Abstract
Background:
The majority of two-stage prepectoral breast reconstruction has been described utilizing acellular dermal matrix (ADM). Although reports of prepectoral breast reconstruction without ADM exist, there is a paucity of comparative studies.
Methods:
A single-institution retrospective review was performed of consecutive patients undergoing immediate prepectoral two-stage breast reconstruction with tissue expanders from 2017 to 2019. Short-term reconstructive and aesthetic complications were compared between cases that utilized ADM for support and those that did not.
Results:
In total, 76 cases (51 patients) were identified, of which 35 cases utilized ADM and 41 did not. Risk factors and demographics were similar between the two cohorts with the exception of body mass index, which was higher in the ADM cohort (29.3 versus 25.4, P = 0.011). Average follow-up length was also longer in patients who received ADM (20.3 versus 12.3 months, P < 0.001). Intraoperative expander fill was higher in patients who did not receive ADM (296.8 cm3 versus 151.4 cm3, P < 0.001) though final implant size was comparable in both cohorts (P = 0.584). There was no significant difference in the rate of any complication between the ADM and no ADM cohorts (25.7% versus 17.1%, respectively P = 0.357), including major mastectomy flap necrosis (P = 0.245), major infection (P = 1.000), seroma (P = 0.620), expander explantation (P = 1.000), capsular contracture (P = 1.000), implant dystopia (P = 1.000), and rippling (P = 0.362).
Conclusions:
Immediate two-stage prepectoral breast reconstruction with tissue expanders has comparable rates of short-term complications with or without ADM support. Safety of prepectoral expander placement without ADM may warrant more selective ADM use in these cases.
INTRODUCTION
Prepectoral breast reconstruction has become increasingly described in the literature over the last several years. This represents a near full-circle journey from prosthesis placement in the “subcutaneous” plane,1,2 to total submuscular coverage,2 dual-plane approaches,3,4 and now muscle-sparing techniques.5,6 Critical differences in today’s procedures include refined mastectomy techniques to minimize any residual breast tissue while preserving the subcutaneous tissue and superficial perfusion,7 as well as newer-generation prosthetic devices and adjunctive tools such as acellular dermal matrix (ADM).8
Many different technical variations of prepectoral prosthesis placement exist. However, the overwhelming majority of studies in the literature utilize ADM in some form.5,6,9–12 Described benefits of ADM use include support of the prosthetic device and pocket definition,13 decreased rippling,14 a reduced inflammatory response15,16 that may minimize capsular contracture rates, and potential protection against the deleterious effects of radiation.17,18
However, studies have also described immediate two-stage prepectoral reconstruction without any ADM with low short- and long-term complication rates.19,20 ADM is additionally associated with a significant cost21 and potential complications such as seroma and infection.22,23 Furthermore, with the advent of tabbed tissue expanders, that allow for fixation of the prosthesis in place, the role of ADM in two-stage prepectoral breast reconstruction has become less clear cut. However, there remains a paucity of data comparing prepectoral techniques without ADM to those with the more standardized anterior ADM support.24
The purpose of this study was to directly compare short-term outcomes in immediate tissue-expander prepectoral breast reconstruction cases with and without ADM. Analysis focused on reconstructive and aesthetic complications to better understand the safety of reconstruction without ADM and further define the role of adjunctive support materials in two-stage prepectoral breast reconstruction.
METHODS
Data Collection and Analysis
A retrospective review was performed of all patients who underwent prepectoral breast reconstruction at a single institution from 2017 to 2019 after institutional review board approval. All consecutive cases of two-stage reconstruction with immediate tissue expander placement were included for analysis. Cases utilizing a dermal flap were excluded from analysis. Cases were divided into “ADM” and “no ADM” cohorts based on if any ADM was utilized at the initial reconstruction, and variables and outcomes were compared between the two cohorts.
Patient demographics, oncologic characteristics, and mastectomy and reconstructive details were analyzed. Specifically, expander type, ADM characteristics, initial expander fill, and implant details were examined. Outcomes included reconstructive complications such as ischemic complications, infection, seroma, and reconstruction failure. Major and minor ischemic complications and infection were defined as previously described.25 Aesthetic complications including capsular contracture, implant dystopia and rippling were evaluated in patients who underwent successful implant exchange.
Surgical Indications and Technique
Ideal candidates for prepectoral reconstruction are determined preoperatively in conjunction with the breast surgeon and patient, as previously described.26 The same five breast and four plastic surgeons were involved throughout the study period with no significant changes in mastectomy or reconstruction techniques other than the variable use of ADM and type of tissue expander. ADM utilization was based on plastic surgeon preference with no specific indication or contraindication for ADM. Prosthesis placement without ADM support began in January 2019 with the availability of tabbed tissue expanders at our institution. Currently only smooth, tabbed expanders are utilized by all surgeons.
