Abstract
Aims/Introduction
Non‐alcoholic fatty liver disease (NAFLD) is becoming more and more prevalent in type 2 diabetes mellitus. Evidence connecting NAFLD to diabetic retinopathy (DR) is increasing, but the results vary. Thus, we undertook a meta‐analysis to explore the effect of NAFLD on diabetic retinopathy in patients with type 2 diabetes mellitus.
Materials and Methods
PubMed, Embase, Cochrane and Scopus database were searched for until September 30, 2019. Original studies analyzing the association between NAFLD and diabetic retinopathy in the type 2 diabetic population were included. This meta‐analysis was processed by RevMan 5.3 software. Subgroup analyses based on countries were carried out. The pooled odds ratios and 95% confidence intervals were used to evaluate the association between NAFLD and diabetic retinopathy incidence. The I 2 test was used to assess heterogeneity of studies.
Results
We retrieved 414 articles, and nine studies involving 7,170 patients were included in the final analysis. The pooled effects estimate suggested that NAFLD was not associated with the risk of diabetic retinopathy in patients with type 2 diabetes mellitus. Subgroup analysis suggested that in China, Korea and Iran, patients with type 2 diabetes mellitus with NAFLD had a decreased risk for diabetic retinopathy compared with the non‐NAFLD individuals. However, in Italy and India, patients with type 2 diabetes mellitus with NAFLD had an increased risk for diabetic retinopathy compared with the non‐NAFLD individuals. In addition, no relevance between NAFLD and diabetic retinopathy was found in America.
Conclusions
On the whole, there was no association between NAFLD and diabetic retinopathy in individuals with type 2 diabetes mellitus. However, subgroup analysis showed that a difference of country may have an influence on the result.
Keywords: Non‐alcoholic fatty liver disease, Diabetic retinopathy, Type 2 diabetes mellitus
It showed that on the whole, NAFLD was not associated with the risk of DR in T2DM patients. However, subgroup analysis suggested that the difference of country may have an influence on the result.
![]()
INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD), one of the causes of chronic liver disease, is a common multiorgan disorder. It affects about 30% of the general adult population and 60–70% of patients with diabetes and obesity1. NAFLD consists of extensive pathological conditions including simple steatosis, nonalcoholic steatohepatitis, liver fibrosis, and cirrhosis. Some researchers have found a correlation between NAFLD and obesity, insulin resistance and diabetes mellitus2. It has been confirmed that insulin resistance can promote the accumulation of triglycerides in the liver and is a key factor in the pathophysiology of NAFLD3, 4. Diabetic retinopathy (DR) is the most common chronic complication of diabetes mellitus and one of the leading causes of acquired vision loss worldwide5. In Western countries, the prevalence of diabetic retinopathy is around 33%6. Research has proven that the main pathogenic mechanism of diabetic retinopathy is hyperglycemia caused by impaired insulin action due to insulin resistance or insulin deficiency7.
Some studies have indicated that NAFLD may increase the incidence of complications in patients with type 2 diabetes mellitus, especially vascular complications, which comprise macrovascular complications and microvascular complications8, 9. Macrovascular complications involve cardiovascular disease and cerebrovascular disease10, 11, while microvascular complications involve retinopathy and chronic kidney disease12. Multiple studies have proved that NAFLD can clearly increase the morbidity of cardiovascular disease in patients with type 2 diabetes mellitus11, 13, 14. Thus, it is speculated that NAFLD may be a risk factor for diabetic retinopathy among patients with type 2 diabetes mellitus. Increasing research has shown that NAFLD and diabetic retinopathy may have common pathogenic mechanisms and interactions15, 16, 17. However, the evidence for a link between NAFLD and diabetic retinopathy is uncertain due to the small study populations and the borderline associations between NAFLD and traditional risk factors for diabetic retinopathy in the published literature. It is extremely important to provide accurate evidence to prove whether there is a correlation between NAFLD and diabetic retinopathy among patients with type 2 diabetes mellitus. Therefore, we performed this meta‐analysis to evaluate this correlation.
