Abstract
Context:
Assessment of peripheral nerves in leprosy by clinical methods is subject to considerable inter-observer variability. High resolution ultrasonography (HRUS) can assess peripheral nerves more objectively.
Aims:
To compare the findings of peripheral nerve involvement in newly diagnosed cases of leprosy by clinical and sonological methods.
Settings and Design:
Cross sectional study in a tertiary care teaching hospital.
Subjects and Methods:
Four pairs of peripheral nerves of 40 newly diagnosed patients with leprosy were examined clinically and by HRUS.
Statistical Analysis Used:
Agreement between clinical examination and HRUS using kappa statistic; sensitivity; specificity; and predictive values.
Results:
Of the 320 nerves examined, 71 (22.18%) were abnormal clinically and 63 (19.7%) sonologically. Sonological abnormalities were increased cross sectional area (n = 63; 100%), hypoechogenicity with loss of fascicular architecture (n = 46; 73%) and increased vascularity (n = 35; 55.6%). There was moderate agreement (Cohen's Ḳ = 0.59) between clinical and sonological findings. HRUS findings were abnormal in 18 (7.2%) nerves that were clinically normal. HRUS was normal in 26 (36.6%) nerves which were clinically abnormal. Sensitivity of HRUS compared to clinical examination was 63%; specificity 92.7%; positive predictive value 71.4%; and negative predictive value 89.9%. Increased cross sectional area agreed with clinical findings the most.
Conclusions:
HRUS has low sensitivity (63%) and high specificity (92.7%) to identify abnormal peripheral nerves in leprosy, compared to clinical examination. It could detect abnormality of some (n = 18, 7.2%) clinically normal nerves, but showed normal findings of some nerves (n = 26, 36.6%), which were considered clinically abnormal.
Keywords: High resolution ultrasonography, leprosy, ultrasonography
Introduction
Clinical examination is still the gold standard in the diagnosis and assessment of nerve involvement in leprosy, but it is prone for considerable inter observer variability.[1] High resolution ultrasonography (HRUS) is a recent investigative modality to assess peripheral nerves.[2] HRUS features of nerve involvement in leprosy include increased cross sectional area indicating nerve enlargement; increased blood flow signals in epineurium and endoneurium indicating inflammation; and changes in echo texture and fascicular architecture of the nerves.[3,4,5,6] Greater objectivity and noninvasive nature of HRUS make it a promising tool in detecting nerve involvement in leprosy.[7]
Probably due to the nonavailability of HRUS and its special probe in most treatment centers in India, it remains underutilized for this purpose. There are a few studies to assess its value to aid or supplement clinical diagnosis. We decided to compare the clinical and sonological characteristics of commonly affected peripheral nerves (ulnar, median, common peroneal, and posterior tibial) in newly detected cases of leprosy in our institution.
Subjects and Methods
This was a cross sectional study among new cases of leprosy attending the department of dermatology, venereology, and leprosy of a tertiary care teaching hospital from December 2015 to May 2017. Leprosy was diagnosed when at least one of the following cardinal signs were present (as per 8th WHO expert committee on leprosy)[8]
Definite loss of sensation in a pale (hypo pigmented) or reddish skin patch.
A thickened or enlarged peripheral nerve, with loss of sensation and/or weakness of the muscles supplied by that nerve.
The presence of acid-fast bacilli in a slit-skin smear.
All new cases meeting this criterion were included in the study. Nerves were considered to be clinically involved if the second criterion was satisfied. Informed written consent was obtained from all participants. Those who did not give consent and those patients who had neurological deficits due to other diseases were excluded. Ethical clearance was obtained from institutional ethical committee.
History taking and clinical examination were done with emphasis to the signs and symptoms related to peripheral nerve involvement of leprosy. This included presence of nerve thickening, tenderness, or nodularity on palpation and sensory or motor deficit along the distribution of the nerve. Four pairs of nerves (ulnar, median, common peroneal, and posterior tibial nerves) were examined by two investigators independently to arrive at a consensus. If there was divergence of opinion between the two investigators, the decision was made based on the opinion of a third (more senior) faculty member. Before starting multi drug therapy, ultrasonography and color doppler evaluation of peripheral nerves were performed in the department of radio diagnosis of our hospital. It was done by a radiologist having 14 years post MD experience in doing nerve ultrasound studies. He was blinded to the clinical examination findings. Ultrasound imaging was done using Logic S8 ultrasound machine, GE Healthcare, USA. ML 6-15 linear array transducer with a frequency range of 4.5–15 mega-hertz was used in assessing peripheral nerves. Due to the superficial location of peripheral nerves, the maximum available frequency of 15 mega-hertz was used.