After mastectomy completion, mastectomy flap thickness27,28 and quality13 are meticulously evaluated to determine appropriateness for prepectoral prosthesis placement based on clinical examination.25 Indocyanine green angiography is not utilized given the judicious infiltration of a dilute epinephrine-containing solution. Any concern for mastectomy flap viability prompted implant placement in the subpectoral position or reconstruction delay. Intraoperative mastectomy flap evaluation did not influence ADM use if prepectoral reconstruction was deemed appropriate.
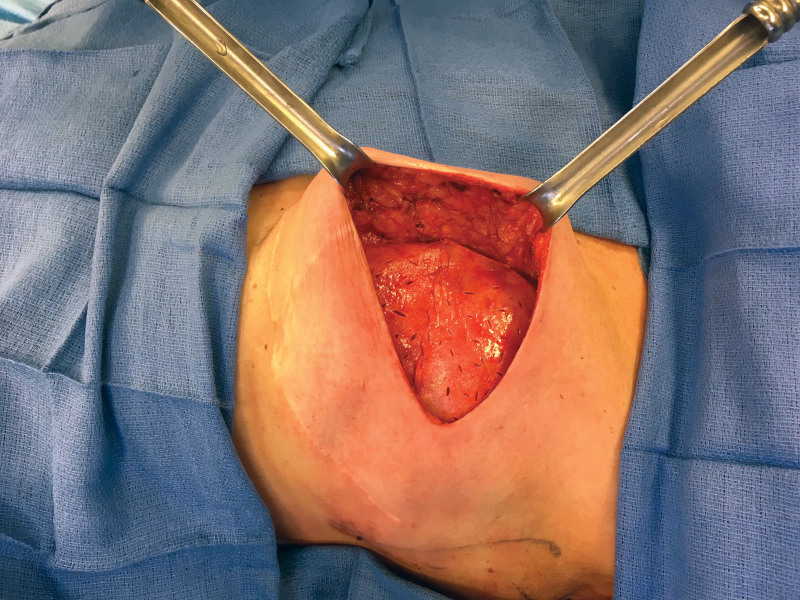
In cases with ADM, either thin medium contour perforated Alloderm (LifeCell Corp., Branchburg, N.J.) or pliable shaped perforated Flex HD (Musculoskeletal Transplant Foundation Biologics, Edison, N.J.) was utilized. ADM fixation techniques included an anterior wrap with fixation of ADM tabs to the chest wall or anterior chest wall fixation of ADM sheets (Fig. 1) with reinforcement of the inframammary fold (IMF) and anterior axillary line. ADM type, number of sheets, and ADM fixation technique was based on surgeon preference.
Fig. 1.
Prepectoral tissue expander in situ with two pieces of fenestrated ADM utilized to define the pocket and support the prosthesis.
In cases without ADM, the mastectomy pocket is meticulously defined to achieve a conforming fit with the prosthesis and minimize the possibility of expander movement. The IMF is reinforced to the chest wall with long-lasting absorbable sutures. The lateral skin and soft tissue is then sutured to the chest wall to delineate the anterior axillary line, obliterate lateral deadspace, and ensure a snug fit with the prosthesis. (See Video [online], which displays the surgical technique for prepectoral breast reconstruction with immediate tissue-expander placement without ADM.) Tabbed tissue expanders were secured to the chest wall in the appropriate position using long-lasting absorbable sutures.
Video 1. Surgical technique. Video 1 from “Do We Need Support in Two-Stage Prepectoral Breast Reconstruction? Comparing Short-Term Outcomes with and Without ADM”.
In all cases, tissue expanders were filled with saline at time of initial reconstruction based on surgeon preference and quality of the mastectomy flaps. Drains were maintained until output was more than 30 cm3/day for 2 consecutive days. Inflation typically began at 2 weeks postoperatively until the desired fill was achieved. At the time of implant exchange, near circumferential capsulotomy was carefully performed with additional radial scoring to expand the implant pocket as needed. Smooth, round implants were utilized in all cases.
Statistical Analysis
A power analysis was performed to estimate the needed sample size. A moderate effect size of w = 0.5 was chosen to represent the predicted difference of any complication between the two groups. Given this, it was found that with an alpha of 0.05 and a power (1-beta) of 0.90, the projected sample size required was 43 cases.
Descriptive statistics and measures of central tendency were used to describe absolute and mean results, respectively. Continuous variables were first tested for normality using the Shapiro-Wilks test. Given the non-normal distribution observed, continuous and ordinal variables were compared using the Mann-Whitney U test. Categorical variables were compared using the chi-Square or Fischer exact test as appropriate. For all analyses, the level of statistical significance was set at an alpha of 0.05. All statistical analyses were performed using SPSS Statistics, Premium v25 (IBM, Armonk, N.Y.).