METHODS
Search strategy
The PubMed, Embase, Cochrane, and Scopus databases were searched to gather related articles until September 30, 2019. We retrieved all the English language articles. Our search terms were as follows: (“Non‐alcoholic Fatty Liver Disease”[Mesh] OR Non alcoholic Fatty Liver Disease OR Nonalcoholic Fatty Liver Disease OR NAFLD OR Fatty Liver, Nonalcoholic OR Liver, Nonalcoholic Fatty OR Nonalcoholic Steatohepatitis OR Steatohepatitis, Nonalcoholic OR NASH) AND (“Retinal Diseases” [Mesh] OR Disease, Retinal OR Retinopathy OR Microvascular complications OR Microangiopathy) AND (“Diabetes Mellitus, Type 2”[Mesh] OR T2DM OR Type 2 Diabetes OR Type 2 Diabetes Mellitus). We also retrieved references from the identified original studies, in order to provide a more complete search.
Study selection and inclusion criteria
Two researchers (DD Song, ZC Wang) independently screened the articles for their eligibility for inclusion. We applied the following criteria as eligibility for inclusion: (i) The article was a cross‐sectional design; (ii) The aims of the original studies were to assess the relationship between NAFLD and diabetic retinopathy in patients with type 2 diabetes; (iii) NAFLD had to be diagnosed by liver histology, imaging (ultrasound, computer tomography, magnetic resonance imaging, or spectroscopy), or biochemistry (elevations in serum AST, ALT, or GGT). Competing causes of steatosis, including alcohol intake (≤140 g/week in women and ≤ 210 g/week in man) and viral hepatitis infection had to be excluded according to standard guidelines1; (iv) Diabetic retinopathy was diagnosed by fundus photography and was evaluated by experienced ophthalmologists based on a guideline for clinical treatment of diabetic retinopathy18. We excluded case reports or review articles. We also reviewed the reported institutions, period of data collection, and the authors of eligible studies to exclude overlapping data. When duplicate publications were identified, we included the most recent article.
Data extraction
Two investigators (DD Song, ZC Wang) independently extracted the following information from the included studies: (i) general characteristics including study design, sample size, and year of publication; (ii) diagnostic methods of NAFLD; (iii) the proportion of NAFLD patients and diabetic retinopathy patients; (iv) adjusted confounders. Disagreements between the two investigators were settled by consensus or consultation with another author (YH Zhao).
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale was used to assess the quality of each study, and it was conducted by two reviewers independently (DD Song, CQ Li). The quality of each article ranged from 1 to 9 stars on the basis of three items: (i) selection of the patients; (ii) comparability of groups or cohorts; and (iii) exposure evaluation for case‐control studies and the outcome evaluation for cohort studies. If there was any discrepancy, it would be resolved by a joint re‐evaluation with a third researcher (YH Zhao). The second author (CQ Li) also assessed the association between quality assessment and meta‐analysis results.
Ethical approval
Our research is in line with the Declaration of Helsinki. As this research is a meta‐ analysis, no prior ethical approval is needed.
Statistical analysis
Data extracted from each study were processed and analyzed using Revman 5.3. We used odds ratio (OR) as the parameter of association between NAFLD and diabetic retinopathy. The random‐effects model was selected, resulting in a more conservative estimate compared with the fixed‐effects model, according to significant heterogeneity among studies. The statistical heterogeneity between summary data was evaluated using the I 2 test, where I 2 ≥ 25%, I 2 ≥ 50%, and I 2 ≥ 75% were defined as low, moderate, and high heterogeneity, respectively. To explore the causes of heterogeneity, we further performed subgroup analyses. A sensitivity analysis was additionally carried out by removing studies one by one to observe the impact on the ultimate effect estimate. Funnel plot analysis was used to evaluate publication bias. A P value of less than 0.05 was considered statistically significant.
RESULTS
Selection results
The details of identifying qualified researches and the exclusion criteria are given in Figure 1. In total, 414 articles were retrieved from PubMed, Embase, Cochrane, and Scopus database, and finally nine articles involving 7,170 patients were included in our analyses. Due to duplication, 191 articles were deleted from 414 articles and the rest were independently reviewed by two researchers. A total of 61 reviews were removed. We reviewed the full texts of the remaining 162 articles, but 153 of them were excluded for the following reasons: 117 studies were inconsistent with our research theme; 31 studies were non‐human experiments; and five studies did not have valid data that we needed. Finally, nine unique observational articles were eligible for inclusion in this meta‐analysis.
Figure 1.