We identified transverse sonological section of the nerves where it appeared to have maximum cross sectional area (CSA). Cross sectional area was measured with the original software of the GE Logic S8 ultrasound machine by continuous tracing technique with calipers, done manually at the inner border of the thin hyperechoic epineural rim in the cross section of the nerve perpendicular to its longitudinal axis. We measured CSA of each nerve three times, using the “area measuring software protocol” in the ultrasound machine and took the average CSA of each nerve rounded off to the nearest whole number. CSA was considered increased if it was more than the mean value of this parameter of the control subjects belonging to an ethnically similar population in a similar study by Jain et al.[5] Accordingly, the selected normal values for cross sectional area of ulnar nerve, median nerve, common peroneal nerve, and posterior tibial nerve were 8.5, 6.2, 5.9, and 6.3 mm2, respectively. Echogenicity of the nerve was assessed subjectively based on the visual observation of the image.[9]
Settings of color doppler ultrasound examination were chosen to identify signals from low flow velocity vessels in the nerves. After B mode imaging of the nerve, a color box is put over a small part of the nerve in its longitudinal axis. Color gain was increased till color bleed (noise) appears in the color box and the color gain is kept just lower to this to avoid the noise. The pulse repetition frequency was kept sufficiently very low to pick up very low blood flow with avoidance of noise in the image and arterial pulsations are searched for. We used wall filter settings of 50 Hz and the lowest possible pulse repetition frequency which did not produce artefacts. Normal nerves are not expected to show significant arterial pulsations.[9] The presence of blood flow signals in the perineural plexus or intrafascicular vessels during color doppler imaging was taken as a sign of hypervascularity of the nerve. The nerves were graded based on the presence or absence of hyper vascularity. Nerves were considered to be sonologically abnormal if at least one of the following parameters was present on cross sectional plane.
Increased cross sectional area.
Hypo echogenicity of the nerve (fascicles and inter fascicular perineurium) with or without loss of architecture.
Hyper vascularity.
Data obtained was first entered in a proforma and then coded and entered to a master sheet. Percentages of descriptive data were calculated. The agreement between clinical examination and HRUS was assessed using kappa statistic. Considering clinical examination as gold standard and HRUS as the test, sensitivity, and specificity of HRUS in assessment of four pairs of peripheral nerves and overall predictive values were calculated.
Results
The clinical profile of the study subjects is given in Table 1. Among the 40 patients studied, the common age groups affected were 41–60 years (n = 17; 42.5%,) and 21–40 years (n = 12; 30%). There were 29 males and 11 females. Most common clinical spectrum was borderline tuberculoid (n-24; 60%). Others were borderline lepromatous (n = 7; 17.5%), lepromatous (n-6; 15%) and indeterminate, histoid, and pure neuritic types of leprosy (n = 1; 2.5% each).
Table 1.