RESULTS
In total, 76 cases were identified (51 patients), of which 35 cases utilized ADM (Fig. 2) and 41 did not (Fig. 3). There were no significant differences in age, comorbidities, tobacco use, neoadjuvant/adjuvant therapies, and oncologic characteristics between the two groups (Table 1). An estimated 5.7% of patients in the ADM group and 14.6% in the no ADM cohort had prior radiation (P = 0.275), and 25.7% versus 22.0% had adjuvant radiation, respectively (P = 0.701). Body mass index (BMI) was higher in the ADM cohort than in the no ADM cohort (29.3 versus 25.4, P = 0.011). Average follow-up length was also longer in patients who received ADM (20.3 versus 12.3 months, P < 0.001).
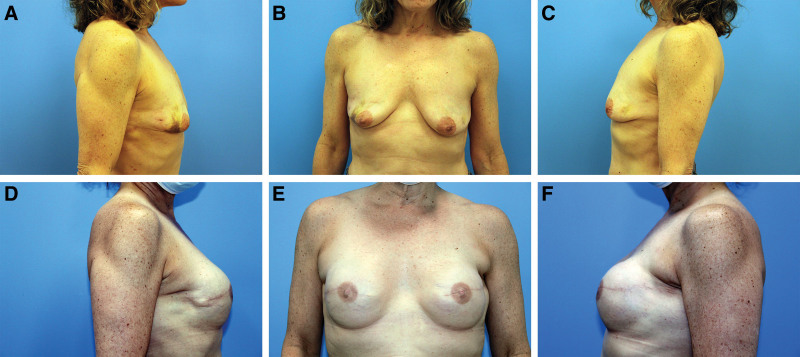
Fig. 2.
Prepectoral breast reconstruction with ADM. A 60-year-old woman with a history of right breast segmental excisions and left breast cancer (A-C) who underwent bilateral skin-sparing mastectomy and immediate prepectoral reconstruction with 250 ml tissue expanders and anterior ADM. The patient underwent subsequent implant exchange with smooth, round highly cohesive 310 ml silicone implants (D-F).
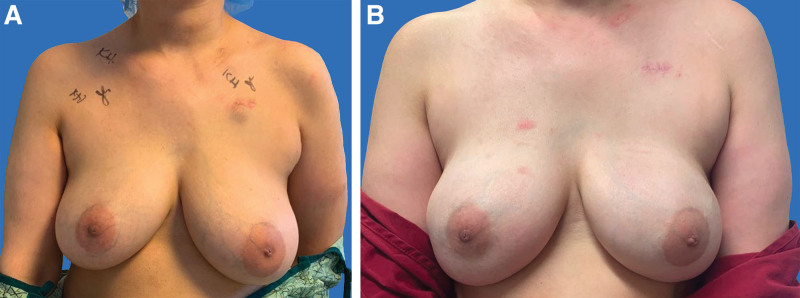
Fig. 3.
Prepectoral breast reconstruction without ADM. A 41-year-old woman with locally advanced right breast cancer (A) who underwent bilateral nipple-sparing mastectomy with inframammary incisions and immediate prepectoral reconstruction with 400 ml tissue expanders. The patient subsequently underwent implant exchange with smooth, round cohesive 560 ml implants (B).
Table 1.
Patient Demographics and Oncologic Characteristics in Two-Stage Prepectoral Reconstruction Cases with and without ADM
| Total | ADM | No ADM | P | |
|---|---|---|---|---|
| Breasts | 76 | 35 | 41 | — |
| Patients | 51 | 23 | 28 | — |
| Age (y)* | 52.1 ± 1.2 | 51.4 ± 1.7 | 52.6 ± 1.7 | 0.735 |
| Mean BMI (kg/m2)* | 27.2 ± 0.8 | 29.3 ± 1.2 | 25.4 ± 0.9 | 0.011 |
| Diabetes mellitus | 6 (7.9%) | 3 (8.6%) | 3 (7.3%) | 1.000 |
| Active tobacco use | 1 (1.3%) | 0 | 1 (2.4%) | 1.000 |
| Former tobacco use | 22 (28.9%) | 9 (25.7%) | 13 (31.7%) | 0.566 |
| Prior breast augmentation | 3 (3.9%) | 0 | 3 (7.3%) | 0.245 |
| Prior breast reduction | 6 (7.9%) | 5 (14.3%) | 1 (2.4%) | 0.089 |
| Prior lumpectomy | 16 (21.1%) | 7 (20.0%) | 9 (22.0%) | 0.835 |
| Previous radiation | 8 (10.5%) | 2 (5.7%) | 6 (14.6%) | 0.275 |
| Previous chemotherapy | 21 (27.6%) | 7 (20.0%) | 14 (34.1%) | 0.169 |
| Postoperative radiation | 18 (23.7%) | 9 (25.7%) | 9 (22.0%) | 0.701 |
| Postoperative chemotherapy | 25 (32.9%) | 13 (37.1%) | 12 (29.3%) | 0.466 |
| Cancer stage | 0.202 | |||
| k 0 | 15 (20.3%) | 4 (12.1%) | 11 (26.8%) | |
| IA/IB | 25 (33.8%) | 13 (39.4%) | 12 (29.3%) | |
| IIA/IIB | 21 (28.4%) | 8 (24.2%) | 13 (31.7%) | |
| IIIA/IIIB/IIIC | 12 (16.2%) | 7 (21.2%) | 5 (12.2%) | |
| IV | 1 (1.4%) | 1 (3.0%) | 0 | |
| Follow-up length (mo)* | 16.0 ± 0.9 | 20.3 ± 1.5 | 12.3 ± 0.8 | 0.000 |
| Follow-up length since exchange (mo)* | 11.1 ± 1.1 | 14.8 ± 1.8 | 8.6 ± 1.2 | 0.008 |
*Mean ± standard error of the mean.
