 months and for doxycycline/minocycline it was 6.3 months. In nine patients, there was a shift from doxycycline to rifampicin–clindamycin due to inadequate response after 8–12 weeks of therapy. Adalimumab was given to only two patients in Hurley stage 3. The dosing protocol followed was 160 mg on Day 1; 80 mg on Day 15; 40 mg on Day 29 followed by 40 mg every week. Once disease activity was controlled, frequency was reduced to 40 mg every two weekly. The two patients received these injections for 1 year and 6 months, respectively, in combination with surgical intervention and oral antibiotics. Due to financial constraints, further doses were stopped. Low-dose steroids or intralesional steroids were used during phases of acute disease exacerbations in 72.7% [Table 2].
Table 2.
Various treatment modalities used across Hurley stages
| Hurley stage 1 | Hurley stage 2 | Hurley stage 3 | Total n, (%) | |
|---|---|---|---|---|
| Medical management | ||||
| Rifampicin plus clindamycin | 2 | 6 | 10 | 18 (81.1) |
| Doxycycline/minocycline | 2 | 5 | 7 | 14 (63.6) |
| Isotretinoin | 3 | 5 | 8 | 16 (72.7) |
| Adalimumab | 0 | 0 | 2 | 2 (9) |
| Surgical intervention | ||||
| Yes | 0 | 2 | 4 | 6 (27.2) |
Surgical management
Plastic surgery intervention (multiple staged sittings) was performed in six patients (27.2%) with stage 2 and 3 disease. Nodules and scarring of axilla had more favorable response to surgery with fewer recurrences and fewer number of sittings compared to perineal lesions. Intermammary lesions took longest time to heal by secondary intention. Among patients with pilonidal sinus, four had undergone surgical treatment for same. Surgical interventions included wide local excision and deroofing with wound left for closure with secondary intention [Figure 3a–d]. Although immediate post-operative period included daily wound care, pain management and a long recovery period of about 4–6 weeks but on follow-up after 1 year, patients had overall better quality of life with only minor recurrences which could be managed with intermittent courses rifampicin/clindamycin combination.
Figure 3.
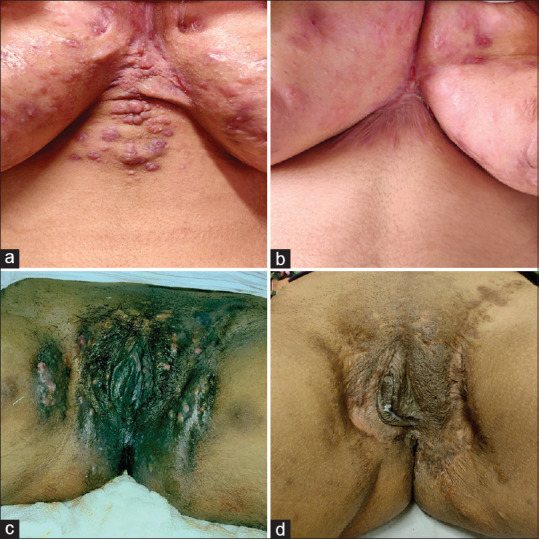
Response to treatment. (a) Hurley stage 3 disease involving the intermammary area. (b) Significant improvement seen after 12 doses of adalimumab and surgical excision of intermammary nodules. (c) Hurley stage 3 disease involving the perineal region and groin resulting in significant disfigurement. (d) Improvement of lesions in groin seen after 20 doses of adalimumab and 3 sittings of serial excision of nodules
Course of disease
Recurrences were noted in all patients within 2–4 weeks of drug discontinuation. Maximum remission on treatment in form of reduction in appearance of new lesions, cessation of pus/serous discharge from preexisting nodules and improvement in quality of life was noted with combination therapy of rifampicin–clindamycin and isotretinoin. Patients who underwent surgical intervention did not show recurrence at site of surgery but continued to develop newer lesions elsewhere.
Discussion
Majority of patients who presented to us had Hurley stage 2 and 3. Patients with stage 1 disease are seldom referred to tertiary center, making it difficult to assess the exact HS prevalence.[2] The mean disease duration was 35 months prior to presentation to us, this long lag-time is attributed to hesitation that patients experience and delay in referral from primary centers.[2,3]
Majority of our patients were overweight which is in concordance with previous reports.[4,5] Mechanical friction and secondary bacterial infections in obese individuals contributes to HS.[4] In our cohort, recalcitrant inter-mammary lesions were noted in overweight women with heavy breasts. Choosing right type of clothing plays a significant part of patient counselling regarding lifestyle changes.[6] Although smoking is a known risk factor, we observed very few (13.6%) active smokers in our cohort. Similar observations were noted in another single center study from Germany.[7]
The prevalence of pilonidal sinus was 31% which is in accordance with previous studies ranging from 4.6% to 30%.[8] A population based study, revealed that likelihood of patients with HS developing PCOS was 2.14 times than general population, hence recommended PCOS screening in all females with HS presenting with features of androgen excess.[9] We did not find any significant association of PCOS and HS in our female patients.
