Abstract
Aims/Introduction
Given the mechanism of action of sodium–glucose cotransporter 2 inhibitors (SGLT2is), these drugs can also reduce bone density and increase fracture risk. We aimed to identify and characterize fracture‐related adverse events that are associated with SGLT2is.
Materials and Methods
In this observational, retrospective, pharmacovigilance, real‐world study, we used disproportionality and Bayesian analyses to compare fracture‐related adverse event reporting in patients who received SGLT2is from the first quarter in 2004 to the fourth quarter in 2019 in the Food and Drug Administration Adverse Event Reporting System. We also compared the effect on combined therapy with SGLT2is and other glucose‐lowering medications (GLMs), and compared their onset times and outcomes.
Results
A total of 317 SGLT2is‐associated fractures were identified. Affected patients tended to be aged >45 years (68.76%) and were more often male than female (58.04% vs 34.07%). SGLT2is‐associated fracture is most commonly reported with canagliflozin (51.10%), dapagliflozin (24.60%) and empagliflozin (23.66%). SGLT2is or SGLT2is combined with GLMs do not show an association with fracture risk under disproportionality and Bayesian analyses. SGLT2i‐associated fractures result in hospitalization in 66.64% of patients and death in 9.38% of patients. GLMs show an increased hospitalization rate compared with SGLT2is (69.72% vs 55.14%, P < 0.0001) and GLMs plus SGLT2is (69.72% vs 61.20%, P = 0.0197).
Conclusions
Based on the Food and Drug Administration Adverse Event Reporting System database, no association is noted between fracture risk and SGLT2is, or SGLT2is combined with GLMs. Long‐term follow up and high‐quality studies need to further verify and explore the relationship between SGLT2is and fractures.
Keywords: Fracture, Pharmacovigilance, Sodium–glucose cotransporter 2 inhibitors
Based on the Food and Drug Administration Adverse Event Reporting System database, no association is noted between fracture risk and sodium–glucose cotransporter 2 inhibitors, or sodium–glucose cotransporter 2 inhibitors combined with glucose‐lowering medications. These findings should be considered in patient care, and support continued surveillance, risk factor identification and comparative studies.
Introduction
Sodium–glucose cotransporter 2 inhibitors (SGLT2is) are insulin‐independent targets that increase glucose excretion in the treatment of type 2 diabetes mellitus1. Currently approved SGLT2is include canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, luseogliflozin and tofogliflozin. Based on recent studies2, 3, 4, 5, 6 of diabetes management and cardiovascular disease, indications for SGLT2is treatment have been expanding. The guidelines7, 8 and consensus9, 10, 11 have undergone significant updates. With an emphasis on treatment strategy, diabetes management should be built on the prevention and treatment of cardiovascular disease. The primary criterion for evaluating hypoglycemic agents is whether the agent is beneficial to the improvement of cardiovascular outcomes. Among them, SGLT2is have become well‐recognized ‘star drugs,’ given their clear cardiovascular and renal benefits12.
Nevertheless, safety issues of SGLT2is also represent an area of concern, such as urogenital infection, fracture, lower‐extremity amputation risk and ketoacidosis risk13, 14, 15, 16. SGLT2is has repeatedly received US Food and Drug Administration (FDA) safety warnings. In September 2015, the FDA strengthened the warning for canagliflozin, related to the increased risk of bone fractures, and added new information about reduced bone mineral density17. Despite the increasing number of studies on SGLT2is‐associated fractures, such as a meta‐analysis based on nine clinical trials that showed that the fracture incidence was higher in the canagliflozin group18, information on a relatively uncommon adverse event remains limited. Some studies even report the opposite view, indicating that SGLT2is did not increase fracture risk in patients compared with dipeptidyl peptidase‐4 inhibitors (DPP‐4) in a case–control study19, and empagliflozin did not increase the fracture risk compared with a placebo or glimepiride in a placebo‐controlled trial20.
Considering the short time‐to‐market for such drugs and, until recently, the lack of pharmacovigilance studies illustrating SGLT2is‐associated fractures, the knowledge about the bone safety profile after treatment with various SGLT2is is limited in real‐world clinical practice. Therefore, we aimed to characterize the fractures potentially caused by various SGLT2is in a large population by using the FDA Adverse Event Reporting System (FAERS). We further examined and compared the timing and outcomes for fractures of different SGLT2is regimens.
Materials and Methods
Data source
We carried out a retrospective pharmacovigilance study using the FAERS database from the first quarter in 2004 to the fourth quarter in 2019. The FAERS is a public spontaneous reporting system that contains information about adverse events and medication error reports provided by health professionals, patients and manufacturers, not only domestically, but also from other regions. FAERS data files comprehensively reflect demographic information, drug information, adverse events, patient outcomes, report sources, therapy dates, indications and deleted cases.