Bold values are statistically significant.
The majority of mastectomies were performed for therapeutic indications in both cohorts (Table 2), though more nipple-sparing mastectomies were performed in the no ADM cohort (P = 0.008). Average mastectomy weight was significantly higher in the ADM cohort than in the no ADM cohort (661.8 g versus 450.8 g, P = 0.024).
Table 2.
Comparison of Mastectomy Characteristics
| ADM | No ADM | P | |
|---|---|---|---|
| Bilateral patients | 12 (52.2%) | 14 (50%) | 0.802 |
| Mastectomy indication | 0.521 | ||
| Therapeutic | 23 (65.7%) | 24 (58.5%) | |
| Prophylactic | 12 (34.3%) | 17 (41.5%) | |
| Mastectomy type | 0.008 | ||
| NSM | 2 (5.7%) | 11 (26.8%) | |
| SSM | 33 (94.3%) | 29 (70.7%) | |
| MRM | 0 | 1 (2.4%) | |
| Mastectomy weight (g)* | 661.8 ± 88.9 | 450.8 ± 44.9 | 0.024 |
*Mean ± standard error of the mean.
NSM, nipple-sparing mastectomy; SSM, skin-sparing mastectomy; MRM, modified racial mastectomy.
Boldface values are statistically significant.
The majority of tissue expanders were textured in both ADM and no ADM cases (74.3% and 65.9%, respectively), though tabbed tissue expanders were more frequently utilized in the no ADM cohort (87.8% versus 34.3%, P < 0.001) (Table 3). In cases that used ADM, most required two sheets (71.4%) of Alloderm (88.6%) using an anterior chest wall fixation technique (85.7%) rather than a wrap (14.3%). Initial intraoperative tissue expander fill was higher in patients who did not receive ADM (296.8 cm3 versus 151.4 cm3, P < 0.001). Implant size was comparable in both cohorts (P = 0.584), though cases without ADM were more likely to receive highly cohesive implants (72.0% versus 42.1%, P = 0.046).
Table 3.
Comparison of Reconstruction Characteristics
| ADM | No ADM | P | |
|---|---|---|---|
| Tabbed tissue expander | 12 (34.3%) | 36 (87.8%) | <0.001 |
| Tissue expander texturing | 0.425 | ||
| Textured | 26 (74.3%) | 27 (65.9%) | |
| Smooth | 9 (25.7%) | 14 (34.1%) | |
| ADM sheets | — | ||
| One | 10 (28.6%) | — | |
| Two | 25 (71.4%) | — | |
| ADM fixation technique | — | ||
| Anterior wrap | 5 (14.3%) | — | |
| Anterior chest wall fixation | 30 (85.7%) | — | |
| Type of ADM | — | ||
| Alloderm | 31 (88.6%) | — | |
| Flex HD | 4 (11.4%) | — | |
| Initial tissue expander fill (cm3)* | 151.4 ± 17.4 | 296.8 ± 19.1 | <0.001 |
| Time to exchange (mo)* | 4.4 (3.5–7.6) | 5.6 (4.0–7.3) | 0.405 |
| Implant size* | 460.3 ± 31.5 | 456.6 ± 20.0 | 0.584 |
| Highly cohesive implant | 8 (42.1%) | 18 (72.0%) | 0.046 |
| Fat grafting at time of exchange | 2 (10%) | 1 (4%) | 0.577 |
*Mean ± standard error of the mean.
There were no significant differences in the rate of any complication between the ADM and no ADM cohorts (P = 0.357) (Table 4). Rates of major ischemic complications were low in both cohorts. The ADM cohort had a major infection rate of 8.6% and seroma incidence of 2.9% (no ADM: 2.4% [P = 0.329] and 7.3% [P = 0.620], respectively). Four cases in each cohort (ADM 11.4%, no ADM 9.8% [P = 1.000]) required explantation of the initial tissue expander due to major mastectomy flap necrosis (25%), major infection (50%), or a combination of seroma and infection (25%). Aesthetic complications were analyzed in 44 cases after implant exchange. Grade III/IV capsular contracture rates were low in both the ADM and no ADM cohorts (0% versus 4.0%, respectively [P = 1.000]). Implant dystopia was present in 11.8% of ADM cases and 8.0% of no ADM cases (P = 1.000) after implant exchange. Sixteen percent of cases in the no ADM cohort had notable rippling compared with 5.0% in the ADM cohort (P = 0.357).