Profile of leprosy patients
| Category | Clinical type | Number of patients with positive AFB smear | Number of patients with lepra reactions | |
|---|---|---|---|---|
|
| ||||
| Type 1 | Type 2 | |||
| Multi bacillary (n=29) | Borderline tuberculoid (n=14) | 0 | 3 | 0 |
| Borderline lepromatous (n=7) | 5 | 3 | 1 | |
| Lepromatous (n=6) | 5 | 0 | 1 | |
| Histoid (n=1) | 1 | 0 | 1 | |
| Pure neuritic (n=1) | 0 | 0 | 0 | |
| Pauci bacillary (n=11) | Borderline tuberculoid (n=10) | 0 | 0 | 0 |
| Indeterminate (n=1) | 0 | 0 | 0 | |
Out of the 320 nerves examined (of 40 patients), 71 (22.18%) were clinically involved. More common among these were left ulnar (n = 15; 37.5%) and right ulnar (n = 14; 35%) nerves. Normal nerves showed fascicular architecture. [Figure 1]. Sonological characteristics of peripheral nerves involved in leprosy were as follows: increased cross sectional area, hypoechogenicity with loss of fascicular architecture [Figure 2], and increased vascularity [Figure 3]. Out of the total 320 peripheral nerves, 63 (19.7%) were sonologically abnormal. All of them showed increased cross sectional area. 46 nerves (14.4%) showed hypoechogenicity with loss of fascicular architecture and 35 nerves (10.9%) showed increased vascularity. [Tables 2 and 3]. Right ulnar (n = 18, 45%) and left ulnar (n = 15; 37.5%) were the more commonly affected nerves sonologically. HRUS detected abnormality in 18 (7.2%) nerves that were not clinically involved. HRUS did not show abnormality in 26 (36.6%) nerves which were clinically involved. Highest sensitivity of HRUS was for right median (100%) and the least was for right and left posterior tibial nerves (40% for each). Highest specificity of HRUS was for right and left common peroneal nerves (100% each) and the least was for right ulnar nerve (73.1%). Overall sensitivity of HRUS as compared to clinical examination was 63% and specificity was 92.7%. Positive predictive value was 71.4% and negative predictive value was 89.9%. There was moderate agreement between clinical and sonological involvement (Cohen's Ḳ = 0.59). Increased cross sectional area was the sonological parameter which agreed most with clinical findings (Cohen's Ḳ = 0.59; moderate agreement). Hypoechogenicity showed moderate agreement (Cohen's Ḳ = 0.43) and hypervascularity showed fair agreement (Cohen's Ḳ = 0.36).
Figure 1.
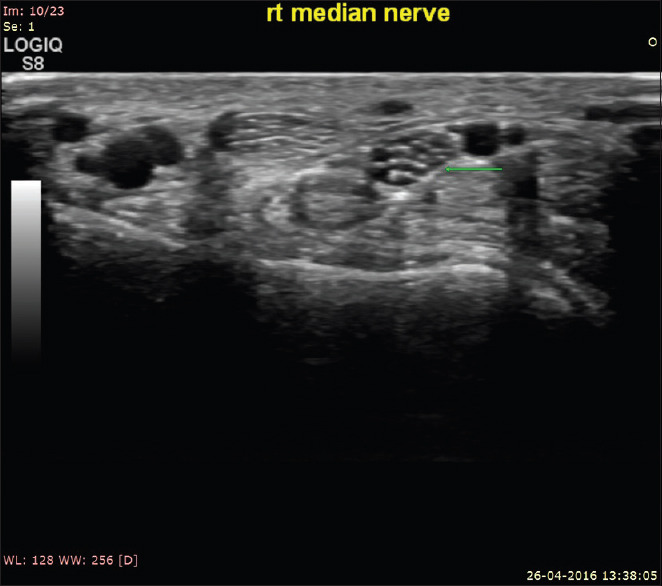
Cross sectional ultrasound image of normal right median nerve showing normal fascicular architecture
Figure 2.
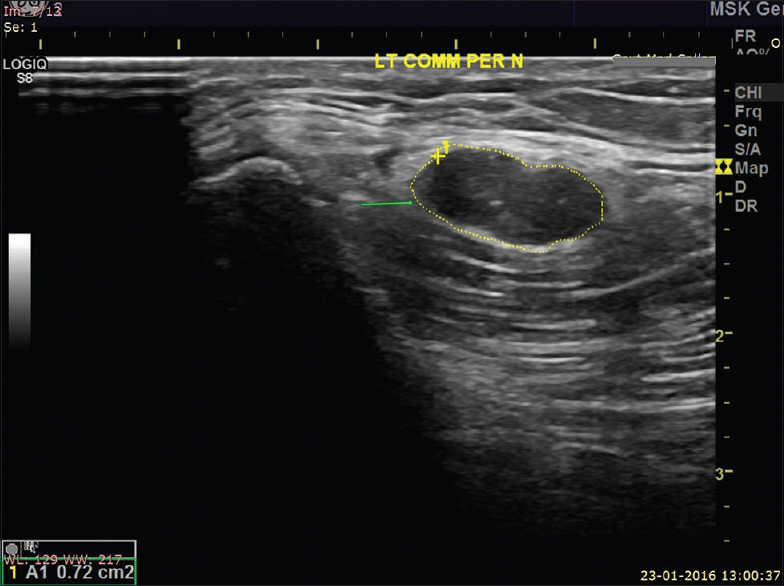
Cross sectional ultrasound image of enlarged hypoechoic left common peroneal nerve (CSA = 72 mm2) showing loss of fascicular architecture
Figure 3.