A total of 13,649,428 reports were obtained from the FAERS database. Records from deleted cases files and duplicated records were removed according to the FDA’s recommendations. In addition, the latest FDA_DT was selected when the CASEIDs were the same, and the higher PRIMARYID was chosen when the CASEID and FDA_DT were the same (Figure 1).
Figure 1.
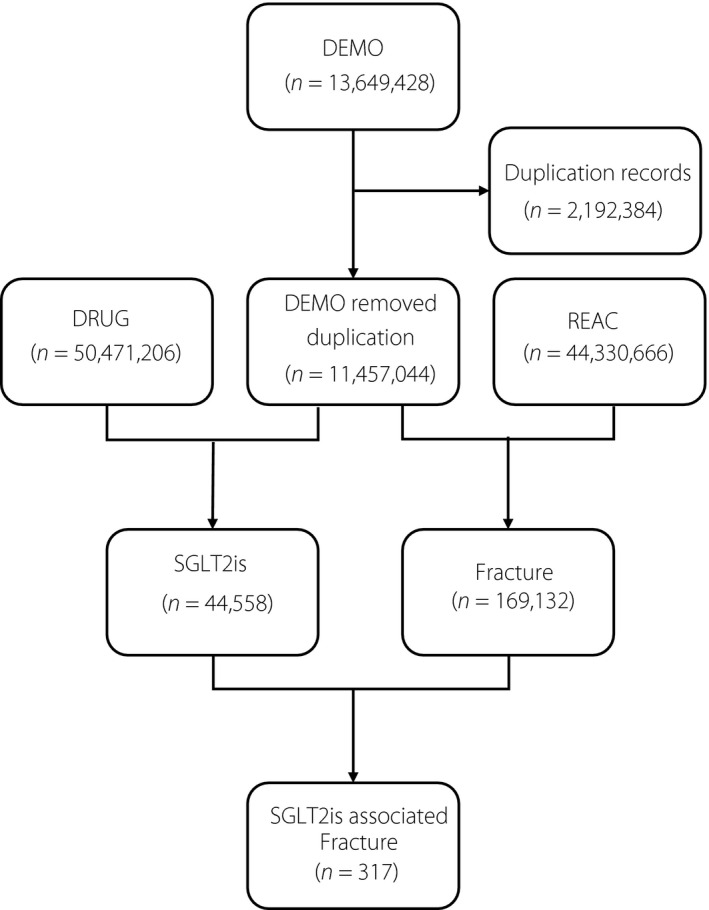
Process of the selection of cases of sodium–glucose cotransporter 2 inhibitors (SGLT2is)‐associated fracture from the Food and Drug Administration’s Adverse Event Reporting System database. DEMO, demographic information; DRUG, drug information; REAC, adverse events.
Adverse event and drug identification
Fracture information was obtained from adverse events files based on the Medical Dictionary for Regulatory Activities (version 23.0) at the preferred term level as follows: ‘Fractures’ [10017076], ‘Joint injury’ [10060820] and ‘Skeletal injury’ [10061363]. We chose generic and brand names of SGLT2is by utilizing the IBM Micromedex as the dictionary in the data mining process (Table 1).
Table 1.
Generic names and brand names of sodium–glucose cotransporter 2 inhibitors
| Generic name | Brand name |
|---|---|
| Canagliflozin | Canaglu®, Invokana® |
| Canagliflozin/metformin Hydrochloride | Invokamet®, Vokanamet® |
| Dapagliflozin propanediol | Edistride®, Farxiga®, Forxiga®, Forziga® |
| Dapagliflozin Propanediol/metformin Hydrochloride | Ebymect®, Xigduo®, Xigduo XR® |
| Dapagliflozin Propanediol/saxagliptin | Qtern® |
| Empagliflozin | Jardiance® |
| Empagliflozin/linagliptin | Glyxambi® |
| Empagliflozin/metformin Hydrochloride | Jardiamet®, Jardiance Duo®, Synjardy® |
| Ertugliflozin | Steglatro® |
| Ertugliflozin/metformin Hydrochloride | Segluromet® |
| Ertugliflozin/sitagliptin | Steglujan® |
| Ipragliflozin | Suglat® |
| Luseogliflozin | Lusefi® |
| Tofogliflozin | Apleway®, Deberza® |
Data mining
Based on the principles of Bayesian analysis and disproportionality analysis, we used the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network and the multi‐item gamma Poisson shrinker algorithms to explore the associations between SGLT2is and fracture. The calculation and criteria21, 22, 23, 24, 25, 26, 27 of the four algorithms are reported as follows: ROR = (a / b) / (c / d), 95% confidence interval (CI) = eln(ROR) ± 1.96(1 / a + 1 / b + 1 / c + 1 / d)^0.5 (criteria: 95% CI >1, n ≥2); PRR = (a / [a + c]) / (b / [b + d]), χ2 = Σ([O – E]^2 / E); (O = a, E = [a + b][a + c] / [a + b + c + d]) (criteria: PRR ≥2, χ2 ≥4, n ≥3); with Bayesian confidence propagation neural network algorithms, IC = log2a(a + b + c + d) / ([a + c][a + b]), IC025 = eln(IC) – 1.96(1 / a + 1 / b + 1 / c + 1 / d)^0.5 (criteria: IC025 >0); with multi‐item gamma Poisson shrinker algorithms, empirical Bayesian geometric mean (EBGM) = a(a + b + c + d) / ([a + c][a + b]), EBGM05 = eln(EBGM) – 1.64(1 / a + 1 / b + 1 / c + 1 / d)^0.5 (criteria: EBGM05 >2, n >0). In these algorithms, ‘a’ is the number of reports containing both the suspect drug and the suspect adverse drug reaction; ‘b’ is the number of reports containing the suspect adverse drug reaction with other medications (except the drug of interest); ‘c’ is the number of reports containing the suspect drug with other adverse drug reactions (except the event of interest); ‘d’ is the number of reports containing other medications and other adverse drug reactions; CI indicates confidence interval; n indicates number of co‐occurrences; χ2 indicates chi‐squared; IC indicates information component; IC025 indicates the lower limit of the 95% two‐sided CI of the IC; EBGM05, the lower limit of the 90% one‐sided CI of the EBGM.
