Abstract
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is a rare condition in which patients develop an isolated dissection of the superior mesenteric artery without traumatic or iatrogenic causes. We present the case of a 52-year-old woman who presented with SISMAD and underwent endovascular stenting as her symptoms failed to respond to medical management. We also spend the bulk of the report discussing the current literature on management of SISMAD.
INTRODUCTION
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is a rare condition in which a superior mesenteric artery (SMA) dissection occurs without trauma, iatrogenic causes or an associated aortic dissection. Numerous case reports in literature have provided insight into the pathophysiology and optimal treatment strategies [1–5]. While SISMAD will typically regress with anticoagulation therapy, especially if it is asymptomatic, the indication to intervene in setting of persistent symptoms remains debated [1, 2]. Despite the increasing number of case reports, the disease remains relatively rare and more data are required to develop robust clinical guidelines and recommendations for treatment. We present a case of symptomatic SISMAD that was treated with endovascular stent placement. Consent for publication of this report was obtained from the patient.
CASE REPORT
A 52-year-old woman presented to the emergency department with 3 days of diffuse abdominal pain that started during a dance class. The pain had a sudden onset with radiation to her back and did not worsen with meals. She had mild associated nausea and bloating but no other symptoms including emesis, hematochezia, melena or diarrhea. Her medical history was notable for former smoking history (10 pack-years, quit 5 weeks prior to presentation), but she was otherwise healthy and was taking no medications. Her surgical history consisted of laparoscopic appendectomy and diagnostic laparoscopy for endometriosis, both of which occurred more than a decade prior to presentation. She was hemodynamically stable with systolic blood pressure ranging from 100 to 120 s mmHg, and her exam was notable for mild diffuse abdominal tenderness without evidence of peritonitis. Her complete blood count and basic metabolic panel were within normal limits, and a venous blood gas demonstrated no evidence of lactic acidosis. A computed tomography (CT) angiogram of the abdomen and pelvis demonstrated a 5–6 cm length proximal SMA dissection with high-grade stenosis of the true lumen as well as a short-segment right external iliac artery dissection with mild narrowing of the true lumen (Fig. 1). There was no radiographic evidence of bowel ischemia.
Figure 1 .
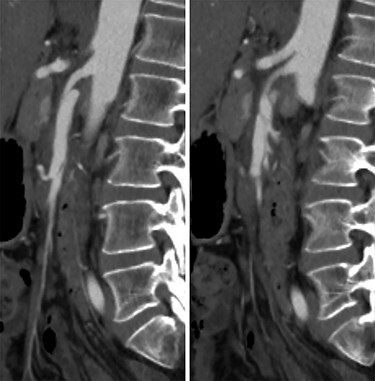
CT angiogram of the SMA dissection demonstrating the Left: false lumen supplying the middle colic artery with Right: the narrowed true lumen supplying the other SMA branches.
She was admitted and maintained on bowel rest and heparin infusion. Given persistent pain after 2 days of non-operative management, an abdominal arteriogram was performed through a left axillary artery cutdown. The dissection was identified 1.5 cm distal to the origin of the SMA (Fig. 2). Intravascular ultrasound was used to determine diameters and landing zones for stent placement (Fig. 3). The dissection was initially treated with a 6 mm × 40 mm self-expanding stent followed by post-dilation with 4 mm × 40 mm and 6 mm × 40 mm balloons. A 5 mm × 19 mm stent graft was deployed proximally with small overlap and ~3-mm extension into the aorta. Completion angiogram showed <10% residual stenosis (Fig. 4). As the right external iliac artery dissection was small and asymptomatic, no intervention was performed.
Figure 2 .
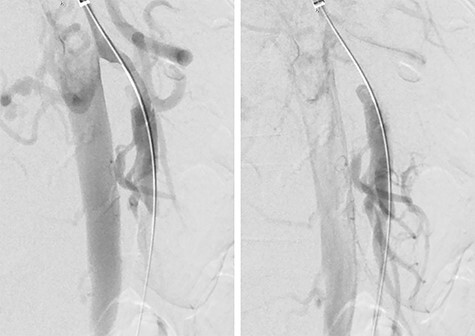
Pre-stenting angiogram of the SMA demonstrating early filling of the false lumen including the middle colic artery.
Figure 3 .
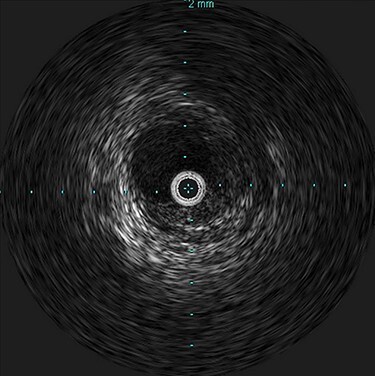
Intravascular ultrasound probe within the true lumen, with false lumen visualized to the bottom right of the probe.
Figure 4 .
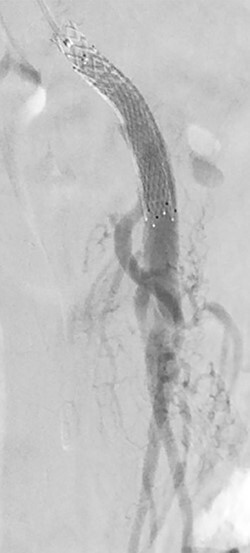
Post-stenting angiogram of the SMA demonstrating preservation of the middle colic artery as well as the distal branches of the SMA.