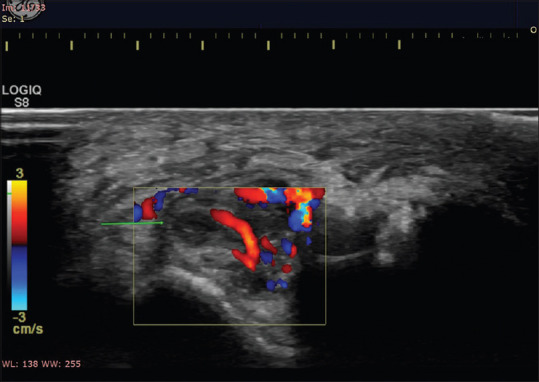
Doppler image of enlarged hypoechoic right ulnar nerve in a patient with neuritis showing loss of fascicular architecture and increased intraneural vascularity
Table 2.
Sonological findings of clinically abnormal peripheral nerves
| Name of the nerve | Increased cross sectional area (n, %) | Hypoechogenicity with loss of fascicular architecture (n, %) | Increased vascularity (n, %) | Sonologically abnormal (n, %) |
|---|---|---|---|---|
| Right ulnar (n=14) | 11 (78.6) | 9 (64.3) | 7 (50) | 11 (78.6) |
| Left ulnar (n=15) | 10 (66.7) | 8 (53.3) | 4 (26.7) | 10 (66.7) |
| Right median (n=4) | 4 (100) | 3 (75) | 2 (50) | 4 (100) |
| Left median (n=5) | 4 (80) | 3 (60) | 3 (60) | 4 (80) |
| Right common peroneal (n=9) | 5 (55.6) | 2 (22.2) | 2 (22.2) | 5 (55.6) |
| Left common peroneal (n=14) | 7 (50) | 4 (28.6) | 4 (28.6) | 7 (50) |
| Right posterior tibial (n=5) | 2 (40) | 1 (20) | 1 (20) | 2 (40) |
| Left posterior tibial (n=5) | 2 (40) | 1 (20) | 1 (20) | 2 (40) |
Table 3.
Sonological findings of clinically normal peripheral nerves
| Name of the nerve | Increased cross sectional area (n, %) | Hypoechogenicity with loss of fascicular architecture (n, %) | Increased vascularity (n, %) | Sonologically abnormal (n, %) |
|---|---|---|---|---|
| Right ulnar (n=26) | 7 (26.9) | 6 (23) | 3 (11.5) | 7 (26.9) |
| Left ulnar (n=25) | 5 (20) | 3 (12) | 2 (8) | 5 (20) |
| Right median (n=36) | 2 (5.6) | 2 (5.6) | 2 (5.6) | 2 (5.6) |
| Left median (n=35) | 1 (2.9) | 1 (2.9) | 1 (2.9) | 1 (2.9) |
| Right common peroneal (n=31) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Left common peroneal (n=26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Right posterior tibial (n=35) | 1 (2.9) | 1 (2.9) | 1 (2.9) | 1 (2.9) |
| Left posterior tibial (n=35) | 2 (5.7) | 2 (5.7) | 2 (5.7) | 2 (5.7) |
Discussion
Fornage (1988) first described the sonological features of peripheral nerves.[10] Silvestri et al. mentioned that peripheral nerves could easily be identified by their predictable anatomic location and characteristic appearance.[11] Lawande et al. described radiological picture of a normal nerve.[12] In transverse section, it showed small hypoechoic areas separated by hyperechoic septae, giving a “honeycomb-like” appearance. The hypoechoic area represented nerve fascicles whereas the echogenic septae represented interfascicular perineurium. The longitudinal sections also revealed the fascicular architecture, showing a “bundle of straws” appearance. On dynamic examination, the nerves show sliding movement over the muscles and tendons. An altered movement or contour deformity during movement of the nerve gave a clue to diagnose the pathology.[12] In the regions where peripheral nerves are closely associated with vascular structures, the use of color doppler helps to distinguish nerves from vessels.