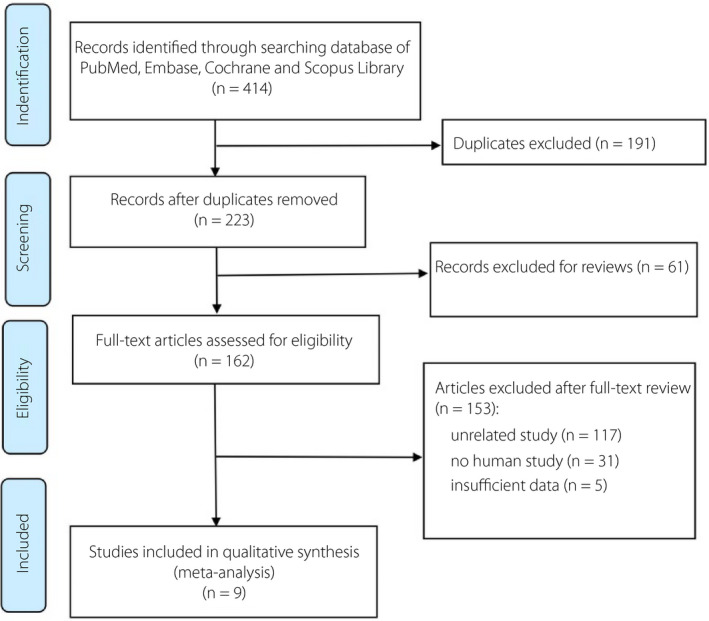
Flow chart of literature selection.
Basic characteristics and quality assessment
The primary characteristics of nine articles15, 16, 17, 19, 20, 21, 22, 23, 24 included are outlined in Table 1. Overall, these nine articles from different countries involved 7,170 patients with type 2 diabetes mellitus (4,075 with NAFLD and 3,095 without NAFLD) and the sample size for each study was between 120 and 2,103. A total of 2,671 diabetic retinopathy events were extracted based on a guideline for the clinical treatment of diabetic retinopathy18. All of these studies were cross‐sectional studies. The countries of these studies included China (3), Korea (1), India (2), Iran (1), Italy (1), and USA (1). All the studies used ultrasound to diagnose non‐alcoholic fatty liver disease. As for quality assessment, we adopted the Newcastle–Ottawa Quality Assessment Scale, and the scores indicated that these nine studies were of high quality. The second author also assessed the association between quality assessment and meta‐analysis results, and the results showed that the two were consistent.
Table 1.
The general characteristics of the nine studies included in the final analysis
| References | Country | Design of study | Sample (n) | Diagnosis of NAFLD | NAFLD patients (%) | DR patients (%) | Age (years) | Duration of DM (years) | BMI (kg/m2) | HbA1c (%) | ALT (U/L) | Study quality† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afarideh et al.15 | Iran | Cross‐sectional | 935 | Liver ultrasound | 26.5 | 15.1 | 57.6 | 8.1 | 29 | 7.8 | 27.6 | 8 |
| Kim et al.16 | Korea | Cross‐sectional | 929 | Liver ultrasound | 63.3 | 46.6 | 57.7 | 6.2 | 24.9 | 8.4 | 35 | 7 |
| Lin et al.19 | US | Cross‐sectional | 945 | Liver ultrasound | 48.6 | 20.5 | 57.9 | NR | NR | 7.6 | NR | 8 |
| Lv et al.17 | China | Cross‐sectional | 1217 | Liver ultrasound | 61 | 46.3 | 63.4 | 9.6 | 26.3 | 8.8 | 22.4 | 7 |
| Somalwar et al.20 | India | Cross‐sectional | 120 | Liver ultrasound | 56.7 | 45.8 | 55.2 | 9.8 | 25.2 | 7.4 | 33 | 7 |
| Targher et al.21 | Italy | Cross‐sectional | 2103 | Liver ultrasound | 67.6 | 46.4 | 58.4 | 13.4 | 26.7 | 7.1 | 23.4 | 7 |
| Viswanathan et al.22 | India | Cross‐sectional | 298 | Liver ultrasound | 52.3 | 20.1 | 49.5 | 8.8 | 28.1 | 9.1 | 26 | 8 |
| Yan et al.23 | China | Cross‐sectional | 212 | Liver ultrasound | 67.5 | 37.7 | 53.7 | 7.5 | 26.9 | 9.1 | 19.2 | 7 |
| Zhang et al.24 | China | Cross‐sectional | 411 | Liver ultrasound | 60.8 | 40.9 | 58.3 | 12.2 | 25.7 | 8.9 | 25.6 | 7 |
ALT, alanine aminotransferase; BMI, body mass index; DM, diabetes mellitus; DR, diabetic retinopathy; HbA1c, glycosylated hemoglobin; NAFLD, non‐alcoholic fatty liver disease; NR, not reported.
