Introduction
The complex of myxomas (cutaneous/mammary/genital/cardiac) with pigmentary and endocrinal abnormalities was first reported by Carney in 1985.[1] Subsequently, superficial angiomyxoma (SAM) was described as an independent entity in the absence of Carney's complex.[2] SAM presents as a gradually progressing, well-defined, soft-nodule, usually on the trunk, limbs or head and neck, and rarely in the genital region.[3] We hereby discuss the clinical, histopathological, and immunohistochemical features of large SAM arising from vulva in a middle-aged woman and an adolescent girl.
Report of Cases
Case 1
A 38-year-old woman presented with a 2-year history of a slowly enlarging, asymptomatic swelling appearing from the vulva. Physical examination revealed a pedunculated, light-pink colored, firm, non-tender swelling, 9 × 4 cm in size with overlying erosions focally, arising from right labium majus [Figure 1a]. The swelling was completely excised with differential diagnoses of giant acrochordon and soft tissue tumor of vulva. Histopathologic examination revealed focally ulcerated epidermis with dermis showing proliferating thin-walled blood-vessels in a myxoid stroma, along with scattered neutrophils. Stromal cells were spindle to stellate shaped with no nuclear atypia [Figure 2a]. Immunohistochemistry showed tumor cells with diffuse expression of vimentin [Figure 2b] and negative for desmin [Figure 2c], smooth muscle actin (SMA), estrogen receptor (ER), progesterone receptor (PR), and CD34. Systemic examination and ultrasound of pelvis were unremarkable. Based on the clinico-pathological findings, a diagnosis of superficial angiomyxoma was rendered. No recurrence occurred after 1-year of follow-up.
Figure 1.
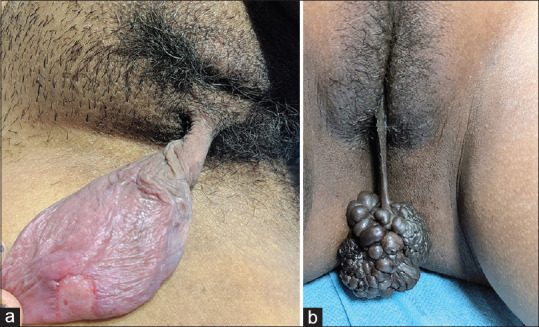
(a) Pedunculated erythematous firm swelling of size 9 × 4 cm with superficial erosion on the posterior surface, arising from the right labium majus. (b) Pedunculated hyperpigmented swelling of size 7 × 5 cm with lobulated surface and rubbery consistency arising from the right labium minus
Figure 2.
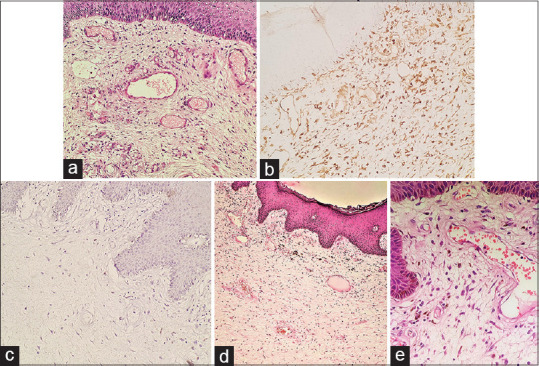
(a) Dermis shows numerous dilated thin-walled small-sized blood vessels in a myxoid stroma. The stromal cells are stellate shaped without nuclear atypia (hematoxylin and eosin, ×200). (b) Tumor cells in the stroma are immunoreactive for vimentin (vimentin, ×200). (c) Stromal cells are negative for desmin (Desmin, ×200). (d) Epidermis is acanthotic. There is a proliferation of variable sized thin-walled blood vessels in a myxoid stroma in the dermis (hematoxylin and eosin, ×100). (e) The stroma contains spindle-shaped cells admixed with inflammatory infiltrate comprising of eosinophils, neutrophils, mast cells and lymphocytes (hematoxylin and eosin, ×400)
Case 2
A 17-year-old girl presented with an asymptomatic dark brown colored vulvar swelling for 1 month. Cutaneous examination revealed a pedunculated, multilobulated, dark brown to black colored rubbery swelling, 7 × 5 cm in size, arising from right labium minus [Figure 1b]. With the provisional diagnoses of giant acrochordon and soft tissue tumor of vulva, the swelling was completely excised. Histopathological examination showed a dermal tumor composed of spindle cells diffusely distributed in a myxoid stroma. Numerous small to medium-sized, thin-walled blood vessels were present, along with mild to moderate inflammatory infiltrate comprising of eosinophils, mast cells, neutrophils, and lymphocytes [Figure 2d and e]. No mitosis was noted. Spindle cells were diffusely positive for vimentin and negative for desmin, SMA, ER, PR, and CD34. Systemic examination and ultrasound of pelvis did not reveal any abnormality. A final diagnosis of superficial angiomyxoma was made. No recurrence was noted after 6 months of follow-up.