Table 4.
Comparison of Reconstructive and Aesthetic Complications
| ADM | No ADM | P | |
|---|---|---|---|
| Minor mastectomy flap necrosis | 0 | 3 (7.3%) | 0.245 |
| Major mastectomy flap necrosis | 1 (2.9%) | 1 (2.4%) | 1.000 |
| Minor NAC necrosis* | 0 | 0 | — |
| Full NAC necrosis* | 0 | 0 | — |
| Minor infection | 1 (2.9%) | 2 (4.9%) | 1.000 |
| Major infection | 3 (8.6%) | 1 (2.4%) | 0.329 |
| Seroma | 1 (2.9%) | 3 (7.3%) | 0.620 |
| Hematoma | 1 (2.9%) | 0 | 0.461 |
| Isolated dehiscence | 2 (5.7%) | 2 (4.9%) | 1.000 |
| Implant exchange | 0 | 1 (2.4%) | 1.000 |
| Tissue expander explantation | 4 (11.4%) | 4 (9.8%) | 1.000 |
| Capsular contracture (Grade III/IV)† | 0 | 1 (4.0%) | 1.000 |
| Implant dystopia† | 2 (11.8%) | 2 (8.0%) | 1.000 |
| Rippling† | 1 (5.0%) | 4 (16.0%) | 0.362 |
| Any complication | 9 (25.7%) | 7 (17.1%) | 0.357 |
*Total 13 nipple-sparing mastectomy cases.
†Total cases: 20 ADM cohort, 24 No ADM cohort.
Boldface values are statistically significant.
DISCUSSION
ADM is one of the many tools of the plastic surgeon and has been a mainstay in the prepectoral breast reconstruction literature.5,6,8,10,13 However, as with all other procedures in plastic surgery, treatment must be individualized to each patient to obtain the optimal results. There is no “one approach for all.” Hidalgo et al recently published a more selective and successful algorithm for using ADM in the treatment of capsular contracture.29 Similarly, more selective use of ADM may be possible in two-stage tissue expander breast reconstruction, as evidence has shown the possibility of successful prepectoral breast reconstruction with low complication rates without ADM.19,20
After the availability of tabbed tissue expanders at our institution in early 2019, the use of ADM decreased significantly (96.1% cases before this date and 20.0% after this date used ADM). The logic behind this transition was that fixation of the tissue expander in place addresses prosthesis support while tailoring of the mastectomy flaps allows for pocket definition. However, concern over a change in overall complications based on certain issues that may be mitigated by ADM such as pressure ischemia of the mastectomy flap, implant dystopia, and capsular contracture was the impetus for this study.
Our results demonstrated a low and comparable rate of complications between the ADM and no ADM cohorts. Importantly, there was no significant difference in major or minor mastectomy flap necrosis in cases without ADM. The most significant potential concern in an “unsupported” expander would be the weight of the tissue expander, particularly with saline fill, on mastectomy flaps that could compromise perfusion. Intraoperative expander fill was actually higher in the no ADM cohort, possibly secondary to increased comfort with the procedure later in the study period, though no differences in any type of ischemic complications were noted. In a prior critical study, Manrique et al. compared two-stage prepectoral reconstructions with and without ADM in a single-surgeon retrospective study, and similarly found low and comparable rates of ischemic and other complications.24 Of note, the authors intraoperatively filled expanders with air, and median intraoperative expansion volume was significantly less in cases without ADM.
The argument for ADM use as prothesis support is important in immediate implant reconstruction,30–32 but may be less relevant with the current generation of tissue expanders. Tabbed tissue expanders allow one to secure prosthesis position, and the use of long-lasting absorbable (or permanent) sutures will minimize prosthesis movement that is more notable with expanders. Textured tissue expanders may help further limit this expander movement and were used early in the study period when smooth expanders were not available. However, our current practice utilizes only smooth tabbed expanders. We have also found adipodermal flaps33,34 to be highly effective in patients with macromastia that undergo Wise-pattern-type incisions; however, these were excluded from this study because this would confound the comparative analysis.
Aside from implications for ischemic complications, support also plays a role in implant migration. Rates of implant dystopia were also comparable between the two cohorts, suggesting that the capsule of a well-defined pocket, along with suture reinforcement at the time of exchange may be adequate to support prostheses. In this regard, pocket definition at the initial stage is critical. This includes restoring the integrity of the IMF if disrupted during mastectomy and limiting lateral deadspace secondary to over dissection. Salibian et al reported implant dystopia in 0.8% of 250 prepectoral cases without ADM, with IMF reinforcement in thin patients receiving large implants at implant exchange.19 There are several additional variables that contribute to implant movement including capsular modifications, capsule thickness, and innate tissue quality, that are difficult to quantify. These factors, in addition to the need for longer-term data on this particular outcome, limit the applicability of our findings. However, an argument can be made that routine use of ADM for inferior support is not needed, and more select application may be warranted based on the aforementioned factors.