The associations between fracture risk and SGLT2is were compared. The effects on combined therapy of SGLT2is with other glucose‐lowering medications (GLMs)7, 28, such as metformin, sulfonylurea (glyburide, glipizide, glimepiride), glucagon‐like peptide‐1 receptor agonists (exenatide, lixisenatide, liraglutide, albiglutide, dulaglutide), thiazolidinedione (pioglitazone, rosiglitazone) and DPP‐4 inhibitors (sitagliptin, saxagliptin, alogliptin) were also evaluated. Polypills that contain SGLT2is and GLM were considered a combined therapy.
In this scenario, GLMs here were considered the ‘primary suspect’ with no SGLT2is listed as the ‘second suspect’ or ‘concomitant’. For the combined therapy of GLMS plus SGLT2is, GLMs were identified as the ‘primary suspect’ in the ROLE_COD field of drug information files, whereas SGLT2is were listed as the ‘second suspect’ or ‘concomitant’. In addition, SGLT2is were identified as the ‘primary suspect’, whereas GLMs were considered the ‘second suspect’ or ‘concomitant’.
Statistical analysis
Descriptive analyses were used to summarize the characteristics of adverse event reports on SGLT2is‐related fractures collected from the FAERS database. The onset time of fracture was defined as the interval between the EVENT_DT (fracture onset date) and the START_DT (start date of SGLT2is use). Reports with input error (EVENT_DT earlier than START_DT) or inaccurate date entry were excluded.
The onset times among SGLT2is were compared using non‐parametric tests (the Mann–Whitney test was used for dichotomous variables, and the Kruskal–Wallis test was used when there were more than two subgroups of respondents). Pearson’s χ2‐test or Fisher’s exact test was used to compare the outcome between SGLT2is. The statistical significance was set at P < 0.05 with 95% confidence intervals. All data mining and statistical analyses were carried out using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Descriptive analysis
In total, 44,558 adverse events related to SGLT2is and 169,132 reports related to fracture were documented in the FAERS database from the first quarter in 2004 to the fourth quarter in 2019 (Figure 1). Ultimately, 317 reports were screened with suspected SGLT2is‐related fractures, and the clinical features of these patients are summarized in Table 2. Most cases were reported from North America (61.83%) and Europe (17.04%), and were reported by consumers (40.06%). Affected patients tend to be older than 45 years (68.76%), and were more often male than female (58.04% vs 34.07%). The most commonly reported SGLT2i‐associated fractures were related to canagliflozin (51.10%) followed by dapagliflozin (24.60%) and empagliflozin (23.66%). Fracture cases are most commonly found in patients with type 2 diabetes mellitus (57.19%).
Table 2.