Discussion
SAM is a benign mesenchymal tumor that frequently occurs in males in the fourth decade of life and uncommonly presents in the genital region.[3] In a study by Fetsch et al., out of 17 cases involving the genitalia, 13 were females and 4 were males. Scrotum was affected in male patients.[2] Among females, labium majus was involved in 6, vulva in 4, groin in 2, and mons pubis in 1 patient.[2] The mean age of presentation is 23 years and clinically manifests as an asymptomatic, soft, pedunculated polyp. The size of tumor ranges from 0.9 to 4 cm, with an average of 2.1 cm and the largest reported being 20 × 10 cm.[3,4] Differential diagnosis for small SAM comprises of skin tag, labial cyst, Bartholin cyst, Gartner's duct cyst and perineal herniation, and of large SAM include aggressive angiomyxoma (AAM) and angiomyofibroblastoma (AMB).[3] The clinical and histopathological differences between SAM, AAM, and AMB are listed in Table 1.[2,3,5,6]
Table 1.
Clinico-pathological features of superficial angiomyxoma, aggressive angiomyxoma and angiomyofibroblastoma
| Superficial angiomyxoma | Aggressive angiomyxoma | Angiomyofibroblastoma | |
|---|---|---|---|
| Age | 15-55 years | 16-70 years | Premenopausal <60 years |
| Gender | More common in males in extra-genital area and females in genital area | More frequent in females | More common in females |
| Site of involvement | Trunk, limbs, head, and neck. Rarely involves the genitalia | Vulvovaginal, perineal, and pelvic region | Vulvovaginal |
| Clinical morphology | Papulo-nodular to polypoidal lobulated mass | Well-defined, pedunculated mass, soft in consistency | Well-defined, soft to firm pedunculated lesion |
| Histopathology | Well-circumscribed and unencapsulated | Poorly circumscribed with infiltrative margins | Well-circumscribed with alternating hypocellular and hypercellular areas |
| Vascularity | Numerous thin-walled blood vessels | Thick-walled blood vessels | Thin-walled blood vessels |
| Tumor cells | Multilobular, lobules of spindle or stellate shaped cells No atypia or mitosis |
Spindle, stellate or oval cells with ill-defined borders entrapping fat, nerve and blood vessels Minimal to no atypia Mitosis is rare |
Epithelioid or plasmacytoid cells with abundant eosinophilic cytoplasm. Accentuation of tumor cells around blood vessels Mitosis and atypia are absent |
| Stroma | Myxoid stroma with abundant neutrophils | Myxoid stroma | Wavy collagen with absence of mucin |
| Immunohistochemical markers | |||
| Vimentin | + | + | + |
| S.100 | + | - | - |
| Desmin | - | + | + |
| ER/PR | - | + | + |
| SMA | Occasionally+ | + | - |
| CD34 | Occasionally + | + | |
| Treatment and prognosis | Simple excision Recurrence rate: 30-40% No metastasis | Wide local excision Hormonal therapy has been tried Recurrence rate: 50-70% Low tendency to metastasize | Simple excision No recurrence No metastasis |
ER: Estrogen receptor; PR: Progesterone receptor; SMA: Smooth muscle actin
SAM is derived from fibroblast-like cells with the ability to transform in response to various cytokines.[2] Though it could be an initial manifestation of Carney's complex, the association between SAM of the genital region and Carney's complex is not established in literature.[2] Our patients did not have other findings of Carney's complex.
Management is surgical excision with clear margins. The rate of local recurrence is 30–40% with incomplete resection. No metastasis or malignant transformation has been reported.[3]
The present cases illustrate that dermatologists should consider SAM as a differential diagnosis for soft-tissue vulval tumors and histopathological evaluation including immunohistochemistry is essential to establish the diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–83. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fetsch JF, Laskin WB, Tavassoli FA. Superficial angiomyxoma (cutaneous myxoma): A clinicopathologic study of 17 cases arising in the genital region. Int J Gynecol Pathol. 1997;16:325–34. doi: 10.1097/00004347-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Kim GY, Lim SJ, Ki KD, Kim HC. Giant superficial angiomyxoma of the vulva: A case report and review of the literature. J Cutan Pathol. 2010;37:672–7. doi: 10.1111/j.1600-0560.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Zhao W, Shi Y, Lin B. Superficial angiomyxoma of the vulva complicated with condyloma acuminatum and Staphylococcus hominis infection. Int J Dermatol. 2014;53:756–8. doi: 10.1111/j.1365-4632.2012.05572.x. [DOI] [PubMed] [Google Scholar]
- 5.Eckhart S, Rolston R, Palmer S, Ozel B. Vaginal angiomyofibroblastoma: A case report and review of diagnostic imaging. Case Rep Obstet Gynecol. 2018;2018:1–8. doi: 10.1155/2018/7397121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar H, Iqbal B, Dogra BB, Chandanwale S. Giant aggressive angiomyxoma of the vulva. J Med Soc. 2016;30:58–60. [Google Scholar]