The most critical variable in reconstructive outcomes of prepectoral reconstruction, regardless of ADM use, is mastectomy flap quality. In the unfortunate situation of full-thickness mastectomy flap necrosis, visualizing nonvascularized ADM is no different than exposure of the prosthesis itself.35,36 Poor ADM adherence is a nidus for complications, which has led certain authors to indicate “ADM-sparing” reconstructions in the setting of compromised mastectomy flaps.37 Relative mastectomy flap thickness is one quantifiable component of quality27; however, adequate imaging data was not available for analysis in this study. Mastectomy flap quality, though, is thoroughly evaluated based on intraoperative clinical examination in all patients who receive prepectoral reconstruction. Cases with any concern for skin envelope viability are converted to submuscular reconstructions or delayed, and therefore do not influence the ADM selection in this study population.
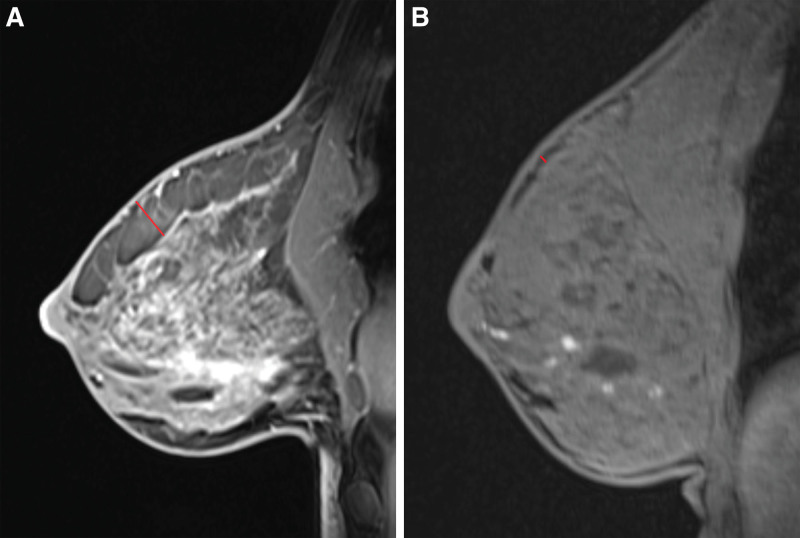
Despite preservation of the subcutaneous layer, the thickness of the subcutaneous tissue is highly variable among patients (Fig. 4). We have previously found a correlation between increased flap thickness and higher BMI.28 Interestingly, patients without ADM had a significantly lower BMI (and mastectomy weights) than those with ADM. This may seem paradoxical, but is likely a reflection of chronological selection for prepectoral reconstruction. Initially thick flaps were likely more frequently selected for prepectoral reconstruction, which was later expanded to thinner, but still well-perfused, flaps. Highly cohesive implants were used more frequently in the no ADM patients to minimize rippling,38 though lower BMI and likely thinner flaps may have contributed to the rippling rate of 16% in this cohort. Autologous fat is also an excellent means of improving soft tissue coverage,39,40 and more liberal use at the time of exchange in this cohort may have further decreased this complication. Lack of ADM may have additionally been a factor as ADM is used to treat rippling41; however, rates of rippling from other prepectoral studies without ADM have been lower (0%–3.6%).19,24 Though rippling was not found to be significantly different between the two cohorts in this study, a larger sample and long-term follow-up is needed to more decisively determine the need for ADM to prevent rippling, particularly in low-BMI patients.
Fig. 4.
A, Preoperative MRI of a patient with a “thick” layer of subcutaneous tissue superficial to breast capsule (red line). B, Preoperative MRI of patient with minimal subcutaneous tissue (red line) superficial to breast capsule.
Capsular contracture is another important outcome given the role of ADM in minimizing the inflammation leading to fibrosis and contracture.15 Rates of Grade III/IV capsular contracture were low in both cohorts, but were limited by short follow-up. Sigla et al reported a similar contracture rate of 3.8%, albeit with textured implants.20 Other long-term studies with smooth, round implants in the prepectoral plane have demonstrated a 7.6% rate of Grade III/IV contracture, suggesting a time-dependent component,19 though dual-plane ADM studies have shown static contracture rates after two years.42 Larger and longer-term comparative studies are needed to further evaluate this outcome, particularly in the setting of more recent stringent contamination prevention measures and smooth tissue expanders.
Tissue expander removal rates were similar between ADM and no ADM cases; however, overall explantation rates were high. While mastectomy techniques have remained standardized among the same five breast surgeons throughout the study period, this trend is likely reflective of an initial learning curve for both breast and plastic surgeons using prepectoral techniques. In the last six months of the study, this rate significantly decreased with two tissue expanders requiring removal (6.7%), and has continued to decline since the study period. These findings reflect the importance of mastectomy flap quality and the lower tolerance of prepectoral prosthesis for wound complications, reinforcing the importance of a coordinated approach between the breast and plastic surgeon, high quality mastectomy flaps and meticulous postoperative surveillance.