Regarding management guidelines, several dermatological societies have published their own guidelines since 2015.[10,11,12,13,14] Broadly, modalities with strong supporting evidence include topical clindamycin, oral tetracyclines, rifampicin plus clindamycin combination, oral retinoids and adalimumab. Procedural modalities recommended are deroofing or wide local excision depending on the stage.[12] The main concern with any of the above treatment is the chronicity of HS and its high recurrence rate on cessation of therapy. For antibiotics the usual period of administration in various guidelines ranges from 12 weeks to as long as 6 months. The dysbiosis of cutaneous microbiome has been proposed as one of the factors involved in the complex pathogenesis of HS. None of the currently used drugs have shown to normalize the resident microbiome in HS and novel drug targets need to be identified.[15]
Adalimumab is only FDA approved agent for HS and can be given for long term, but widespread application of this is not practical in our set up as most patients are not covered under health insurance. Moreover, studies have shown that efficacy in HS decreases over time.[14] There is no guideline which gives a stringent stepwise management approach and finally treatment has to be individualized. In our study, for stage 2 and 3 disease; a combination of medical and surgical management was found most optimal. This benefit of combination therapy has also been observed in other studies.[10] Given the high psychological morbidity coupled with its deleterious impact on sexual health,[16] it is important to emphasize on the long term benefits of a combination therapy rather than pharmacotherapy alone. Although expensive, immunomodulatory drugs in combination with surgery was found to optimally target scarring, inflammation and perceived pain. These drugs were also found to reduce the wound healing time.[17]
The retrospective study design, low patient count, and fewer follow-up were main limitations.
Conclusion
Herein we observed that treatment strategy needs to be modified as per patient requirements. The most frequent first line treatments given were oral antibiotics–rifampicin–clindamycin combination with isotretinoin being next. The duration of therapy was highly variable and was decided based on clinical response. Steroids worked well to reduce intermittent disease exacerbations. We found that patients undergoing procedural intervention in addition to pharmacotherapy had best overall outcomes. The role of a multidisciplinary team has to be emphasized upon with involvement of dermatologist, plastic surgeon, dietician, psychiatrist and gynecologist for the holistic management of a patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389–92. doi: 10.1046/j.1468-3083.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 2.Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546–9. doi: 10.1111/bjd.14038. [DOI] [PubMed] [Google Scholar]
- 3.Zimman S, Comparatore MV, Vulcano AF, Absi ML, Mazzuoccolo LD. Hidradenitis suppurativa: Estimated prevalence, clinical features, concomitant conditions, and diagnostic delay in a University Teaching Hospital in Buenos Aires, Argentina. Actas Dermo-Sifiliográficas Engl Ed. 2019;110:297–302. doi: 10.1016/j.ad.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrocini G, De Vita V, Donnarumma M, Russo G, Monfrecola G. South Italy: A privileged perspective to understand the relationship between hidradenitis suppurativa and overweight/obesity. Skin Appendage Disord. 2016;2:52–6. doi: 10.1159/000447716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalom G, Freud T, Harman-Boehm I, Polishchuk I, Cohen AD. Hidradenitis suppurativa and metabolic syndrome: A comparative cross-sectional study of 3207 patients. Br J Dermatol. 2015;173:464–70. doi: 10.1111/bjd.13777. [DOI] [PubMed] [Google Scholar]
- 6.Loh TY, Hendricks AJ, Hsiao JL, Shi VY. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2019:1–6. doi: 10.1159/000501611. [DOI] [PubMed] [Google Scholar]
- 7.Wollina U, Langner D, Heinig B, Nowak A. Comorbidities, treatment, and outcome in severe anogenital inverse acne (hidradenitis suppurativa): A 15-year single center report. Int J Dermatol. 2017;56:109–15. doi: 10.1111/ijd.13393. [DOI] [PubMed] [Google Scholar]
- 8.Benhadou F, Van der Zee HH, Pascual JC, Rigopoulos D, Katoulis A, Liakou AI, et al. Pilonidal sinus disease: An intergluteal localization of hidradenitis suppurativa/acne inversa: A cross-sectional study among 2465 patients. Br J Dermatol. 2019;181:1198–206. doi: 10.1111/bjd.17927. [DOI] [PubMed] [Google Scholar]
- 9.Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: A population-based analysis in the United States. J Invest Dermatol. 2018;138:1288–92. doi: 10.1016/j.jid.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks AJ, Hsiao JL, Lowes MA, Shi VY. A Comparison of international management guidelines for hidradenitis suppurativa. Dermatology. 2019:1–16. doi: 10.1159/000503605. [DOI] [PubMed] [Google Scholar]
- 11.Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:91–101. doi: 10.1016/j.jaad.2019.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunger RE, Laffitte E, Läuchli S, Mainetti C, Mühlstädt M, Schiller P, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatol Basel Switz. 2017;233:113–9. doi: 10.1159/000477459. [DOI] [PubMed] [Google Scholar]
- 13.Magalhães RF, Rivitti-Machado MC, Duarte GV, Souto R, Nunes DH, Chaves M, et al. Consensus on the treatment of hidradenitis suppurativa-Brazilian Society of Dermatology. An Bras Dermatol. 2019;94:7–19. doi: 10.1590/abd1806-4841.20198607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619–44. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 15.Langan EA, Recke A, Bokor-Billmann T, Billmann F, Kahle BK, Zillikens D. The role of the cutaneous microbiome in hidradenitis suppurativa-light at the end of the microbiological tunnel. Int J Mol Sci. 2020;21:1205. doi: 10.3390/ijms21041205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alavi A, Farzanfar D, Rogalska T, Lowes MA, Chavoshi S. Quality of life and sexual health in patients with hidradenitis suppurativa. Int J Womens Dermatol. 2018;4:74–9. doi: 10.1016/j.ijwd.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollina U, Brzezinski P, Koch A, Philipp-Dormston WG. Immunomodulatory drugs alone and adjuvant to surgery for hidradenitis suppurativa/acne inversa-A narrative review. Dermatol Ther. 2020;19:e13877. doi: 10.1111/dth.13877. [DOI] [PubMed] [Google Scholar]


