Abstract
Despite progress in the treatment of diabetic macular edema and diabetic retinopathy, the rate of lower fundus examination due to limitations of medical resources delays the diagnosis and treatment of diabetic retinopathy. Therefore, implementation of automated diabetic retinopathy screening program and the identification of more specific and sensitive biomarkers are important for facilitating the earlier detection of diabetic macular edema and diabetic retinopathy to decrease the prevalence of poor vision and blindness.
Keywords: Diabetes retinopathy, Epidemiology, Risk factors, Screening, Treatment
Epidemiology
Diabetic retinopathy (DR) is a common microvascular complication of diabetes mellitus and is the leading cause of visual loss in the elderly. Hyperglycemia and altered metabolic pathways lead to oxidative stress and the development of neurodegeneration in the initial stage of diabetic retinopathy1. Vascular endothelial damage, the development of microaneurysms, and dot intraretinal hemorrhage are early hallmarks of non‐proliferative diabetic retinopathy (NPDR). Disruption of the blood–retinal barrier and leakage of multiple inflammatory cytokines and plasma proteins lead to the hard exudates observed under fundoscopy. As disease progresses, vasoconstriction and capillary occlusions lead to tortured capillaries and retinal ischemia. The presence of ‘cotton wool spots’ can be seen during this stage. At the end stage of diabetic retinopathy, severe hypoxia leads to neovascularization, vitreous hemorrhage, and retinal detachment1.
In Taiwan, the prevalence of diabetic eye disease ranged from 3.75 to 3.95%, and the prevalence of poor vision and blindness ranged from 0.29 to 0.35% during 2005 to 20142. In Korea, the prevalence of diabetic retinopathy increased from 14.3% in 2006 to 15.9% in 20133. Both studies revealed that women with type 2 diabetes had a higher prevalence of diabetic retinopathy than men, but men suffered from more severe retinopathy, poor vision, or blindness. The severity of diabetic retinopathy not only impacted the quality of life, but also predicted all‐cause, vascular, and non‐cancer mortality4. Another study found that diabetic retinopathy is associated with prolonged QT interval, which may play a role in life‐threatening arrhythmia5.
Risk factors, preventive factors, and biomarkers
The development of diabetic retinopathy strongly correlates with a longer duration of diabetes, greater hyperglycemia, and hypertension. A higher HbA1c level is significantly associated with the progression of diabetic retinopathy6, 7 and intensive glycemic control reduces the incidence and deterioration of retinopathy8. In recent studies, glycemic variability was found to be strongly associated with diabetic retinopathy in type 2 diabetes9. Therefore, correcting postprandial hyperglycemia is also important for the prevention of diabetic retinopathy10. Moreover, there is clear evidence regarding the relationship between hypertension and diabetic retinopathy. Tight blood pressure control reduces the deterioration of retinopathy11. Other risk factors include nephropathy, dyslipidemia, smoking, and higher body mass index, which are also modifiable to prevent the progression of diabetic retinopathy12, 13, 14.
Despite the above mentioned risk factors, studies revealed a substantial variation in the development and severity of diabetic retinopathy that could not be fully explained by the known risk factors. Thus, identifying more biomarkers to stratify the risk or to evaluate the therapeutic response of diabetic retinopathy is important.
Systemic biomarkers include markers of inflammation such as C‐reactive protein (CRP)15 and homocysteine16, and the advanced glycation end products (AGE)17 are related to the pathogenic process and the risk of diabetic retinopathy. Many more biomarkers have been identified by plasma proteomic approach. For example, retinol‐binding protein 1 (RBP1), diphosphoinositol polyphosphohydrolase 3 alpha, neuroglobin (NGB) were downregulated and hemoglobin subunit gamma 2 (HBG2) and CD 160 antigen18 were upregulated in diabetic retinopathy. Among the above mentioned five proteins, the plasma level of neuroglobin may be a potential biomarker for diabetic retinopathy due to the significant difference between the control and the diabetic retinopathy group18. Furthermore, metabolomics19, micro RNA20, and genetic biomarkers are also under extensive investigation.