This study has several limitations, including its retrospective nature that restricts the assessment of outcomes to data retrieved from chart review. Certain reconstruction characteristics differed between the two cohorts, including a higher percentage of NSM and greater initial fill size in the non-ADM cohorts. Greater initial expander fill in the non-ADM cohort may have been secondary to multiple variables (including less tension on the skin closure), as this cohort had a higher rate of NSM, lower BMI in an attempt to achieve a larger initial breast mound, and potential differences in mastectomy flap thickness, which were unable to be objectively analyzed retrospectively. However, despite these differences, relevant complication rates such as mastectomy flap necrosis were similar between the two cohorts. Sample size was also limited in post-exchange patients to analyze certain differences in complications such as rippling, which may result in a type II error in comparison of these outcomes. Additionally, there is an aspect of selection bias in ideal candidates chosen for prepectoral reconstruction. However, there was no selection for ADM use that would have affected comparison of the outcomes of interest. A chronological “learning curve” likely contributed to certain observed differences between the two groups (BMI, mastectomy weight, initial tissue expander fill), though this did not affect the comparative rate of complications. Finally, long-term outcomes are needed, particularly with regard to aesthetic analysis and patient-reported outcomes.
CONCLUSIONS
Comparative outcomes in two-stage prepectoral breast reconstruction with and without ADM demonstrated the safety of immediate prepectoral expander placement without ADM support. Although certain benefits of ADM in implant-based breast reconstruction are well-known, these data suggest that more selective use of ADM may be warranted in the appropriate patients, rather than a blanket approach to these cases. In this regard, individualizing treatment choices based on the particular patient and mastectomy defect may help optimize both reconstructive and aesthetic outcomes, while minimizing cost and avoiding potential ADM-associated complications. Long-term data and comparative objective aesthetic and patient-reported outcomes from multiple institutions are needed to further refine the indications and implications of ADM use in two-stage prepectoral breast reconstruction.
Footnotes
Published online 10 August 2021.
Disclosure: Dr. Choi and Dr. Karp are consultants for Allergan. All the other authors have no financial interest to declare in relation to the content of this article. This study did not receive any funding.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Artz JS, Dinner MI, Foglietti MA, et al. Breast reconstruction utilizing subcutaneous tissue expansion followed by polyurethane-covered silicone implants: A 6-year experience. Plast Reconstr Surg. 1991;88:635–9;discussion 640. [PubMed] [Google Scholar]
- 2.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: A comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317. [DOI] [PubMed] [Google Scholar]
- 3.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. [DOI] [PubMed] [Google Scholar]
- 4.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. [DOI] [PubMed] [Google Scholar]
- 5.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: A safe alternative to submuscular prosthetic reconstruction following Nipple-Sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 6.Nahabedian MY, Cocilovo C. Two-Stage prosthetic breast reconstruction: A comparison between prepectoral and partial subpectoral techniques. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):22S–30S. [DOI] [PubMed] [Google Scholar]
- 7.Storm-Dickerson T, Sigalove N. Prepectoral breast reconstruction: The breast surgeon’s perspective. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):43S–48S. [DOI] [PubMed] [Google Scholar]
- 8.Nahabedian MY. Innovations and advancements with prosthetic breast reconstruction. Breast J. 2018;24:586–591. [DOI] [PubMed] [Google Scholar]
- 9.Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):51S–59S. [DOI] [PubMed] [Google Scholar]
- 10.Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: A new dimension to implant-based breast reconstruction. Clin Breast Cancer. 2017;17:266–271. [DOI] [PubMed] [Google Scholar]
- 11.Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon acellular dermal matrix: A new muscle-sparing breast reconstruction. ANZ J Surg. 2017;87:493–498. [DOI] [PubMed] [Google Scholar]
- 12.Momeni A, Remington AC, Wan DC, et al. A matched-pair analysis of prepectoral with subpectoral breast reconstruction: Is there a difference in postoperative complication rate? Plast Reconstr Surg. 2019;144:801–807. [DOI] [PubMed] [Google Scholar]
- 13.Sbitany H. Important considerations for performing prepectoral breast reconstruction. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):7S–13S. [DOI] [PubMed] [Google Scholar]
- 14.Nahabedian MY, Glasberg SB, Maxwell GP. Introduction to “prepectoral breast reconstruction”. Plast Reconstr Surg. 2017;140:4S–5S. [DOI] [PubMed] [Google Scholar]