The Newcastle–Ottawa scale was used for quality assessment, with scores of 1–3, 4–6, and 7–9 for low‐quality, intermediate‐quality, and high‐quality studies, respectively.
Outcome meta‐analysis
NAFLD and risk of diabetic retinopathy
Nine studies evaluated the relationship between NAFLD and diabetic retinopathy among patients with type 2 diabetes mellitus. Overall, this analysis indicated no correlation between NAFLD and diabetic retinopathy in patients with type 2 diabetes mellitus (OR = 0.94, 95% CI 0.51–1.71; P = 0.83) (Figure 2). However, the heterogeneity test showed I 2 = 96%, indicating a large heterogeneity. Therefore, we utilized the random‐effects model to draw a more conservative conclusion.
Figure 2.
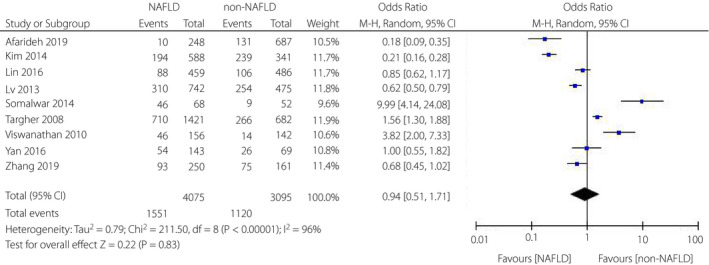
Meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of diabetic retinopathy in type 2 diabetes mellitus.
NAFLD and risk of NPDR
Three articles16, 19, 21 explored the impacts of NAFLD on the incidence of non‐proliferative diabetic retinopathy (NPDR). There was no statistical difference in the incidence of NPDR between NAFLD and non‐NAFLD patients (OR = 0.74, 95% CI 0.36–1.51; P = 0.40) (Figure 3). However, the heterogeneity test showed I 2 = 95%, indicating a considerable heterogeneity.
Figure 3.
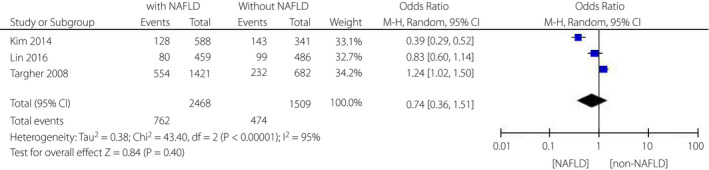
Meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of non‐proliferative diabetic retinopathy in type 2 diabetes mellitus.
NAFLD and risk of PDR
Three of nine articles16, 19, 21 reported the impacts of NAFLD on the risk of proliferative diabetic retinopathy (PDR) among patients with type 2 diabetes mellitus. We discovered that there was also no correlation between PDR and NAFLD among individuals with type 2 diabetes mellitus (OR = 0.96, 95% CI 0.22–4.30; P = 0.96) (Figure 4). Similarly, the heterogeneity test showed I 2 = 97%, indicating that a considerable heterogeneity also existed.
Figure 4.
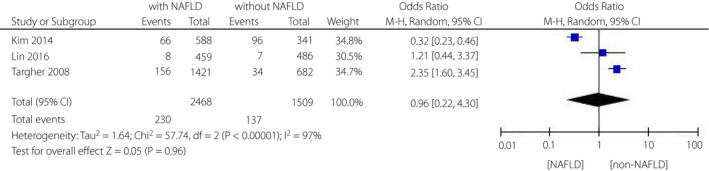
Meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of proliferative diabetic retinopathy in type 2 diabetes mellitus.
Subgroup analyses
Meanwhile, a subgroup analysis was carried out based on the country of the participants. As shown in Figure 5, in Chinese studies, patients with type 2 diabetes mellitus with NAFLD had a decreased risk for diabetic retinopathy (OR = 0.67, 95% CI: 0.55–0.82; P = 0.0001) compared with the non‐NAFLD individuals. The heterogeneity test showed that the result had great stability (I 2 = 7%). Similarly, NAFLD was linked to a decreased incidence for diabetic retinopathy in Korean studies (OR = 0.21, 95% CI: 0.16–0.28; P < 0.00001) and Iranian studies (OR = 0.18, 95% CI: 0.09–0.35; P < 0.00001). However, among Italian studies (OR = 1.56, 95% CI: 1.30–1.88; P < 0.00001) and Indian studies (OR = 5.89, 95% CI: 2.31–15.04; P = 0.0002) patients with NAFLD had an increased incidence for diabetic retinopathy compared with non‐NAFLD patients. There was a potential heterogeneity among the Indian studies (I 2 = 66%). Moreover, no correlation between NAFLD and diabetic retinopathy was found in American studies (OR = 0.85, 95% CI: 0.62–1.17; P = 0.32).