Ocular biomarkers include sampling the vitreous and tears as well as ocular imaging. Increases in angiogenic factors, such as vascular endothelial growth factor (VEGF)21 and platelet‐derived growth factor (PDGF)22, and decreases in anti‐angiogenic factors, e.g., pigment epithelium‐derived factor (PEDF)23, were detected in the vitreous in diabetic retinopathy. Also, an increased microaneurysm turnover rate24 and larger retinal vessel diameters25 detected by retinal fundus photos and retinal neurodegeneration recognized by optical coherence tomography (OCT)26 were associated with the development of diabetic retinopathy. However, most of the novel biomarkers have yet to be applied in clinical practice and require more validation studies for predicting diabetic retinopathy and its clinical outcomes.
Screening
As diabetic retinopathy remains the leading cause of visual impairment, screening for diabetic retinopathy is important to early detect preventable blindness. Most patients with developed diabetic retinopathy have no symptoms until macular edema (ME) or proliferative diabetic retinopathy (PDR) presents. Although panretinal laser photocoagulation (PRP) and intraocular VEGF inhibitor injection are effective for ME or PDR related visual impairment, they benefit more in preventing visual loss than in reversing deteriorated visual acuity. Therefore, a timely screening program for diabetic retinopathy could assist individuals with diabetes to preserve their vision.
According to current guidelines for the screening of diabetic retinopathy, reported by the International Council of Ophthalmology (ICO) and American Diabetes Association (ADA) in 2018, the timing of the first eye examination and minimum screening examinations are required for appropriate referral to an ophthalmologist27, 28. For type 2 diabetes patients, the first eye examination should be initiated once a diagnosis of diabetes is confirmed, as for type 1 diabetes, the timing being extended to 5 years after the onset of diabetes. Minimum screening, on the other hand, includes visual acuity examination and retinal examination27. Both examinations should be performed by well‐trained personnel, and retinal imaging could be analyzed either by human‐based telemedicine or an automated computer system29. Thanks to the advances of artificial intelligence (AI) and a deep learning algorithm, the first automated diabetic retinopathy screening program has been approved by FDA since April 201830, achieving a 96.8% sensitivity and 87% specificity for detecting referable diabetic retinopathy31. Recently, implementation of a smartphone‐based retinal camera in low‐resource settings also showed great potential in the detection of diabetic retinopathy when combined with telemedicine32. To maximize the cost‐effectiveness of diabetic retinopathy screening, several studies introduced an individualized screening schedule based on the patient’s risk of proliferative diabetic retinopathy or macular edema29, 33, 34. It was estimated that it would save $1 billion over 20 years, compared with routine annual screening in type 1 diabetes in the USA33. However, the individualized screening schedule in type 1 diabetes needs further validation in type 2 diabetes.
Treatments
In addition to optimal medical control of blood glucose, blood pressure and serum cholesterol level, several intraocular managements have become standard treatments for diabetic retinopathy27, 28. As for patients with diabetic macular edema (DME), the use of anti‐VEGF therapy has reformed its management, including agents such as ranibizumab, bevacizumab, and aflibercept. Since 2010, several large randomized trials have demonstrated the efficacy of all three anti‐VEGF agents in the reduction of diabetic macular edema and in the improvement of vision35, 36, 37, 38, 39, 40. However, in eyes with diabetic macular edema with initial worse visual acuity (20/50 or worse), aflibercept could result in better visual acuity than did bevacizumab at 2 years41. Despite the progress in anti‐VEGF therapy, the optimal frequency of injections and the duration of the treatment course are still largely unknown. Most patients might require frequent injection of intravitreous anti‐VEGF during the first year of treatment, and fewer injections are needed in following years for the maintenance of remission27.
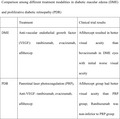
For patients with proliferative diabetic retinopathy, on the other hand, panretinal laser photocoagulation has been demonstrated to be effective in reducing the risk of vision loss42, 43. PRP, therefore, is considered the preferred treatment for patients with all stages of PDR and severe NPDR44, 45. In addition to PRP, recent studies have also provided evidence that intravitreous injection of anti‐VEGF may be a safe alternative treatment for PDR. The clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy (CLARITY) study demonstrated that eyes assigned to anti‐VEGF group (aflibercept) had better visual‐acuity results than eyes assigned to the PRP group at 1 year46. The Diabetic Retinopathy Clinical Research Network randomized trial showed that visual‐acuity outcomes in the anti‐VEGF group (ranibizumab) were non‐inferior to those in the PRP group at both 2 years and 5 years47, 48. The comparison among different treatment modalities in diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) is summarized in Table 1. However, adherence to frequent follow‐up, treatment burden, and patients’ preference must be considered when applying the above mentioned clinical trial results to clinical practice in the real world.