Clinical characteristics of patients with fracture sourced from the US Food and Drug Administration’s Adverse Event Reporting System database (2004q1 to 2019q4)
| Characteristics | Reports, n (%) |
|---|---|
| Reporting region | |
| Europe | 54 (17.04%) |
| North America | 196 (61.83%) |
| South America | 8 (2.52%) |
| Asia | 55 (17.35%) |
| Oceania | 4 (1.26%) |
| Reporters | |
| Consumer | 127 (40.06%) |
| Other health professional | 38 (11.99%) |
| Pharmacist | 21 (6.62%) |
| Physician | 115 (36.28%) |
| Unknown or missing | 16 (5.05%) |
| Reporting year | |
| 2019 | 66 (20.82%) |
| 2018 | 95 (29.97%) |
| 2017 | 52 (16.40%) |
| 2016 | 65 (20.50%) |
| 2015 | 28 (8.83%) |
| 2014 | 8 (2.52%) |
| 2013 | 2 (0.63%) |
| Unknown or missing | 1 (0.32%) |
| Sex of patients | |
| Male | 184 (58.04%) |
| Female | 108 (34.07%) |
| Unknown or missing | 25 (7.89%) |
| Age groups (years) | |
| <18 | 1 (0.32%) |
| 18–44 | 8 (2.52%) |
| 45–64 | 105 (33.12%) |
| 65–74 | 65 (20.50%) |
| >75 | 48 (15.14%) |
| Unknown or missing | 90 (28.39%) |
| SGLT2is | |
| Canagliflozin | 162 (51.10%) |
| Dapagliflozin | 78 (24.60%) |
| Empagliflozin | 75 (23.66%) |
| Ertugliflozin | 2 (0.63%) |
| Ipragliflozin | 0 (0%) |
| Luseogliflozin | 0 (0%) |
| Remogliflozin | 0 (0%) |
| Tofogliflozin | 0 (0%) |
| Indications | |
| Cardiac disorder | 5 (1.64) |
| Chronic kidney disease | 2 (0.65) |
| Diabetes mellitus | 46 (15.04) |
| Glycosylated hemoglobin increased | 4 (1.31) |
| Obesity | 1 (0.33) |
| Type 1 diabetes mellitus | 3 (0.98) |
| Type 2 diabetes mellitus | 175 (57.19) |
| Unknown | 70 (22.88) |
SGLT2is, sodium–glucose cotransporter 2 inhibitors.
Disproportionality analysis and Bayesian analysis
No positive signals were detected among SGLT2is and GLMs plus SGLT2is. Just three of 14 GLMs posed one or two positive signals based on the criteria for the four algorithms: which are pioglitazone in ROR: 1.24 (1.10, 1.40) and IC: 0.31 (0.27), lixisenatide in PRR: 2.2 (3.32) and IC: 1.14 (0.47), and alogliptin in IC: 0.24 (0.13; Table 3).
Table 3.
Association of sodium–glucose cotransporter 2 inhibitors and glucose‐lowering medications with fracture
| Class | Drug | n | ROR | PRR | IC | EBGM |
|---|---|---|---|---|---|---|
| (95% two‐sided CI) | (χ2) | (IC025) | (EBGM05) | |||
| SGLT2is | Canagliflozin | 162 | 0.49 (0.42–0.57) | 0.49 (86.54) | −1.02 (−) | 0.49 (0.43) |
| Dapagliflozin | 78 | 0.54 (0.43–0.67) | 0.54 (30.66) | −0.88 (−) | 0.54 (0.45) | |
| Empagliflozin | 75 | 0.42 (0.33–0.52) | 0.42 (60.56) | −1.25 (−) | 0.42 (0.35) | |
| Ertugliflozin | 2 | 0.31 (0.08–1.26) | 0.32 (3.00) | −1.66 (−) | 0.32 (0.10) | |
| Ipragliflozin | 0 | – | – | – | – | |
| Luseogliflozin | 0 | – | – | – | – | |
| Remogliflozin | 0 | – | – | – | – | |
| Tofogliflozin | 0 | – | – | – | – | |
| GLMs | Metformin | 767 | 0.38 (0.36–0.41) | 0.39 (753.68) | −1.36 (−) | 0.39 (0.37) |
| Glyburide | 7 | 0.48 (0.23–1.01) | 0.48 (3.92) | −1.05 (−) | 0.48 (0.26) | |
| Glipizide | 9 | 0.26 (0.14–0.51) | 0.27 (18.30) | −1.90 (−) | 0.27 (0.15) | |
| Glimepiride | 14 | 0.52 (0.31–0.88) | 0.53 (6.06) | −0.93 (−) | 0.53 (0.34) | |
| Exenatide | 407 | 0.42 (0.38–0.46) | 0.42 (321.92) | −1.23 (−) | 0.43 (0.39) | |
| Lixisenatide | 5 | 2.24 (0.92–5.46) | 2.2 (3.32)† | 1.14 (0.47)† | 2.20 (1.04) | |
| Liraglutide | 60 | 0.15 (0.12–0.19) | 0.15 (287.36) | −2.71 (−) | 0.15 (0.12) | |