Figure 5.
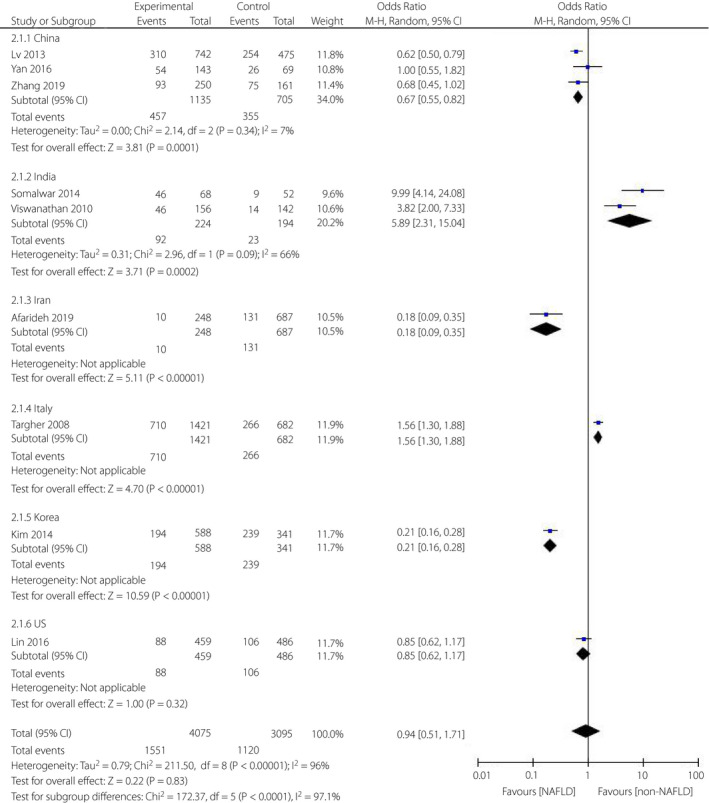
Meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of diabetic retinopathy in type 2 diabetes mellitus based on the different countries.
In addition, we performed analysis in subgroups based on mean age, duration of diabetes, ALT (alanine aminotransferase), BMI (body mass index) and HbA1c (glycosylated hemoglobin), respectively, and no heterogeneous sources were found.
Sensitivity analysis and publication bias
Furthermore, we also performed a sensitivity test by removing a study one by one, and the results did not change. In evaluating publication bias, funnel plots were roughly symmetrical, suggesting no obvious publication bias (Figure 6, S1 and S2).
Figure 6.
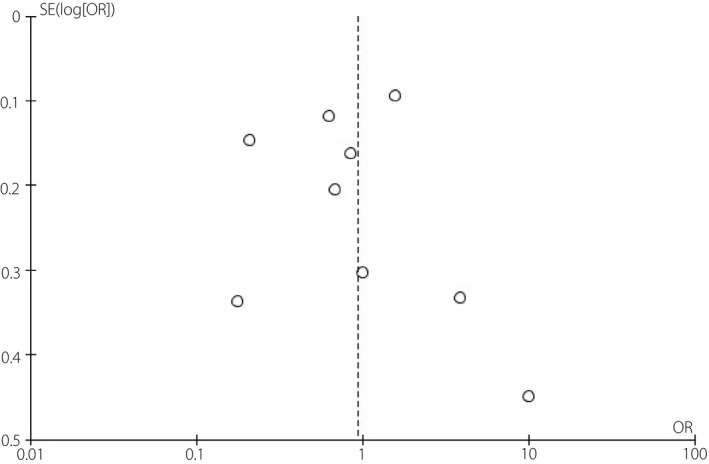
Funnel plots for meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of diabetic retinopathy in type 2 diabetes mellitus.