Our study shows that the sonological features of peripheral nerves in leprosy are increased cross sectional area, hypoechogenicity with or without loss of architecture and hypervascularity. Increased cross sectional area was the sonological parameter which agreed most with clinical findings. This is consistent with findings of several studies from different parts of the world. A study by Afsal et al. from New Delhi showed significant thickening of both ulnar nerves and median nerves both in carpal tunnel and forearm in patients with leprosy as compared to controls.[13] They found that the nerve thickening was more extensive and involved more nerves than those were clinically diagnosed. Lugao et al. in their study showed that asymmetry was a characteristic of leprosy neuropathy regardless of its classification.[6] Martinoli found that color doppler imaging of the nerves showed increased vascularization in reactions probably indicating inflammation.[4]
In our study, out of the 320 nerves, 71 (22.18%) were clinically involved. Out of the total 320 peripheral nerves, 63 nerves (19.7%) were sonologically abnormal. Increased cross sectional area was the most frequently associated sonological parameter. In a study by Ashwini et al., out of the 210 nerves examined, 86 (41%) were clinically thickened and 138 (65.7%) were sonologically thickened.[14] In this study also cross sectional area of ulnar, median, and common peroneal nerves were significantly increased in cases than controls and a value above 0.08 cm2 was considered to be a good predictor of nerve thickening. In our study HRUS detected sonological abnormality in 18 (7.2%) nerves that were not clinically involved. If we add the HRUS evidence of peripheral nerve involvement to clinical findings, 89 (27.8%) nerves would have been considered to be abnormal—an increase of more than 5%, over and above the number of abnormal nerves based on only clinical examination. In a study by Kumaran et al., 41 out of 240 nerves (17.1%) which were clinically normal were found to be enlarged on sonography.[15] This shows that at least in some cases, HRUS can detect nerve involvement earlier than clinical examination. Jain et al. reported significant correlation between clinical parameters such as the grade of thickening, sensory loss, and muscle weakness with sonological abnormalities such as nerve echotexture, endoneurial flow, and cross sectional area.[5] In our study HRUS was normal in 26 (36.6%) nerves which were clinically involved. The proportion of clinically abnormal nerves being normal sonologically was higher in the studies by Jain et al. (39/86; 45.35%) and lower in Kumaran et al. (21/130; 16.15%).[5,15] But as long as sonological methods have not been established to be superior to clinical methods, it would be prudent to consider a nerve as abnormal if either clinical or sonological methods suggest so. Therefore, it is reasonable to infer that a combination of clinical methods and HRUS would help to improve the ascertainment of peripheral nerve involvement in Hansen's disease.
Absence of a control group is a limitation of our study. Also, the normal values for cross sectional area of ulnar nerve, median nerve, common peroneal nerve, and posterior tibial nerve were taken from a previous study from south India[5] As our study population is ethnically similar, we believe that this would not have affected the validity of our findings much. Subjective assessment of the echogenicity of the nerves may depend on the echogenicity of the surrounding tissues and the depth of the nerve from the skin surface and these can be sources of error.[9] Quantitative methods of nerve echogenicity by various automated methods would have avoided such a bias. Another limitation is that we have not analyzed the relationship of the sonological findings with clinical findings such as thickening, tenderness, and loss of nerve function, separately.
Early nerve changes may show only minimal clinical involvement. HRUS may reveal early nerve changes in such cases and can be more sensitive than clinical assessment. Still, due to the unavailability of HRUS and easiness of clinical assessment, the latter remains the gold standard for assessment of peripheral nerves in leprosy and therefore we decided to compare the relatively newer HRUS with it. In several previous studies, the main parameter assessed was the agreement between clinical and sonological findings using the kappa statistics. We used sensitivity, specificity, and predictive values, in addition to kappa statistics. We found that correlation between clinical and HRUS is better for upper limb nerves compared to lower limb nerves. A possible explanation for this is that, compared with upper limb nerves, sonological assessment of lower limb nerves can be more difficult especially in obese patients with thick subcutaneous fat or lower limb oedema.