| Albiglutide | 12 | 0.10 (0.05–0.17) | 0.1 (102.95) | −3.37 (−) | 0.10 (0.06) | |
| Dulaglutide | 88 | 0.25 (0.20–0.31) | 0.25 (200.15) | −1.99 (−) | 0.25 (0.21) | |
| Pioglitazone | 267 | 1.24 (1.10–1.40)† | 1.24 (12.20) | 0.31 (0.27)† | 1.24 (1.12) | |
| Rosiglitazone | 606 | 0.46 (0.42–0.50) | 0.46 (384.27) | −1.11 (−) | 0.46 (0.43) | |
| Sitagliptin | 202 | 0.47 (0.41–0.54) | 0.48 (117.88) | −1.07 (−) | 0.48 (0.42) | |
| Saxagliptin | 21 | 0.43 (0.28–0.66) | 0.44 (15.56) | −1.20 (−) | 0.44 (0.30) | |
| Alogliptin | 11 | 1.18 (0.65–2.14) | 1.18 (0.30) | 0.24 (0.13)† | 1.18 (0.71) | |
|
GLMs+ SGLT2is |
Metformin + SGLT2is | 105 | 0.47 (0.39–0.57) | 0.47 (63.07) | −1.08 (−) | 0.47 (0.40) |
| Glyburide + SGLT2is | 2 | 0.38 (0.10–1.54) | 0.39 (1.96) | −1.37 (−) | 0.39 (0.12) | |
| Glipizide + SGLT2is | 10 | 0.71 (0.38–1.33) | 0.71 (1.16) | −0.48 (−) | 0.71 (0.42) | |
| Glimepiride + SGLT2is | 7 | 0.42 (0.20–0.88) | 0.42 (5.59) | −1.24 (−) | 0.42 (0.23) | |
| Exenatide + SGLT2is | 12 | 0.55 (0.31–0.96) | 0.55 (4.47) | −0.86 (−) | 0.55 (0.34) | |
| Lixisenatide + SGLT2is | 0 | – | – | – | – | |
| Liraglutide + SGLT2is | 10 | 0.44 (0.24–0.83) | 0.45 (6.93) | −1.16 (−) | 0.45 (0.27) | |
| Albiglutide + SGLT2is | 0 | – | – | – | – | |
| Dulaglutide + SGLT2is | 11 | 0.39 (0.22–0.71) | 0.39 (10.35) | −1.34 (−) | 0.39 (0.24) | |
| Pioglitazone + SGLT2is | 10 | 0.73 (0.39–1.37) | 0.74 (0.97) | −0.44 (−) | 0.74 (0.44) | |
| Rosiglitazone + SGLT2is | 0 | – | – | − | – | |
| Sitagliptin + SGLT2is | 22 | 0.45 (0.29–0.68) | 0.45 (15.07) | −1.15 (−) | 0.45 (0.32) | |
| Saxagliptin + SGLT2is | 10 | 0.95 (0.51–1.78) | 0.95 (0.02) | −0.07 (−) | 0.95 (0.57) | |
| Alogliptin + SGLT2is | 1 | 0.80 (0.11–5.78) | 0.81 (0.05) | −0.31 (−) | 0.81 (0.15) |
CI, confidence interval; EBGM, empirical Bayes geometric mean; EBGM05, the lower 90% one‐sided confidence interval of empirical Bayes geometric mean; GLMs, glucose‐lowering medications; IC, information component; IC025, the lower limit of the 95% two‐sided confidence interval of the information component; n, the reported number of drug‐associated fractures; PRR, proportional reporting ratio; ROR, reporting odds ratio; SGLT2is, sodium‐glucose cotransporter 2 inhibitors.
Positive signal.
Onset times of fracture
Overall, the median onset times of SGLT2is, GLMs and GLMs plus SGLT2is were 150 days (interquartile range [IQR] 56–313 days), 534 days (IQR 153–1,247 days) and 184 days (IQR 58–481 days), respectively. The onset times of fracture for each regimen are described in Figure 2. Of note, a significant difference in median onset times of fracture was found between GLMs versus GLMs plus SGLT2is (P < 0.0001). However, the median onset times of fracture for SGLT2is versus GLMs (P = 0.7050) or SGLT2is versus GLMs plus SGLT2is (P = 0.6299) were not significantly different. Significant differences were noted for SGLT2is versus metformin (P < 0.0001), SGLT2is versus thiazolidinedione (P < 0.0001), metformin versus glucagon‐like peptide‐1 receptor agonists (P < 0.0001), metformin versus DPP‐4 inhibitors (P < 0.0001), metformin versus metformin plus SGLT2is (P < 0.0001), metformin versus DPP‐4 inhibitors plus SGLT2is (P = 0.0109), glucagon‐like peptide‐1 receptor agonists versus thiazolidinedione (P < 0.0001), thiazolidinedione versus DPP‐4 inhibitors (P < 0.0001) and thiazolidinedione versus metformin plus SGLT2is (P < 0.0001).
Figure 2.