Table 1.
Comparison among different treatment modalities in diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR)
| Treatment | Clinical trial results | |
|---|---|---|
| DME | Anti‐vascular endothelial growth factor (VEGF): ranibizumab, evacizumab, aflibercept35, 36, 37, 38, 39, 40 | Aflibercept resulted in better visual acuity than bevacizumab in DME eyes with initial worse visual acuity41 |
| PDR | Panretinal laser photocoagulation (PRP)42, 43, Anti‐VEGF: ranibizumab, evacizumab, aflibercept | Aflibercept group had better visual acuity than PRP group46; Ranibuzumab was non‐inferior to PRP group47, 48 |
The molecular basis of diabetic retinopathy are still under investigation, such as the involvement of (pro)renin receptor in the retinal renin‐angiotensin system (RAS) activation pathway49. These studies might help the discovery of novel drug targets and provide more individualized therapies.
Conclusion
In summary, despite progress in the treatment of diabetic macular edema and diabetic retinopathy, the lower fundus examination rate due to the limitation of medical resources delayed the diagnosis and treatment of diabetic retinopathy. Therefore, implementation of an automated diabetic retinopathy screening program and identification of more specific and sensitive biomarkers are important to facilitate the earlier detection of diabetic macular edema and diabetic retinopathy to decrease the prevalence of poor vision and blindness.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig. 2021
References
- 1.Wong TY, Cheung CMG, Larsen M, et al. Diabetic retinopathy. Nat Rev Dis Prim 2016; 2: 16012. [DOI] [PubMed] [Google Scholar]
- 2.Lin KD, Hsu CC, Ou HY, et al. Diabetes‐related kidney, eye, and foot disease in Taiwan: an analysis of nationwide data for 2005–2014. J Formos Med Assoc 2019; 118(Suppl. 2): S103–S110. [DOI] [PubMed] [Google Scholar]
- 3.Song SJ, Han K, Choi KS, et al. Trends in diabetic retinopathy and related medical practices among type 2 diabetes patients: Results from the National Insurance Service Survey 2006–2013. J Diabetes Investig 2018; 9: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takao T, Suka M, Yanagisawa H, et al. Combined effect of diabetic retinopathy and diabetic kidney disease on all‐cause, cancer, vascular and non‐cancer non‐vascular mortality in patients with type 2 diabetes: a real‐world longitudinal study. J Diabetes Investig 2020; 11: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi S, Nagao M, Asai A, et al. Severity and multiplicity of microvascular complications are associated with QT interval prolongation in patients with type 2 diabetes. J Diabetes Investig 2018; 9: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hainsworth DP, Bebu I, Aiello LP, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 2019; 42: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song KH, Jeong JS, Kim MK, et al. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J Diabetes Investig 2019; 10: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Ma X, Zhang L, et al. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J Diabetes Investig 2019; 10: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takao T, Takahashi K, Yoshida Y, et al. Effect of postprandial hyperglycemia at clinic visits on the incidence of retinopathy in patients with type 2 diabetes: an analysis using real‐world long‐term follow‐up data. J Diabetes Investig 2020; 11: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner R, Holman R, Stratton I, et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 1998; 317: 703–713. [PMC free article] [PubMed] [Google Scholar]
- 12.Estacio RO, McFarling E, Biggerstaff S, et al. Overt albuminuria predicts diabetic retinopathy in hispanics with NIDDM. Am J Kidney Dis 1998; 31: 947–953. [DOI] [PubMed] [Google Scholar]
- 13.Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the ACCORD eye study. Ophthalmology 2014; 121: 2443–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaštelan S, Tomić M, Gverović Antunica A, et al. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm 2013; 2013: 436329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Chen S, Liu X, et al. Relationship between C‐reactive protein level and diabetic retinopathy: a systematic review and meta‐analysis. PLoS One 2015; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tawfik A, Mohamed R, Elsherbiny N, et al. Homocysteine: a potential biomarker for diabetic retinopathy. J Clin Med 2019; 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Chen LJ, Yu J, et al. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem 2018; 48: 705–717. [DOI] [PubMed] [Google Scholar]