DISCUSSION
Recently, there have been more and more studies designed to explore the impacts of non‐alcoholic fatty liver disease on the incidence of diabetic retinopathy in patients with type 2 diabetes mellitus, but opinions vary. As far as we know, this is the first systematic review and meta‐analysis aimed at investigating the relationship between NAFLD and diabetic retinopathy excluding the effects of type 2 diabetes mellitus. On the basis of nine observational studies involving 7,170 type 2 diabetes mellitus, 4,075 NAFLD, and 2,671 diabetic retinopathy cases, our research was designed to explore the correlation between NAFLD and diabetic retinopathy of patients with type 2 diabetes. This analysis showed that on the whole, NAFLD had nothing to do with the risk of diabetic retinopathy among the type 2 diabetes mellitus population, mostly consistent with the findings of the American study19. However, subgroup analysis suggested that a difference of country may have an influence on the result. Since considerable heterogeneity was observed in this analysis and a difference in country may be one of the sources of heterogeneity, the results should be treated with caution and confirmed in further research.
An observed phenomenon that no correlation was discovered between NAFLD and diabetic retinopathy among patients with type 2 diabetes mellitus is somewhat surprising. The underlying mechanisms responsible for the association between NAFLD and diabetic retinopathy in patients with type 2 diabetes mellitus remain to be elucidated. Targher et al.21 believed that the possible molecular mediators linking NAFLD with diabetic retinopathy might include an increased release of some pathogenic mediators from the liver, such as advanced glycation end‐products, reactive oxygen species, CRP, interleukin (IL)‐6 and tumor necrosis factor (TNF)‐α. Some researchers have proven that these potential mediators of vascular and/or renal injury are higher in obese and/or diabetic patients with NAFLD than in those without NAFLD25, 26, 27, 28. However, these studies were mostly based in patients with non‐alcoholic steatohepatitis (NASH), and there has been little evidence that inflammatory mediators are increased in patients with simple steatosis. The pathophysiological mechanism of NAFLD is recognized as a condition of insulin resistance, the hepatic manifestation of the metabolic syndrome29. In the study by Targher et al.21, HbA1c was higher in patients with type 2 diabetes mellitus with NAFLD than in those without NAFLD. Insulin resistance associated with NAFLD might have led to poorer glycemic control and a higher prevalence of diabetic complications. In contrast, in the study by Kim et al.16, no statistical difference in HbA1c was observed between the NAFLD group and the non‐NAFLD group in Korean people with type 2 diabetes mellitus. A possible explanation is that Asians have relatively lower insulin secretion compared with Westerners30, 31, 32. Higher serum C‐peptide and insulin levels in the patients with NAFLD might reflect a relatively preserved β‐cell function, which could have beneficial effects on glycemic control, thus decreasing the occurrence of diabetic complications. In the study of Afarideh et al.15, patients with NAFLD had a lower average age and a shorter duration of type 2 diabetes mellitus compared with those without NAFLD. He also speculated that patients with NAFLD might participate in more regular and intense physical activity than non‐NAFLD patients to reduce the occurrence of microvascular complications. In our study, no correlation was observed between NAFLD and diabetic retinopathy among the type 2 diabetes mellitus population. There are some potential explanations for the non‐significant relationship between NAFLD and diabetic retinopathy. The characteristics of NALFD and metabolic syndrome are similar33, 34. Metabolic syndrome is believed to decrease insulin effects due to insulin resistance, thus influencing the function to suppress plasma free fatty acids35. The excess accumulation of fatty acids in the liver may be the reason for causing fatty liver. In a previous study, a recent meta‐analysis (n = 8075 DM patients; 12 clinical studies) found no association between metabolic syndrome (or any single metabolic syndrome component) and the risk of diabetic retinopathy for either type of diabetes mellitus36. Therefore, hyperglycemia and high blood pressure appear to be key risk factors for diabetic retinopathy, and may explain the pathogenesis better than metabolic syndrome or NAFLD.
However, the subgroup analysis showed that in China, Korea, and Iran, the NAFLD group had lower retinal morbidity than the non‐NAFLD group in patients with type 2 diabetes mellitus, while in Italy and India, the NAFLD group had a higher retinal morbidity than the non‐NAFLD group. No relevance between NAFLD and diabetic retinopathy was found in America. Possible explanations are as follows. First, different pathophysiological characteristics of patients with type 2 diabetes mellitus in different countries may be one reason. As mentioned above, Asians have a lower insulin secretion capacity than Westerners30, 31, 32, thereby reducing the incidence of diabetic retinopathy. Second, differences in the characteristics of the study participants may be another reason for the different results between countries. Kim et al.16 found that the Korean participants had a lower percentage of men and lower body mass index, and were younger patients with poor glycemic control than the Italian participants21. Third, discrepancies in the duration of diabetes and glycemic control levels between studies from different countries may contribute to differences in the results between countries. Sasongko et al.37 demonstrated that the duration of diabetes, fasting blood glucose level, and hypertension were independent risk factors for diabetic retinopathy in patients with type 2 diabetes mellitus. Yun et al.38 also discovered that there is a significant association between diabetic retinopathy and blood glucose control, duration of diabetes, age, and proteinuria.