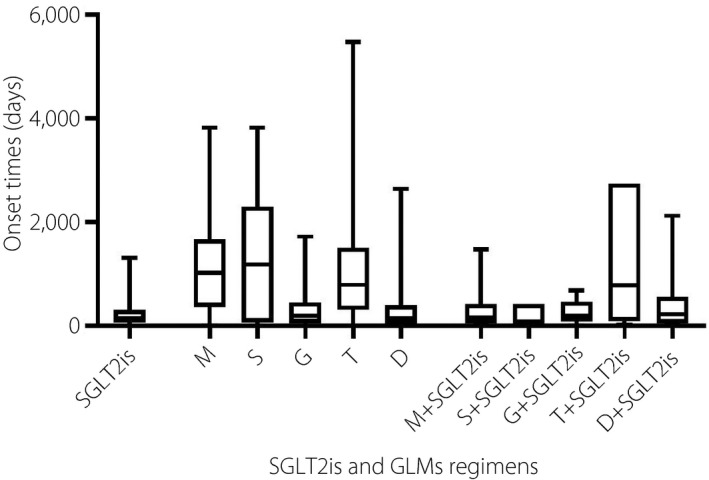
Onset times of sodium–glucose cotransporter 2 inhibitors (SGLT2is) and glucose‐lowering medications regimens. D, dipeptidyl peptidase‐4 inhibitors; G, glucagon‐like peptide 1 receptor agonists; M, metformin; S, sulfonylurea; T, thiazolidinedione.
Outcomes due to fracture
To analyze the prognosis of SGLT2is‐associated fractures, we assessed the rate of outcomes (death, disability, hospitalization, life‐threatening, other serious and required intervention) due to fracture after various SGLT2is and GLMs treatments, and the results are shown in Table 4. The outcome of fracture tends to be poor, resulting in hospitalization in 66.64% patients and death in 9.38%. GLMs result in a higher hospitalization rate (69.72%) than SGLT2is (55.14%, P < 0.0001) and GLMs plus SGLT2is (61.20%, P = 0.0197). No significant difference was noted between SGLT2is and GLMs + SGLT2is (P = 0.2619). Similarly, fatality rates for GLMs (11.10%) were significantly higher than SGLT2is (5.61%, P < 0.0001) and GLMs plus SGLT2is (0.55%, P < 0.0001).
Table 4.
Outcome events of fractures
| Outcome events | Reports (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SGLT2is | M | S | G | T | D | M + SGLT2is | S + SGLT2is | G + SGLT2is | T + SGLT2is | D + SGLT2is | |
| Congenital Anomaly | 1 (0.47) | 0 (0.00) | 1 (3.70) | 0 (0.00) | 0 (0.00) | 1 (0.48) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Death | 12 (5.61) | 87 (11.68) | 1 (3.70) | 4 (0.99) | 85 (9.84) | 73 (34.76) | 0 (0.00) | 0 (0.00) | 1 (3.70) | 0 (0.00) | 0 (0.00) |
| Disability | 22 (10.28) | 35 (4.70) | 2 (7.41) | 15 (3.69) | 63 (7.29) | 25 (11.90) | 11 (11.34) | 1 (6.25) | 2 (7.41) | 2 (20.00) | 2 (6.06) |
| Hospitalization | 118 (55.14) | 518 (69.53) | 21 (77.78) | 259 (63.79) | 604 (69.91) | 168 (80) | 53 (54.64) | 13 (81.25) | 17 (62.96) | 5 (50.00) | 24 (72.73) |
| Life‐Threatening | 11 (5.14) | 20 (2.68) | 1 (3.70) | 6 (1.48) | 9 (1.04) | 36 (17.14) | 8 (8.25) | 1 (6.25) | 1 (3.70) | 0 (0.00) | 3 (9.09) |
| Other Serious | 126 (58.88) | 284 (38.12) | 10 (37.04) | 232 (57.14) | 278 (32.18) | 157 (74.76) | 59 (60.82) | 5 (31.25) | 14 (51.85) | 5 (50.00) | 19 (57.58) |
| Required Intervention | 1 (0.47) | 8 (1.07) | 1 (3.70) | 1 (0.25) | 79 (9.14) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (10.00) | 0 (0.00) |
D, dipeptidyl peptidase‐4 inhibitors (sitagliptin, saxagliptin, and alogliptin); G, glucagon‐like peptide‐1 receptor agonists (exenatide, lixisenatide, liraglutide, albiglutide, and dulaglutide); M, metformin; S, sulfonylurea (glyburide, glipizide, and glimepiride); SGLT2is, sodium–glucose cotransporter 2 inhibitors; T, thiazolidinedione (pioglitazone and rosiglitazone).
Discussion
It is believed that SGLT2is might change the balance of calcium and phosphorus in the body, leading to decreased bone density and increased fracture risk. The mechanism causing this imbalance is potentially attributed to the fact that SGLT2is inhibit sodium and glucose transporters, and increase serum phosphorus levels, leading to increased levels of fibroblast growth factor‐23 and parathyroid hormone, and ultimately causing osteomalacia29. The incidence of all fractures for canagliflozin was increased compared with a placebo (15.4 vs 11.9 fracture patients per 1,000 patient‐years; HR 1.26, 95% CI 1.04–1.52) in the Canagliflozin Cardiovascular Assessment Study (CANVAS) trial, which involved a total of 10,142 participants with type 2 diabetes and high cardiovascular risk12. Another study showed that during the first year of canagliflozin administration, the incidence of fractures increases in the second year30. In a 104‐week follow up of 716 diabetes patients, canagliflozin significantly reduced bone mineral density in patients compared with a placebo, which might be related to fracture events31.