Postoperatively, her abdominal pain resolved and she was discharged on postoperative Day 1 with aspirin 81 mg and clopidogrel 75 mg daily. A 4-week and 6-month follow-up abdominal duplex ultrasound demonstrated patent SMA stents without dissection or stenosis. Ankle-brachial indices at 6 months were normal bilaterally.
DISCUSSION
SISMAD lacks the strongly associated predisposing factors seen with aortic dissections, with the greatest risk factors being male gender (70–80%) and hypertension (30–50%) [2]. The precise etiology of SISMAD remains unclear, although its typical location 1–3 cm from the ostium of the SMA has led researchers to postulate that the transition from the fixed retropancreatic segment to the mobile mesenteric segment at the sharp angulation of the SMA generates a high shear stress that contributes to the intimal tear [6]. The abdominal pain associated with SISMAD frequently occurs without bowel ischemia, with Yun et al. [7] reporting post-prandial pain in only 27% of symptomatic patients. One proposed mechanism for this pain is that the reactive inflammation to the dissection stimulates the visceral nerve plexus [8].
Several studies have proposed treatment algorithms for SISMAD [1, 3, 5]. In the absence of bowel ischemia, a trial of non-operative treatment is recommended. Subhas et al. [1] recommended administering anticoagulation for all patients with SISMAD until radiographic resolution of the dissection. Meanwhile, Yun et al. [7] reported no significant difference in outcome without anticoagulation and suggested anticoagulation may delay thrombosis of the false lumen, which may lead to further propagation of the dissection.
The indication to intervene on SISMAD typically consists of bowel ischemia, progression of the dissection or aneurysm, narrowing or thrombosis of the true lumen and saccular aneurysm at risk of causing rupture or embolization [1, 5]. Persistent pain beyond 7–14 days and concurrent celiac artery dissection is also a common indication for intervention, and Takach and colleagues reported using >80% diameter compromise of the true lumen as an indication to intervene [3–5]. Morgan et al. [2] treated 77 patients with spontaneous celiac or SMA dissections, including 50 symptomatic patients, demonstrating the viability of medical management in both symptomatic and asymptomatic patients. However, symptomatic patients have a higher risk of failing medical therapy at 16% compared with 3% for asymptomatic patients [5].
If the decision is made to intervene, there are numerous options available for revascularization depending on the anatomic characteristics of the SISMAD. Evidence of bowel ischemia mandates investigation with laparoscopy or laparotomy, with resection of infarcted bowel segments [1, 4]. Subhas et al. [1] recommended endovascular balloon angioplasty or stent placement for focal proximal stenosis and open intimectomy with patch angioplasty for long segment stenosis involving multiple branches. Mesenteric bypass or SMA transposition may be more appropriate if the dissection is not amenable to endovascular intervention [2, 4, 5].
In this case we started with non-operative management and anticoagulation and then chose to perform endovascular stenting when her symptoms did not improve in setting of an ~80% diameter narrowing of the true lumen. Her symptoms rapidly resolved postoperatively, and the stent remained patent at 6-month follow up. We plan to follow up with abdominal duplex every 6 months for 2 years, then yearly after. The patient will be on lifetime clopidogrel and statin therapy, and reintervention will be considered if she develops recurrent symptoms with in-stent stenosis >80%.
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Yonjae J Kim, General Surgery, Carle Foundation Hospital, Urbana, IL 61801, USA.
Brian R Beeman, Vascular Surgery, Carle Foundation Hospital, Urbana, IL 61801, USA.
References
- 1.Subhas G, Gupta A, Nawalany M, Oppat WF. Spontaneous isolated superior mesenteric artery dissection: a case report and literature review with management algorithm. Ann Vasc Surg 2009;23:788–98. [DOI] [PubMed] [Google Scholar]
- 2.Morgan CE, Mansukhani NA, Eskandari MK, Rodriguez HE. Ten-year review of isolated spontaneous mesenteric arterial dissections. J Vasc Surg 2018;67:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerbib P, Perot C, Lambert M, Seblini M, Pruvot FR, Chambon JP. Management of isolated spontaneous dissection of superior mesenteric artery. Langenbecks Arch Surg 2010;395:437–43. [DOI] [PubMed] [Google Scholar]
- 4.Takach TJ, Madjarov JM, Holleman JH, Robicsek F, Roush TS. Spontaneous splanchnic dissection: application and timing of therapeutic options. J Vasc Surg 2009;50:557–63. [DOI] [PubMed] [Google Scholar]
- 5.Garrett HE. Options for treatment of spontaneous mesenteric artery dissection. J Vasc Surg 2014;59:1433–1439.e2. [DOI] [PubMed] [Google Scholar]
- 6.Park YJ, Park C-W, Park KB, Roh YN, Kim D-I, Kim Y-W. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg 2011;53:80–6. [DOI] [PubMed] [Google Scholar]
- 7.Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg 2009;37:572–7. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa H, Moriyama N. Spontaneous dissection of the superior mesenteric artery diagnosed on multidetector helical CT. J Comput Assist Tomogr 2002;26:143–4. [DOI] [PubMed] [Google Scholar]