HRUS could detect abnormality in several nerves that were clinically normal. At the same time, sonological evaluation was normal in several nerves which were clinically abnormal. This suggests that HRUS cannot replace clinical examination in evaluation of peripheral nerve involvement in leprosy. Rather, it would be prudent to supplement clinical examination with HRUS wherever such facilities are available. This would help to confirm the diagnosis in a greater number of patients with suspected leprosy as peripheral nerve involvement is one of the cardinal diagnostic criteria. Future studies may explore if combination of HRUS with clinical examination can provide earlier and more reliable detection of nerve involvement in patients with leprosy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chen S, Wang Q, Chu T, Zheng M. Inter-observer reliability in assessment of sensation of skin lesion and enlargement of peripheral nerves in leprosy patients. Lepr Rev. 2006;77:371–6. [PubMed] [Google Scholar]
- 2.Wilder Smith EP, Van Brakel WH. Nerve damage in leprosy and its management. Nat Clin Pract Neurol. 2008;4:656–63. doi: 10.1038/ncpneuro0941. [DOI] [PubMed] [Google Scholar]
- 3.Rai D, Malhotra HS, Garg RK, Goel MM, Malhotra KP, Kumar V, et al. Nerve abscess in primary neuritic leprosy. Lepr Rev. 2013;84:136–40. [PubMed] [Google Scholar]
- 4.Martinoli C, Derchi LE, Bertolotto M, Gandolfo N, Bianchi S, Fiallo P, et al. US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol. 2009;29:142–50. doi: 10.1007/s002560050584. [DOI] [PubMed] [Google Scholar]
- 5.Jain S, Visser LH, Praveen TL, Rao PN, Surekha T, Ellanti R, et al. High resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498. doi: 10.1371/journal.pntd.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugão HB, Nogueira-Barbosa MH, Marques W Jr, Foss NT, Frade MA. Asymmetric nerve enlargement: A characteristic of leprosy neuropathy demonstrated by ultrasonography. PLoS Negl Trop Dis. 2015;9:e0004276. doi: 10.1371/journal.pntd.0004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beekman R, Schoemaker MC, Van Der Plas JP, Van Den Berg LH, Franssen H, Wokke JH, et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62:767–73. doi: 10.1212/01.wnl.0000113733.62689.0d. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1–61. [PubMed] [Google Scholar]
- 9.Im Suk J, Walker FO, Cartwright MS. Ultrasonography of peripheral nerves. Curr Neurol Neurosci Rep. 2013;13:328. doi: 10.1007/s11910-012-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fornage BD. Peripheral nerves of the extremities: Imaging with US. Radiology. 1988;167:179–82. doi: 10.1148/radiology.167.1.3279453. [DOI] [PubMed] [Google Scholar]
- 11.Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: Correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291–6. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 12.Lawande AD, Warrier SS, Joshi MS. Role of ultrasound in evaluation of peripheral nerves. Indian J Radiol Imaging. 2014;24:254–8. doi: 10.4103/0971-3026.137037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afsal M, Chowdhury V, Prakash A, Singh S, Chowdhury N. Evaluation of peripheral nerve lesions with high-resolution ultrasonography and color Doppler. Neurol India. 2016;64:1002–9. doi: 10.4103/0028-3886.190269. [DOI] [PubMed] [Google Scholar]
- 14.Ashwini B, NandaKishore B, Basti RS, Martis J, Hundi GK, Jayaraman J. Ultrasound as a diagnostic modality for the involvement of peripheral nerves in leprosy. Indian J Lepr. 2018;90:1–14. [Google Scholar]
- 15.Kumaran MS, Thapa M, Narang T, Prakash M, Dogra S. Ultrasonography versus clinical examination in detecting leprosy neuropathy. Lepr Rev. 2019;90:364–70. [Google Scholar]