However, neither empagliflozin nor dapagliflozin increased the risk of fracture compared with a placebo32, 33, 34. Of note, although the experimental SGLT2is used in these studies were different, the proportion of women and obese patients, and the type 2 diabetes mellitus duration in the CANVAS trial was higher and longer. Considering that being female, obesity and longer duration of type 2 diabetes mellitus are risk factors for fractures, these differences might increase fracture risk, as observed in the CANVAS trial. In a meta‐analysis study that included 20 randomized controlled trials of 8,266 patients reported that SGLT2is did not increase fracture risk (relative risk 0.67, 95% CI 0.42–1.07) compared with a placebo35. Another systematic review showed similar results in which the use of SGLT2i (relative risk 1.02, 95% CI 0.91–1.16) was not associated with the risk of fracture36. Therefore, based on the results of existing clinical trials, it is difficult to determine the correlation between SGLT‐2 inhibitors and fracture occurrence.
Similarly, we cannot exclude the possibility that a patient had been using GLM for many years, and fractures occur after replacement with SGLT2is and may be attributed to SGLT2is as noted in the study. However, these fractures are likely to be the result of a combination of GLM and disease.
Clinical trials studies still lack enough power to draw definitive conclusions about drug safety due to the strict inclusion criteria, limited sample sizes and relatively short observation periods. In our pharmacovigilance analysis, surprisingly, no association between SGLT2is and fracture was detected. Additionally, the association between GLMs plus SGLT2is with fracture was not detected.
Interestingly, we found positive signals of pioglitazone in ROR and IC. When pioglitazone was combined with SGLT2is, the signals became negative. This finding is consistent with a systematic review that showed that the use of thiazolidinediones, including both pioglitazone and rosiglitazone, was associated with an increased risk of fracture37. However, this result might not be sufficient to show that SGLT2is help prevent bone fractures. A possible explanation for this finding might be that pioglitazone was approved for marketing in 1999, and there are a large number of patients with long‐term use. In contrast, SGLT2is has been on the market for just a few years. Therefore, in the combined therapy group, the possibility of shorter durations of pioglitazone in some patients cannot be excluded. Furthermore, as diabetes progresses, the disease itself might also cause osteoporosis and fractures. Similarly, the short‐term use of SGLT2is might not pose a fracture risk.
Another finding is that the median onset times of fracture are 242 (IQR 56–313) days for SGLT2is. This time is considerably reduced compared with that reported in another study carried out by Meier et al., showing that the median duration between diabetes mellitus onset and fracture date was 4.5 years based on a large population of 354,438 type 2 diabetes mellitus patients38. This result is also increased compared with the data of the other two groups in the present study; that is, 534 (IQR 153–1247) for GLMs and 184 (IQR 58–481) for GLMs plus SGLT2is. The difference in results might be explained by the fact that the present study used data on adverse drug events, namely, fractures suspected to be caused by the drug rather than those caused by trauma. Nevertheless, Meier’s study used a UK‐based primary care database and identified patients with a low‐trauma fracture (non‐vertebral fractures of the proximal and distal upper and lower extremities, ribs and thorax, hip and foot) during the study period.
The predictable increase in the number of patients who will receive SGLT2is in the future implies that close monitoring and constant epidemiological surveillance are required, even if the adverse event of fracture occurs rarely. It is still recommended to include fractures in post‐marketing risk management plans, especially in the context of falls, reduced bone density, advanced age, alcoholism, low weight, presence of certain diseases (such as electrolyte disorders, epilepsy, chronic obstructive pulmonary disease etc.) and the simultaneous use of certain drugs (such as glucocorticoids, antidepressants, anti‐epileptic drugs etc.).
We acknowledge certain limitations of the present study. First, data mining technology fails to fully reflect all clinical information from patients. It also requires detailed information from clinical follow up and other surveys to verify data mining hypotheses. Also, most of the patients came from North America (61.83%), Asia (17.35%) and Europe (17.04%). There was indeed some uncertainty in other regions and ethnicities. Second, data mining technology cannot remedy the inherent limitations of adverse drug reaction reporting systems, such as underreporting, false reporting, incomplete reporting, inaccuracy and arbitrariness. Third, some relevant statistics, such as the incidence rate for each suspicious drug, cannot be calculated due to the lack of total numbers of patients receiving treatment.
The present study showed that SGLT2is used in type 2 diabetes mellitus seems to have no safety profile regarding fracture events. It is recommended to assess fractures as the primary outcome, at long‐term follow up, and in high‐quality real‐world studies to further verify and explore the relationship between SGLT2is and fractures.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank St. Bernard Flake for the inspiration in this manuscript. No funding was received for this study.
J Diabetes Investig. 2021
Bin Zhao and Juan Shen contributed equally to this work.