Our meta‐analysis also has several potential limitations. First, the diagnosis of NAFLD in the included studies was dependent on ultrasound imaging instead of histopathological examination, which is the gold standard for diagnosing and determining the degree of NAFLD. Second, it is impossible to differentiate simple steatosis from steatohepatitis, in the light of the potential that diverse severity of NAFLD could affect diabetic retinopathy events differently. Third, the design of all studies is cross‐sectional, lacking any causal or temporal relationship between NAFLD and diabetic retinopathy in patients with type 2 diabetes. Thus, larger longitudinal studies are urged to confirm the associations between NAFLD and diabetic retinopathy in type 2 diabetes mellitus individuals. Finally, our results should be interpreted with caution on account of residual confounders including unknown or unmeasured risk factors as well as potential selection and information bias. Meanwhile, there is considerable heterogeneity in this meta‐analysis, which was likely to have been a result of the study design, ethnic differences in the burden of diabetic retinopathy, and the clinical characteristics of the patients.
In conclusion, our study results suggested that on the whole, there was no association between non‐alcoholic fatty liver disease and diabetic retinopathy in individuals with type 2 diabetes mellitus. However, subgroup analysis showed that the difference of country may have an influence on the result. What is more, more researchers are urged to elucidate the correlation between NAFLD and diabetic retinopathy in type 2 diabetes.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1 | Funnel plots for meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of non‐proliferative diabetic retinopathy in type 2 diabetes mellitus.
Figure S2 | Funnel plots for meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of proliferative diabetic retinopathy in type 2 diabetes mellitus.
Figure S3 | PRISMA checklist for this meta‐analysis.
Figure S4 | PRISMA checklist for this meta‐analysis.
Acknowledgments
All authors would like to thank to Professor Zhao for her instructions on this meta‐analysis. Our research received no funding support.
J Diabetes Investig. 2021
REFERENCES
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142: 1592–1609. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Ji X, Wang Q, et al. New insight into inter‐organ crosstalk contributing to the pathogenesis of non‐alcoholic fatty liver disease (NAFLD). Protein Cell 2018; 9: 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelmoemen G, Khodeir SA, Zaki AN, et al. Overexpression of Hepassocin in diabetic patients with nonalcoholic fatty liver disease may facilitate increased hepatic lipid accumulation. Endocr Metab Immune Disord Drug Targets 2019; 19: 185–188. [DOI] [PubMed] [Google Scholar]
- 4.Zelber‐Sagi S, Ivancovsky‐Wajcman D, Fliss Isakov N, et al. High red and processed meat consumption is associated with non‐alcoholic fatty liver disease and insulin resistance. J Hepatol 2018; 68: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 5.Campos EJ, Campos A, Martins J, et al. Opening eyes to nanomedicine: Where we are, challenges and expectations on nanotherapy for diabetic retinopathy. Nanomedicine 2017; 13: 2101–2113. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi‐ethnic cohort in the United States. Am J Ophthalmol 2006; 141: 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner TW, Abcouwer SF, Barber AJ, et al. An integrated approach to diabetic retinopathy research. Arch Ophthalmol 2011; 129: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazlehurst JM, Woods C, Marjot T, et al. Non‐alcoholic fatty liver disease and diabetes. Metabolism 2016; 65: 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017; 23: 8263–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkagiet S, Papagiannis A, Tziomalos K. Associations between nonalcoholic fatty liver disease and ischemic stroke. World J Hepatol 2018; 10: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdeldyem SM, Goda T, Khodeir SA, et al. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol 2017; 11: 915–919. [DOI] [PubMed] [Google Scholar]
- 12.Mima A. Incretin‐based therapy for prevention of diabetic vascular complications. J Diabetes Res 2016; 2016: 1379274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007; 30: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Padovani R, et al. Increased prevalence of cardiovascular disease in type 2 diabetic patients with non‐alcoholic fatty liver disease. Diabet Med 2006; 23: 403–409. [DOI] [PubMed] [Google Scholar]