- 18.Gopalakrishnan V, Purushothaman P, Bhaskar A. Proteomic analysis of plasma proteins in diabetic retinopathy patients by two dimensional electrophoresis and MALDI‐Tof‐MS. J Diabetes Complications 2015; 29: 928–936. [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Qiao S, Shi C, et al. Metabolomics window into diabetic complications. J Diabetes Investig 2018; 9: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zampetaki A, Willeit P, Burr S, et al. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes 2016; 65: 216–227. [DOI] [PubMed] [Google Scholar]
- 21.Han L, Zhang L, Xing W, et al. The associations between VEGF gene polymorphisms and diabetic retinopathy susceptibility: a meta‐analysis of 11 case‐control studies. J Diabetes Res 2014; 2014: 805801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Praidou A, Klangas I, Papakonstantinou E, et al. Vitreous and serum levels of platelet‐derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res 2009; 34: 152–161. [DOI] [PubMed] [Google Scholar]
- 23.Ogata N, Nishikawa M, Nishimura T, et al. Unbalanced Vitreous levels of pigment epithelium‐derived factor and vascular endothelial growth factor in diabetic retinopathy. Jpn J Clin Ophthalmol 2003; 57: 769–772. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro ML, Nunes SG, Cunha‐VazJ G. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care 2013; 36: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung N, Rogers SL, Donaghue KC, et al. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care 2008; 31: 1842–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeClerck EEB, Schouten JSAG, Berendschot TTJM, et al. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol 2015; 3: 653–663. [DOI] [PubMed] [Google Scholar]
- 27.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow‐up, referral, and treatment based on resource settings. Ophthalmology 2018; 125: 1608–1622. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017; 40: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 2020; 8: 337–347. [DOI] [PubMed] [Google Scholar]
- 30.FDA permits marketing of artificial intelligence‐based device to detect certain diabetes‐related eye problems | FDA. Available at: https://www.fda.gov/news‐events/press‐announcements/fda‐permits‐marketing‐artificial‐intelligence‐based‐device‐detect‐certain‐diabetes‐related‐eye. Published April 11, 2018. Accessed on September 7, 2020.
- 31.Abràmoff MD, Lou Y, Erginay A, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig Ophthalmol Vis Sci 2016; 57: 5200–5206. [DOI] [PubMed] [Google Scholar]
- 32.Constable IJ, Yogesan K, Eikelboom R, et al. Fred hollows lecture: digital screening for eye disease. Clin Exp Ophthalmol 2000; 28: 129–132. [DOI] [PubMed] [Google Scholar]
- 33.Nathan DM, Bebu I, Hainsworth D, et al. Frequency of evidence‐based screening for retinopathy in type 1 diabetes. N Engl J Med 2017; 376: 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takagi H. Novel strategy for screening of diabetic retinopathy. J Diabetes Investig 2018; 9: 726–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badr H, Carmack CL, Kashy DA. Massimo Cristofanilli and TAR. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 23: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT Study). 12‐Month Data: Report 2. Ophthalmology 2010; 117(1078–1086): 1078–1086.e2. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P, Bandello F, Schmidt‐Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 39.Brown DM, Schmidt‐Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100‐week results from the VISTA and VIVID studies. Ophthalmology 2015; 122: 2044–2052. [DOI] [PubMed] [Google Scholar]
- 40.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema two‐year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016; 123: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diabetic T, Study R . Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976; 81: 397–402.944536 [Google Scholar]
- 43.Riaskoff S. Photocoagulation treatment of proliferative diabetic retinopathy. Ophthalmology 1981; 197: 9–17. [PubMed] [Google Scholar]
- 44.Palanker D, Blumenkranz MS. Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med 2011; 365: 1520–1526. [DOI] [PubMed] [Google Scholar]
- 45.Tan GS, Cheung N, Simó R, et al. Diabetic macular oedema. Lancet Diab Endocrinol 2017; 5: 143–155. [DOI] [PubMed] [Google Scholar]
- 46.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single‐blinded, randomised, controlled, phase 2b, non‐inferiority trial. Lancet 2017; 389: 2193–2203. [DOI] [PubMed] [Google Scholar]
- 47.Gross JG, Glassman AR, Liu D, et al. Five‐Year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol 2018; 136: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. J Am Med Assoc 2015; 314: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda A, Ishida S. (Pro)renin receptor: involvement in diabetic retinopathy and development of molecular targeted therapy. J Diabetes Investig 2019; 10: 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