References
- 1.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015; 12: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: The CVD‐REAL 2 Study. J Am Coll Cardiol 2018; 71: 2628–2639. [DOI] [PubMed] [Google Scholar]
- 3.Cavender MA, Norhammar A, Birkeland KI, et al. SGLT‐2 Inhibitors and cardiovascular risk: an analysis of CVD‐REAL. J Am Coll Cardiol 2018; 71: 2497–2506. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 5.Bonaventura A, Carbone S, Dixon DL, et al. Pharmacologic strategies to reduce cardiovascular disease in type 2 diabetes mellitus: focus on SGLT‐2 inhibitors and GLP‐1 receptor agonists. J Intern Med 2019; 286: 16–31. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: The CVD‐REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium‐Glucose Cotransporter‐2 Inhibitors). Circulation 2017; 136: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Canada Clinical Practice Guidelines Expert Committee , Lipscombe L, Booth G, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes 2018; 42 (Suppl 1): S88–S103. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020; 63: 221–228. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, D’Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018; 72: 3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New Eng J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 13.Musso G, Gambino R, Cassader M, et al. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta‐analysis of randomised controlled trials. BMJ 2019; 365: l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirabelli M, Chiefari E, Caroleo P, et al. Long‐term effectiveness and safety of SGLT‐2 inhibitors in an Italian cohort of patients with type 2 diabetes mellitus. J Diabetes Res 2019; 2019: 3971060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Yang J, Zhao B. A survey of the FDA’s adverse event reporting system database concerning urogenital tract infections and sodium glucose cotransporter‐2 inhibitor use. Diabetes Ther 2019; 10: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Sarangdhar M, De Ponti F, et al. Pharmacovigilance assessment of the association between Fournier’s gangrene and other severe genital adverse events with SGLT‐2 inhibitors. BMJ Open Diabetes Res Care 2019; 7: e000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food & Drug Administration . FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. 2015. https://www.fda.gov/drU.S. drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐fda‐revises‐label‐diabetes‐drug‐canagliflozin‐invokana‐invokamet. Accessed Feb 25, 2020.
- 18.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of Canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2015; 101: 157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmedt N, Andersohn F, Walker J, et al. Sodium‐glucose co‐transporter‐2 inhibitors and the risk of fractures of the upper or lower limbs in patients with type 2 diabetes: a nested case‐control study. Diabetes Obes Metab 2019; 21: 52–60. [DOI] [PubMed] [Google Scholar]
- 20.Kohler S, Kaspers S, Salsali A, et al. Analysis of fractures in patients with type 2 diabetes treated with Empagliflozin in pooled data from placebo‐controlled trials and a head‐to‐head study versus glimepiride. Diabetes Care 2018; 41: 1809–1816. [DOI] [PubMed] [Google Scholar]
- 21.van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 2002; 11: 3–10. [DOI] [PubMed] [Google Scholar]
- 22.Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry 2010; 19: 227–229. [PMC free article] [PubMed] [Google Scholar]
- 23.Ooba N, Kubota K. Selected control events and reporting odds ratio in signal detection methodology. Pharmacoepidemiol Drug Saf 2010; 19: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 24.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001; 10: 483–486. [DOI] [PubMed] [Google Scholar]
- 25.Hauben M, Madigan D, Gerrits CM, et al. The role of data mining in pharmacovigilance. Exp Opin Drug Safety 2005; 4: 929–948. [DOI] [PubMed] [Google Scholar]
- 26.Noren GN, Bate A, Orre R, et al. Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med 2006; 25: 3740–3757. [DOI] [PubMed] [Google Scholar]
- 27.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher‐than‐expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 2002; 25: 381–392. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care 2020; 43(Suppl 1): S98–S110. [DOI] [PubMed] [Google Scholar]
- 29.Meier C, Schwartz AV, Egger A, et al. Effects of diabetes drugs on the skeleton. Bone 2016; 82: 93–100. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015; 3: 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with Canagliflozin. J Clin Endocrinol Metab 2016; 101: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 33.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 34.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 35.Ruanpeng D, Ungprasert P, Sangtian J, et al. Sodium‐glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: A meta‐analysis. Diabetes Metab Res Rev 2017; 33. [DOI] [PubMed] [Google Scholar]
- 36.Hidayat K, Du X, Shi BM. Risk of fracture with dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists, or sodium‐glucose cotransporter‐2 inhibitors in real‐world use: systematic review and meta‐analysis of observational studies. Osteoporos Int 2019; 30: 1923–1940. [DOI] [PubMed] [Google Scholar]
- 37.Hidayat K, Du X, Wu MJ, et al. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: systematic review and meta‐analysis of observational studies. Obes Rev 2019; 20: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 38.Vavanikunnel J, Charlier S, Becker C, et al. Association between glycemic control and risk of fracture in diabetic patients: a nested case‐control study. J Clin Endocrinol Metab 2019; 104: 1645–1654. [DOI] [PubMed] [Google Scholar]