- 15.Afarideh M, Aryan Z, Ghajar A, et al. Association of non‐alcoholic fatty liver disease with microvascular complications of type 2 diabetes. Prim Care Diabetes 2019; 13: 505–514. [DOI] [PubMed] [Google Scholar]
- 16.Kim BY, Jung CH, Mok JO, et al. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non‐alcoholic fatty liver disease. J Diabetes Investig 2014; 5: 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv WS, Sun RX, Gao YY, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol 2013; 19: 3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med 2013; 30: 387–398. [DOI] [PubMed] [Google Scholar]
- 19.Lin TY, Chen YJ, Chen WL, et al. The relationship between nonalcoholic fatty liver disease and retinopathy in NHANES III. PLoS One 2016; 11: e0165970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somalwar A, Raut A. Study of association of non alcoholic fatty liver disease (NAFLD) with micro and macrovascular complications of type 2 diabetes mellitus (T2DM). Int J Res Med Sci 2014; 2: 493–497. [Google Scholar]
- 21.Targher G, Bertolini L, Rodella S, et al. Non‐alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser‐treated retinopathy in type 2 diabetic patients. Diabetologia 2008; 51: 444–450. [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan V, Kadiri M, Medimpudi S, et al. Association of non‐alcoholic fatty liver disease with diabetic microvascular and macrovascular complications in South Indian diabetic subjects. Int J Diabetes Dev Countries 2010; 30: 208–212. [Google Scholar]
- 23.Yan LH, Mu B, Guan Y, et al. Assessment of the relationship between non‐alcoholic fatty liver disease and diabetic complications. J Diabetes Investig 2016; 7: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Li L, Chen J, et al. Presence of diabetic retinopathy is lower in type 2 diabetic patients with non‐alcoholic fatty liver disease. Medicine (Baltimore) 2019; 98: e15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Targher G, Bertolini L, Scala L, et al. Plasma PAI‐1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care 2007; 30: e31–e32. [DOI] [PubMed] [Google Scholar]
- 26.Abiru S, Migita K, Maeda Y, et al. Serum cytokine and soluble cytokine receptor levels in patients with non‐alcoholic steatohepatitis. Liver Int 2006; 26: 39–45. [DOI] [PubMed] [Google Scholar]
- 27.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004; 99: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 28.Albano E, Mottaran E, Vidali M, et al. Immune response towards lipid peroxidation products as a predictor of progression of non‐alcoholic fatty liver disease to advanced fibrosis. Gut 2005; 54: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37: 917–923. [DOI] [PubMed] [Google Scholar]
- 30.Yoon KH, Ko SH, Cho JH, et al. Selective beta‐cell loss and alpha‐cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003; 88: 2300–2308. [DOI] [PubMed] [Google Scholar]
- 31.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 32.Rhee SY, Woo JT, Chon S, et al. Characteristics of insulin resistance and insulin secretory capacity in Korean subjects with IFG and IGT. Diabetes Res Clin Pract 2010; 89: 250–255. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal AJ, American GA. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 1705–1725. [DOI] [PubMed] [Google Scholar]
- 34.De Alwis NM, Day CP. Non‐alcoholic fatty liver disease: the mist gradually clears. J Hepatol 2008; 48(Suppl 1): S104–S112. [DOI] [PubMed] [Google Scholar]
- 35.Cortez‐Pinto H, Camilo ME, Baptista A, et al. Non‐alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr 1999; 18: 353–358. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Wang C, Shi K, et al. Relation of metabolic syndrome and its components with risk of diabetic retinopathy: a meta‐analysis of observational studies. Medicine (Baltimore) 2018; 97: e12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasongko MB, Widyaputri F, Agni AN, et al. Prevalence of diabetic retinopathy and blindness in Indonesian adults with type 2 diabetes. Am J Ophthalmol 2017; 181: 79–87. [DOI] [PubMed] [Google Scholar]
- 38.Yun JS, Lim TS, Cha SA, et al. Clinical course and risk factors of diabetic retinopathy in patients with type 2 diabetes mellitus in Korea. Diabetes Metab J 2016; 40: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Funnel plots for meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of non‐proliferative diabetic retinopathy in type 2 diabetes mellitus.
Figure S2 | Funnel plots for meta‐analysis of association between non‐alcoholic fatty liver disease and the risk of proliferative diabetic retinopathy in type 2 diabetes mellitus.
Figure S3 | PRISMA checklist for this meta‐analysis.
Figure S4 | PRISMA checklist for this meta‐analysis.