- 15.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg. 2010;126:1842–1847. [DOI] [PubMed] [Google Scholar]
- 16.Tevlin R, Borrelli MR, Irizarry D, et al. Acellular dermal matrix reduces myofibroblast presence in the breast capsule. Plast Reconstr Surg Glob Open. 2019;7:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: Defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg. 2014;134:396–404. [DOI] [PubMed] [Google Scholar]
- 18.Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg. 2009;123:807–816. [DOI] [PubMed] [Google Scholar]
- 19.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39. [DOI] [PubMed] [Google Scholar]
- 20.Singla A, Singla A, Lai E, et al. Subcutaneously placed breast implants after a skin-sparing mastectomy: Do we always need ADM? Plast Reconstr Surg Glob Open. 2017;5:e1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg. 2012;69:516–520. [DOI] [PubMed] [Google Scholar]
- 22.Smith JM, Broyles JM, Guo Y, et al. Human acellular dermis increases surgical site infection and overall complication profile when compared with submuscular breast reconstruction: An updated meta-analysis incorporating new products. J Plast Reconstr Aesthet Surg. 2018;71:1547–1556. [DOI] [PubMed] [Google Scholar]
- 23.Kim JYS, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. [DOI] [PubMed] [Google Scholar]
- 24.Manrique OJ, Huang TC, Martinez-Jorge J, et al. Prepectoral two-stage implant-based breast reconstruction with and without acellular dermal matrix: Do we see a difference? Plast Reconstr Surg. 2020;145:263e–272e. [DOI] [PubMed] [Google Scholar]
- 25.Frey JD, Salibian AA, Bekisz JM, et al. What is in a number? Evaluating a risk assessment tool in immediate breast reconstruction. Plast Reconstr Surg Glob Open. 2019;7:e2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salibian AA, Frey JD, Karp NS. Strategies and considerations in selecting between subpectoral and prepectoral breast reconstruction. Gland Surg. 2019;8:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey JD, Salibian AA, Choi M, et al. Mastectomy flap thickness and complications in nipple-sparing mastectomy: Objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2017;5:e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey JD, Salibian AA, Choi M, et al. Optimizing outcomes in nipple-sparing mastectomy: Mastectomy flap thickness is not one size fits all. Plast Reconstr Surg Glob Open. 2019;7:e2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidalgo DA, Weinstein AL. Surgical treatment for capsular contracture: A new paradigm and algorithm. Plast Reconstr Surg. 2020;146:516–525. [DOI] [PubMed] [Google Scholar]
- 30.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):31S–38S. [DOI] [PubMed] [Google Scholar]
- 31.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: A new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. [DOI] [PubMed] [Google Scholar]
- 32.Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon. Gland Surg. 2017;6:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705. [DOI] [PubMed] [Google Scholar]
- 34.Mosharrafa AM, Mosharrafa TM, Zannis VJ. Direct-to-implant breast reconstruction with simultaneous nipple-sparing mastopexy utilizing an inferiorly based adipodermal flap: Our experience with prepectoral and subpectoral techniques. Plast Reconstr Surg. 2020;145:1125–1133. [DOI] [PubMed] [Google Scholar]
- 35.Salibian AA, Frey JD, Bekisz JM, et al. Ischemic complications after nipple-sparing mastectomy: predictors of reconstructive failure in implant-based reconstruction and implications for decision-making. Plast Reconstr Surg Glob Open. 2019;7:e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SY, Bang SI. Impact of acellular dermal matrix (ADM) use under mastectomy flap necrosis on perioperative outcomes of prosthetic breast reconstruction. Aesthetic Plast Surg. 2017;41:275–281. [DOI] [PubMed] [Google Scholar]
- 37.Safran T, Al-Halabi B, Viezel-Mathieu A, et al. Direct-to-implant, prepectoral breast reconstruction: A single-surgeon experience with 201 consecutive patients. Plast Reconstr Surg. 2020;145:686e–696e. [DOI] [PubMed] [Google Scholar]
- 38.Panettiere P, Marchetti L, Accorsi D. Soft cohesive silicone gel breast prostheses: A comparative prospective study of aesthetic results versus lower cohesivity silicone gel prostheses. J Plast Reconstr Aesthet Surg. 2007;60:482–489. [DOI] [PubMed] [Google Scholar]
- 39.Maxwell GP, Gabriel A. Bioengineered breast: Concept, technique, and preliminary results. Plast Reconstr Surg. 2016;137:415–421. [DOI] [PubMed] [Google Scholar]
- 40.Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: The management of acquired contour deformities. Plast Reconstr Surg. 2009;124:409–418. [DOI] [PubMed] [Google Scholar]
- 41.Maxwell GP, Gabriel A. Acellular dermal matrix for reoperative breast augmentation. Plast Reconstr Surg. 2014;134:932–938. [DOI] [PubMed] [Google Scholar]
- 42.Salzberg CA, Ashikari AY, Berry C, et al. Acellular dermal matrix-assisted direct-to-implant breast reconstruction and capsular contracture: A 13-year experience. Plast Reconstr Surg. 2016;138:329–337. [DOI] [PubMed] [Google Scholar]