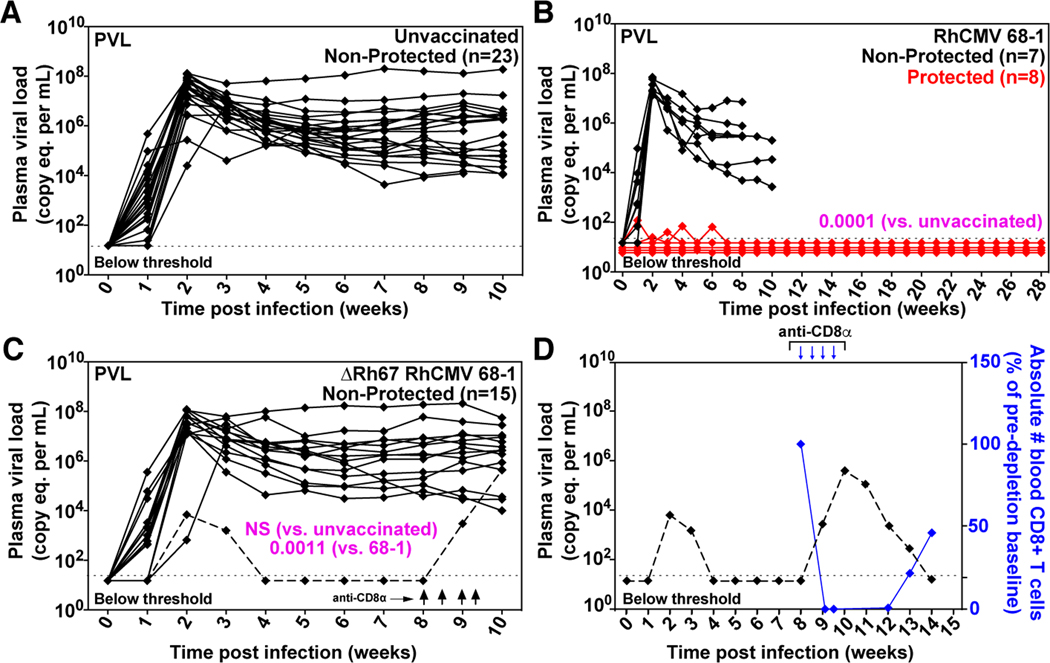
Figure 8. Efficacy of ΔRh67 68–1 RhCMV/SIV vectors.
(A, B, C) Assessment of the outcome of SIV infection after repeated, limiting dose SIVmac239 challenge of the ΔRh67 68–1 RhCMV/SIV vector vaccinated RM, relative to RM vaccinated with the Rh67-intact parent 68–1 vectors [red delineates RM with documented SIV replication arrest; reproduced from (14)], and to unvaccinated controls (including RM contemporaneously challenged with both vaccinated cohorts). (D) The dashed line in panel C delineates the one of 15 ΔRh67 68–1 RhCMV/SIV vector set-vaccinated RM that showed abbreviated SIV viremia post-infection, prompting us to perform CD8α cell depletion at day 57 post-infection to distinguish conventional elite control (susceptible to CD8α cell depletion) to RhCMV/SIV vector vaccination induced SIV replication arrest (not susceptible to CD8α cell depletion; see fig. S6). In panels A-C, binomial exact P-values are shown where the proportion of RM with SIV replication arrest (protected) in one vaccine group differs significantly from the designated comparison group. NS = not significant.